A Potential Effect of Rh-BMP2 on P53 Gene Expression in the Proceeding Oral Squamous Cell Carcinoma in Hamsters
Download
Abstract
Introduction: Bone morphogenetic proteins (BMPs), belonging to the transforming growth factor-β superfamily, regulate many cellular activities. The Use of recombinant human bone morphogenic protein‑2 (rhBMP‑2) in oral and maxillofacial surgery has seen a tremendous increase, yet the relation between RH-BMP2 and oral squamous cell carcinoma cells (OSCC) is not fully clear. This study aimed to investigate the effect of RH-BMP2 on p53, a tumor suppressor gene, which is reported to be the most frequent target for genetic alterations leading to cancer during the progression of OSCC in the golden Syrian hamster Buccal pouches.
Materials and methods: Buccal pouches of 34 hamsters were painted with 0.5% DMBA in liquid paraffin three times per week for 14 weeks. 0.25μg of rhBMP-2 were injected in the 1st, 7th and 49th days in the Test group (BMP2 group: 17 hamsters), then biopsies were extracted. Expression of P53 was immunohistochemically investigated in the cancerous tissues.
Results: p53 expression was noticed in 33.11% of “BMP2 group” samples and in 23.19% of “control group” samples, a valuable statistical difference was noticed P<0.05between the two groups.
Conclusion: 0.25μg of rhBMP-2 affects clearly on p53 expression within OSCC cells. BMPs should not be utilized in the presence of a developed oral cancer till more investigations clarify its fully roles and functions in this type of malignancies.
Introduction
p53- the most famous tumor suppressor protein- is a transcription factor playing an important role in regulating the cell cycle and the induction of apoptosis in the most types of living cells. In vivo, this protein functions as a tumor suppressor, and more than 50% of all human cancers have been characterized with mutations in the p53 expression [1].
The cell cycle is negatively regulated by p53 gene and its mutations lead the cells to lose the normal function and becomes unable to suppress the tumor; thus p53 perturbing the control of cell cycle progression and leads to enhanced tumor development [2]. The production of wild-type p53 prevents uncontrolled cellular proliferation after DNA damage via G1 arrest of the cell cycle. This arrest functions either to provide extra time for DNA repair by mechanisms activated simultaneously by p53 or, if the repair fails, to trigger cell suicide by apoptosis[3].
Bone morphogenetic proteins (BMPs) was discovered as bone inductive proteins by Urist in the 1960s [4], since that time many investigators have shown that BMPs induce stem and mesenchymal cell differentiation into osteogenic cells which has the function of bone formation. Modern molecular biology theories state that BMPs are morphogenetic proteins, namely, molecules which stimulates the genome to start the construction of a morphogenetic area. BMPs diffuse through a concentration gradient, thereby altering the developmental process[5, 6].
In response to this stimulus, the cells proliferate according to a programmed pattern and spatial arrangement. From a physical and chemical point of view, BMPs are proteins secreted by cells, which act as ligands for receptors existed on the cellular membrane of several types of cells (autocrine or paracrine effects), thus establishing cell and tissue organization[7, 8].
The role of Bone morphogenetic proteins in bone arrangement during the development and fractures repair has been well established. BMPs have the ability to induce the formation of bone tissue in ectopic sites and in critical-sized defects of the bone in many animal models[9].
Recent research revealed that BMPs also are involved in tumor development, showing antitumorigenic effects in the initial stages and protumorigenic effects in progressive carcinogenesis [10].
Although (BMPs) are considered as multifunctional proteins, implantation of osteogenic BMPs such as BMP-2 at an osseous or extraosseous locations leads to bone formation. BMP-2 molecules are soluble, local-acting signaling proteins can bind to definite receptors located on the cellular membrane. These receptors transfer the signal through a group of proteins called Smads, which in turn stimulate specific genes[11].
BMP signaling plays a pivotal role in the balance between differentiation and proliferation of active muscular cells. Initially, BMP signals preserve satellite cells descendants in a proliferating state thereby increasing the numbers of the cells [12].
Therefore, the objective of the present study was to clarify some of the basic aspects of the cellular and molecular mechanisms of action of RH-BMP2 and its affection on P53 in the during the development and progression of OSCC which are characterised by imbalances of cell proliferation, differentiation and death, all of which are believed to be the result of alterations in cell cycle control.
Materials and methods
Animals
34 survived male golden Syrian hamsters -divided into two equal groups-, (8 to10) weeks old, weighing between (80 and120 g), were maintained in the Animal Houses of the faculty of pharmacy, Damascus University, Syria. The animals were preserved in polypropylene enclosed cages and were subjected to a standard nutritional system comprised of 21% protein, 5% lipids, 4% crude fiber, 8% ash, 1% calcium, 0.6% phosphorus, 3.4% glucose, 2% vitamins, 55% nitrogen-free extract and water. The animals were kept under controlled temperature (27±11C) and humidity (55±5%) with a 12 h light/dark cycle. One hamster in the BMP2 group and two in the control did not survive to the end of the experiment.
Materials
7,12-dimethylbenz[a]anthracene (DMBA) (95% purity, CAS NO.: 57–97–6 soluble in liquid paraffin) were purchased from Sigma Aldrich Chemical Pvt. Ltd. rhBMP-2 (0.25µg/ml , soluble in physiological serum) were purchased from Cowellmedi Co.,Ltd Korea . p53 (DO7; Bio-SB; Concentration of stock solution (CSS): 35ug/ml, Dilution 1:50)
Tumor induction and experimental procedures
The carcinogenesis process in the hamster’s buccal pouches was induced in the 34 golden Syrian hamsters by the applying of 0.5% DMBA in liquid paraffin topically for three times a week for 14 weeks. rhBMP-2 (0.25µg/ml) was injected in the Buccal pouch submucosal tissue of 17 animals (test group) at the 1st, 7th and 49th day, then animals were sacrificed and tissues samples were extracted and preserved in 10% buffered formalin.
Histopathological studies
Buccal pouches mucosa tissues were examined histopathologically in both groups animals -the control and experimental -. Tissue samples were fixed in 10% buffered formalin then processed and embedded in paraffin wax;
Two serial slices 4μm thick were prepared and placed on silanized slides. The sections were deparaffinized and rehydrated via xylene and three descending grades of alcohol. Antigen retrieval was carried out in a pressure cooker in 10 mM citrate buffer (pH 6.0) for 2 to 5min. The sections were
then incubated after covering them with 3% hydrogen peroxide for 15min to block any endogenous peroxidase activity, and then 34 slides incubated with the primary anti-p53 monoclonal antibody (Bio-SB, USA) for 4h at 27C temperature using an optimal dilution of 6 µg/ml. After additional incubation with the secondary antibody (45min) and streptavidin-peroxidase (30min), visualization was performed using freshly prepared diaminobenzidine (DAB) chromogen for 10min. The slides were finally counterstained with Harris hematoxylin. Then the sections were examined under the microscope to investigate the staining patterns and expression of p53 in each group.
Five of the most invading islands of a tumor were captured and the total number of a tumor epithelial cells within these islands were counted vertically from one side to another and examined for the p53 expression under magnification of 40X. Tumor cells were considered positive for p53 antibodies if there was brown intranuclear DAB staining irrespective of its intensity, while cells without brown staining were considered negative.
The labeling index for each field (field LI), was calculated as the total number of positive cells divided by the total number of cells in that field and for each case, the mean value of the 5 islands was calculated to represent the mean expression value of each case. For statistical analysis, the samples were divided into two groups using the median value of the mean LI as a cut-point; low expression (≤the median) and high expression groups (>the median)[3]. SPSS version 17.0 for Windows was used for statistical analysis.
Results
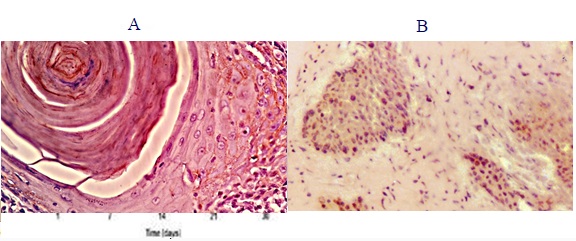
A total of 34 induced squamous cell carcinoma biopsies were evaluated for the expression of the tumor suppressor gene p53. Positive tumor cells were mainly located at the periphery of the invading tumor islands whereas the inner more mature and differentiated cells were negative (Fig. 1-2).
Figure 1 :Hamster buccal pouch after 14 days of DMBA application
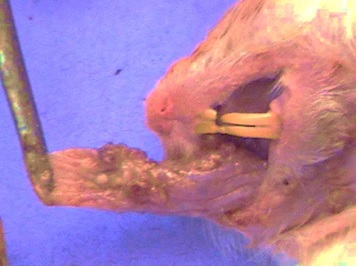
Figure 2 :The Nuclear Expression of p53 A, in the BMP2 group; B, in the control group.
with 17 hamster Buccal pouches injected with rhBMP2 during the tumoral induction and 17 did not receive any additional treatment. We determined p53 staining level in all of the 34 cases of both groups.
The median value of p53 expression was 26.81% (mean 27.2, range 4-77.3).
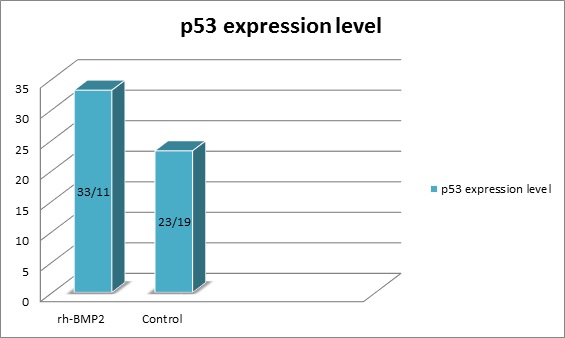
The mean of p53 expression in the (RH-BMP2 group) was 33.11%±25.31 and in the (control group) was 23.19% ± 18.27, (Fig. 3). Statistically, we found a significant difference between the two groups, Kruskal–Wallis = 55.656, P = 0.001.
Figure 3 :the expression percentage of p53 in the study groups.
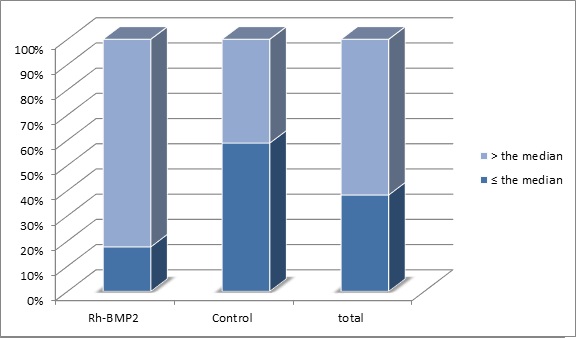
In the RH-BMP2 (n = 17 cases), 3 cases had p53 expression ≤ the median (26.81%) while 14 cases had been higher than the median. In the control group (n = 17 cases), 10 cases had p53 expression ≤ the median while only 7 cases had an expression higher than the median (Tabl 1)(Fig. 4).
| Study groups | |||
| Rh-BMP2 | Control | Total | |
| ≤ the median | 3 | 10 | 13 |
| > the median | 14 | 7 | 21 |
| total | 17 | 17 | 34 |
Figure 4 :Distribution of Cases According to the Median of P53 Expression
Discussion
Overexpression of p53 protein in OSCC has been reported in many studies [13, 14, 15] However, no study -as we know- of these studies attempted to determine a correlation between the level of p53 expression and the utilizing of RH-BMP2. A variety of the Clinicopathological investigations have targeted the Squamous Cells Carcinoma (SCC), which is the most common malignancy in the oral cavity and composes About 95% of oral cancers in some countries [16].
Bone morphogenic proteins are very important factors for tissues growth, organizing multiple processes during the embryonic development, hematopoiesis, formation of nervous tissue, in addition to determining the plan of differentiation and proliferation in the embryonic cells.
This sort of proteins was discovered in the 1960's and subjected to a huge number of investigations and researches over the past 60 years, however, there is still a lot of points that need to be clarified [9].
In the current study oral squamous cell carcinoma was induced in the golden Syrian hamsters' Buccal pouches using 7,12-dimethylbenz[a]anthracene (DMBA) which stimulates malignancy with molecular, histological and biochemical alteration similar to the alterations that occur in the human oral squamous cells carcinomas [17].
P53 expressed in 33.11% of the cells in the group which has been injected with RH-BMP2 during the induction of OSCC and most of the cases showed an expression higher than the median expression of the whole sample. Also, P53 expressed in 23.19% of the control group cells which did not receive any additional treatment and most of the cases showed expression under the median expression. The current study shows that an association between treatment with RH-BMP2 and p53 expression levels.
It has been well documented that alterations of levels of p53 expression are early events in the development of squamous carcinomas. Expressions of p53 have been found in dysplastic oral epithelial cells [18, 19]. There is no doubt that p53 expression is an important early step in the development of tongue carcinoma [20].
The link between RH-BMP2 and p53 may be explained by the alterations induced by BMP2 in the Wnt Pathway. The interaction between BMP2 and Wnt pathway where leads β-catenin to translocate from the cytoplasm to the nuclei. As β-catenin translocates into the cellular nuclei, it creates a complex with the transcriptional factor Tcf/Lef. This complex enhances the expression of many targeted genes - it may involve p53- and activates of the Wnt signaling pathway [21]. Thus, RH-BMP2 leads to cellular proliferation, migration, invasion, and EMT through the nuclear translocation of β-catenin [9].
Mutation and overexpression of p53 are common in head and neck cancers. The p53 mutation has been reported in 8 cell lines and 2 xenografts of head and neck cancers[22]. Expression of p53 has been reported in 78% of laryngeal carcinomas[23], 37% of hypopharyngeal carcinomas [24], 45% to 55% of oral cavity cancers[25], and 27% to 61% of tongue carcinomas[20]. In premalignant oral dysplasia or carcinoma in situ, 46% of the lesions had mutations and 20% had overexpression of p53 [26, 27, 28].In conclusion, 0.25µg of rhBMP-2 promotes significantly p53 expression within OSCC cells. this type of growth factors should not be utilized in patients with a developed oral cancer till more investigations clarify its fully roles and functions with this kind of patients.
References
[1]. Mesaeli N, Phillipson C. Impaired p53 expression, function, and nuclear localization in calreticulin-deficient cells. Mol Biol Cell. 2004;15(4):1862-70.
[2]. Khattak F, Haseeb M, Fazal S, Bhatti AI, Ullah M. Mathematical Modeling of E6-p53 interactions in Cervical Cancer. Asian Pac J Cancer Prev. 2017;18(4):1057-61.
[3]. Sawair F, Hassona Y, Irwin C, Stephenson M, Hamilton P, Maxwell P, et al. p53, Cyclin D1, p21 (WAF1) and Ki-67 (MIB1) Expression at Invasive Tumour Fronts of Oral Squamous Cell Carcinomas and Development of Local Recurrence. Asian Pac J Cancer Prev. 2016;17(3):1243-9.
[4]. Urist MR. Bone: formation by autoinduction. Science. 1965;150(3698):893-9.
[5]. Carreira AC, Alves GG, Zambuzzi WF, Sogayar MC, Granjeiro JM. Bone Morphogenetic Proteins: structure, biological function, and therapeutic applications. Arch Biochem Biophys. 2014;561:64-73.
[6]. Granjeiro JM, Oliveira RC, Bustos-Valenzuela JC, Sogayar MC, Taga R. Bone morphogenetic proteins: from structure to clinical use. Braz J Med Biol Res. 2005;38(10):1463-73.
[7]. Lee KB, Folger JK, Rajput SK, Smith GW. Temporal regulation of mRNAs for select bone morphogenetic proteins (BMP), BMP receptors and their associated SMAD proteins during bovine early embryonic development: effects of exogenous BMP2 on embryo developmental progression. Reprod Biol Endocrinol. 2014;12:67.
[8]. Cuellar A, Inui A, James MA, Borys D, Reddi AH. Immunohistochemical Localization of Bone Morphogenetic Proteins (BMPs) and their Receptors in Solitary and Multiple Human Osteochondromas. J Histochem Cytochem. 2014;62(7):488-98.
[9]. Zaid KW, Chantiri M, Bassit G. Recombinant Human Bone Morphogenetic Protein-2 in Development and Progression of Oral Squamous Cell Carcinoma. Asian Pac J Cancer Prev. 2016;17(3):927-32.
[10]. Blanco Calvo M, Bolos Fernandez V, Medina Villaamil V, Aparicio Gallego G, Diaz Prado S, Grande Pulido E. Biology of BMP signalling and cancer. Clin Transl Oncol. 2009;11(3):126-37.
[11]. Ebara S, Nakayama K. Mechanism for the action of bone morphogenetic proteins and regulation of their activity. Spine (Phila Pa 1976). 2002;27(16 Suppl 1):S10-5.
[12]. Friedrichs M, Wirsdoerfer F, Flohe SB, Schneider S, Wuelling M, Vortkamp A. BMP signaling balances proliferation and differentiation of muscle satellite cell descendants. BMC Cell Biol. 2011;12:26.
[13]. Solomon MC, Vidyasagar MS, Fernandes D, Guddattu V, Mathew M, Shergill AK, et al. The prognostic implication of the expression of EGFR, p53, cyclin D1, Bcl-2 and p16 in primary locally advanced oral squamous cell carcinoma cases: a tissue microarray study. Med Oncol. 2016;33(12):138.
[14]. Swaminathan U, Joshua E, Rao UK, Ranganathan K. Expression of p53 and Cyclin D1 in oral squamous cell carcinoma and normal mucosa: An Immunohistochemical study. J Oral Maxillofac Pathol. 2012;16(2):172-7.
[15]. Lingen MW, Chang KW, McMurray SJ, Solt DB, Kies MS, Mittal BB, et al. Overexpression of p53 in squamous cell carcinoma of the tongue in young patients with no known risk factors is not associated with mutations in exons 5-9. Head Neck. 2000;22(4):328-35.
[16]. Krishna A, Singh RK, Singh S, Verma P, Pal US, Tiwari S. Demographic risk factors, affected anatomical sites and clinicopathological profile for oral squamous cell carcinoma in a north Indian population. Asian Pac J Cancer Prev. 2014;15(16):6755-60.
[17]. Miyata M, Furukawa M, Takahashi K, Gonzalez FJ, Yamazoe Y. Mechanism of 7,12-dimethylbenz[a]anthracene-induced immunotoxicity: role of metabolic activation at the target organ. Jpn J Pharmacol. 2001;86(3):302-9.
[18]. Kudo Y, Takata T, Ogawa I, Sato S, Nikai H. Expression of p53 and p21CIP1/WAF1 proteins in oral epithelial dysplasias and squamous cell carcinomas. Oncol Rep. 1999;6(3):539-45.
[19]. Hogmo A, Lindskog S, Lindholm J, Kuylenstierna R, Auer G, Munck-Wikland E. Preneoplastic oral lesions: the clinical value of image cytometry DNA analysis, p53 and p21/WAF1 expression. Anticancer Res. 1998;18(5B):3645-50.
[20]. Yuen PW, Chow V, Choy J, Lam KY, Ho WK, Wei WI. The clinicopathologic significance of p53 and p21 expression in the surgical management of lingual squamous cell carcinoma. Am J Clin Pathol. 2001;116(2):240-5.
[21]. Ebert MP, Yu J, Hoffmann J, Rocco A, Rocken C, Kahmann S, et al. Loss of beta-catenin expression in metastatic gastric cancer. J Clin Oncol. 2003;21(9):1708-14.
[22]. Ndoye A, Dolivet G, Hogset A, Leroux A, Fifre A, Erbacher P, et al. Eradication of p53-mutated head and neck squamous cell carcinoma xenografts using nonviral p53 gene therapy and photochemical internalization. Mol Ther. 2006;13(6):1156-62.
[23]. Farhadieh RD, Smee R, Rees CG, Salardini A, Eggleton S, Yang JL, et al. Mutant p53 and cyclin A1 protein expression in primary laryngeal squamous cell carcinomas do not correlate to second primary tumours of the head and neck. ANZ J Surg. 2009;79(1-2):48-54.
[24]. Miyahara H, Yane K, Naitoh H, Konishi N, Kitahori Y, Matsunaga T, et al. p53 tumor suppressor gene and ras oncogene mutations in hypopharyngeal squamous cell carcinomas. Int J Oncol. 1997;11(1):133-7.
[25]. Sarkar J, Dominguez E, Li G, Kusewitt DF, Johnson DG. Modeling gene-environment interactions in oral cavity and esophageal cancers demonstrates a role for the p53 R72P polymorphism in modulating susceptibility. Mol Carcinog. 2014;53(8):648-58.
[26]. Rosa EA, Lia EN, Macedo SB, Amorim RF. In situ carcinoma developed over oral lichen planus: a case report with analysis of BUB3, p16, p53, Ki67 and SOX4 expression. J Appl Oral Sci. 2015;23(4):442-7.
[27]. Asgari M, Nabi Maybodi M, Abolhasani M. Differential diagnosis of urothelial carcinoma in situ from non-neoplastic urothelia: Analysis of CK20, CD44, P53 and Ki67. Med J Islam Repub Iran. 2016;30:400.
[28]. Cuevas Gonzalez JC, Gaitan Cepeda LA, Borges Yanez SA, Cornejo AD, Mori Estevez AD, Huerta ER. p53 and p16 in oral epithelial dysplasia and oral squamous cell carcinoma: A study of 208 cases. Indian J Pathol Microbiol. 2016;59(2):153-8.
License
Copyright
© ,
Author Details