Carbon Disulfide (CS2) Induced Chromosomal Alterations and Apoptosis in Circulated Blood Lymphocytes of Personnel Working in Viscose Industry
Download
Abstract
Background and objective: Carbon disulfide (CS2) is a naturally occurring chemical substance in the environment and also an important endogenous substance in the human body. This study was done to collect data on the effects and to find a possible relationship between in-vivo CS2 induced apoptosis and genotoxic effects.
Material and method: Circulated blood lymphocytes (PBL) of 41 workers occupationally exposed to Carbon disulfide (CS2) in viscose industry were investigated. The participants involved three groups. The first group included 41 participants exposed to CS2 together with various confounding factors; the second group comprised of 41 participants who were inhabitant of viscose industry and were partially exposed to CS2 in long periods; and third group consisting 41 participants as control group who were not exposed to any kind of chemicals and radiation hazards. Ambient air concentrations of CS2 were measured in different workplaces. Measures of genotoxicity included the determination of the frequencies of chromosomal aberrations (CA), sister-chromatid exchange (SCE), HPRT mutations (variant frequency, VF), and measurement of UV-induced unscheduled DNA-repair synthesis (UDS). The percentages of premature centromere division (PCD) and of cells with a high frequency of SCE (HF/SCE) were also scored. Apoptosis and c l proliferation were determined by flow cytometry.
Results: In both CS2 exposed groups, the apoptotic activity and the CA levels in PBLs were significantly higher than in controls. CA was mostly breaks of the chromatid type. In group II, CA was slightly lower in comparison with group I, which can be attributed to a different rate of elimination of damaged lymphocytes as a consequence of CS2 induced apoptotic activity.
Conclusion: The results demonstrate that exposure to CS2 induced apoptosis and CA, indicating an excess cancer risk among participants occupationally exposed to CS2. The results also emphasized the importance of the measurement of occupationally exposed pollutants such as CS2 in order to avoid genotoxic effects in the workers.
Introduction
Carbon disulfide (CS2) is a naturally occurring chemical substance in the environment and also an important endogenous substance in the human body. CS2 is also an industrial toxicant and solvent mainly used in the viscose, rubber, and chemical manufacturing industries [1]. The largest use of CS2 is in the viscose industry where it is used to obtain alkali cellulose. Exposure to high CS2 concentrations in these industries may cause severe effects on human body [2, 3]. Chronically revelation to CS2 can cause eye, ear, cardiovascular, nervous, and reproductive system disturbances [4, 5, 6, 7, 8, 9, 10, 11].
Currently, CS2 exposure level is mostly below 31 mg/m3. According to Guidotti et al (1999), Hoffman
(1990), and Keil et al., (1996), short time exposure also caused deceleration of intra-cardiac impulse conduction and modified arrhythmia in the coronary occlusion. An extensive literature is available on the toxic effects and genetic and chromosomal damage of CS2 on circulating blood lymphocytes and human sperm samples [12, 13, 14]. In addition, Bao et al., (1996) reported an increased incidence of chromosomal aberrations in the pronuclei of zygotes in adult female mice exposed to 10/100 mg/m3 of CS2 for 3 weeks and mated with unexposed male mice. In -vitro exposure of human sperm to CS2 concentrations at 10 micromole/liter(μmol/L) resulted in increased occurrences of chromosomal aberrations [15]. Furthermore, the mutagenicity and genotoxicity potentials of CS2 have been evaluated in vitro and in-vivo experiments using crucial confounding factors [14, 16, 17, 18]. Previously, CS2 does not exhibit mutagenic activity in bacteria with or without the presence of activation system [19]. Additional in vitro tests, including host-mediated assay, unscheduled DNA synthesis in human fibroblasts and primary cultures of human leucocytes are unconvincing. However, the significance of these tests cannot be properly evaluated because of methodological problems such as the lack of proper positive controls [20].
Therefore, insufficient data are currently available to evaluate the mutagenic and genotoxic potentials of CS2. A quantitative assessment of published data on workers heavily exposed to CS2 can provide some evidence for/against the hypothesis regarding it association with numerous diseases. Current meta-analyses showed significant genetic changes induced by the potential confounding factors like smoking and alcohol. Today, chromosomal alterations in human peripheral lymphocytes (PBL) are recognized as a valuable biomarker of the effect of toxic chemicals, probably the only one which has been standardized and validated [21] method to detect the genetic level damage. SCE frequency in human PBL has been used as the best indicator of chromosome damage [22]. To assess early carcinogenic effects of various genotoxic markers involved, DNA alterations [23] have been used in molecular epidemiological studies [24, 25].
Cytogenetic studies on occupational exposure have reported genetic damage among viscose industry workers [18, 26] even though the results in this regard are rather contradictory. However, there is still no consistent evidence supporting that exposure to CS2 causes chromosome damage. The inconsistent genotoxicity data could be due to differences in levels of direct and indirect exposures or in the utilized end-points. Hence, there is a need to evaluate different populations to analyze various genotoxic parameters of CS2. Early identification of hazards is crucial to reduce the exposure and carcinogenic risk. A literature survey has revealed that no investigation has been conducted among these workers with different cytogenetic tools considering different levels of CS2 exposure.
The focal aim of the present study was to identify genetic alterations of viscose factory workers exposed to CS2 occupationally in Tamil Nadu, South India, by using the relevant cytogenetic tools such as apoptosis induced factors, chromosomal aberrations (CA), and Sister Chromatid exchange (SCE) assay and considering various confounding factors. To evaluate the possibility of toxic chemicals effect at the workplace, confounding factors and other influences associated with work duration must be taken into consideration.
Materials and Methods
Selection of subjects
Altogether 123 subjects from various viscose industries in Tamilnadu state especially Tiruppur and Erode districts were recruited for current study. Subjects were divided into three groups; namely group I consisting of 41 subjects directly involved and exposed to CS2 in viscose industry, group II comprising 41 subjects who were not exposed to CS2 but they were inhabitants near viscose industries, group III consisting of 41 subjects in the control group. Additionally, group I were divided based on their duration of exposure (0–3; 4–6; 7–9; 10–12; 13–15 years) and they were known to be exposed to CS2 for a minimum of 8 hrs per day. Group II were those who were not directly exposed to CS2, but they were inhabitants nearby viscose industry during past 2 decades and they were further categorized based on the year of residence (20–30; 31–40; 41–50; 51–60; and 61–70 years). Control participants were normal and healthy individuals who were not exposed to any kind of chemicals and radiation hazards, Control participants were matched to exposed groups with respect to age. Experimental groups were further categorized based on their age (Group I <40 years; Group II >40 years). A face to face questionnaire which recorded standard demographic profile as well as questions relating to lifestyle, consumption habits such as smoking, alcohol intake, medication, recent viral infections, vaccinations, diagnostic tests or previous occupational exposures to chemicals was completed by all the participants. It was also ensured that all the participants do not take any medicines nor been exposed to any kind of hazards for at least 12 months before sampling. Individuals (both experimental and controls) who smoked for at least 1 year (more than 15 cigarettes/day) were considered as smokers. Informed consent was obtained from all the participants.
CS2 concentrations in ambient air were measured at fixed locations in the industries, and the concentrations were in the range of 0.13–1.20 mg corresponding to an 8-h time-weighted average exposure (TWA-8) of 0.9 mg/m3, which exceeds the limits of both the short-term exposure (0.6 mg/m3) and TWA-8 (0.6 mg/m3). All participants complained about acute toxic effects (eye irritation) and the presence of CS2 was perceivable in each industry. Data concerning the ambient air concentration measurements for these chemicals were not available, but ambient air concentrations for each of these chemicals were much below both the 8-h time weighted average exposure (TWA-8) and short-term exposure limits in the selected region. Only active smokers were considered to be smokers.
The determination of HPRT gene mutations and UV-induced DNA synthesis (UDS) in PBL
To determine HPRT gene mutations, PBLs were separated from the blood samples on Histopaque 1,077 gradients and cultured in RPMI-1640 medium supplemented with 20% fetal calf serum and 0.5% phytohemagglutinin -P (PHA) without antibiotics in a standard thermostat at 37oC in a humidified atmosphere containing 5% CO2. The determination of the frequency of gene mutations at the HPRT locus (variant frequency, VF) was performed by autoradiography of the cells cultured in the presence of 3H-thymidine reagent (3H-TdR) and selective agent (10−4 M 6-thioguanine) applying the modified method of Strauss and Albertini (1979). The lectin labeling index (LI) and the HPRT mutation frequency were determined from the same samples, as previously described [27, 28]. Finally, the VF was calculated according to the equation of Strauss and Albertini (2000).
The measurement of UDS was carried out according to Bianchi et al. (1981), as previously described briefly. PBLs were separated on Histopaque 1,077 gradients by density centrifugation. After separation, PBLs were irradiated in open petri-dishes by UV light (24 J/m2) and then incubated for 3 hrs with 10 pC i/ml3H-TdR in the presence of 2.5 m Mhydroxy urea. The degree of ‘de novo’ UDS was measured by scintillometry based on 3H-TdR incorporation in separated lymphocytes. UDS was calculated as the difference between radioactivities of the incorporated 3H-TdR in UV-irradiated and control participants.
Determination of CA and SCE frequencies
Whole blood samples were processed for studying CA and SCE. The cell culture methods were identical in both protocols: samples of 0.8 ml heparinized blood were cultured in duplicate at 37 oC, in 5% CO2 atmosphere, in 10 ml RPMI-1640 supplemented with 20% fetal calf serum and 0.5% PHA without antibiotics. For CA and SCE analyses, the cultures were incubated for 50 and 72 hrs, respectively. 5- Bromo-2µ- deoxyuridine (BrdU), used in SCE analysis to identify the first and subsequent metaphases, was added at 5 µg/ml concentration for 22 hrs. Culture harvest, slide preparation, and staining were conducted following the standard methods using 5% Giemsa stain for CA (Moorhead et al, 1960) and according to the Fluorescence-plus-Giemsa method of Perry and Wolff (1974) for SCE. CA characterization was carried out in 100 metaphases with 46±1 chromosomes per subject according to Carrano and Natarajan (1988). Mitoses with unmatched (45 or 47) chromosomes were considered as aneuploidy cells. Mitoses containing only achromatic lesions (gaps) and/or aneuploidy were not considered aberrant. Premature centromere division (PCD) was scored according to Mehes and Bajnoczky (1981). Total PCD and mitoses with more than three chromosomes with PCD (PCD/CSG, centromere separation general) were also scored. Cells with a higher number of SCE per cell than the 95 percentile of the control (≥10) were considered as high- frequency SCE cells (HFC/SCE) according to Tates et al., (1991) and Major et al., (1996).
Flow-cytometric analysis of apoptosis and cell proliferation in PBL
For the measurement of the percentage apoptosis and the S-phase, PBL were separated from the blood samples on Histopaque 1,077 gradients and cultured in RPMI-1640 medium supplemented with 20% fetal calf serum and 0.5% phytohemagglutinin-P (PHA) for 50 hrs without antibiotics in a standard thermostat at 37 oC in a humidified atmosphere containing 5% CO2. One hour prior to termination of the incubations, 5 µg/ml BrdU was added to the cultures. Cells were washed twice with PBS, fixed in 1ml ice-cold 70% ethanol, and stored at -20 oC until further processing. DNA denaturation prior to propidium iodide (PI) and fluorescein isothiocyanate (FITC) labeled monoclonal anti-BrdU staining was performed at room temperature with 2MHCl containing 0.2 mg/ml pepsin according to the method proposed by van Erp et al. (1988). DNA was stained with PI and the incorporated BrdU was detected by use of immunocytochemistry with FITC labeled monoclonal antibody.
Flow-cytometric analysis was performed with a FACS Calibur flow cytometer. Data for at least 10,000 lymphocytes per sample were acquired; CellQuestPro Software was used for the analysis.
Cell proliferation was also characterized by flow-cytometric measurement of the expression of the cell-activation marker CD71 on T- lymphocytes (Biro et al., 2002). Briefly, heparinized whole blood was mixed and incubated at room temperature for 20 min with the appropriate amount of fluorescence-labeled monoclonal antibodies against surface antigens. The following monoclonal antibodies were used: peridinin–chlorophyll–protein complex (PerCP)-labelled anti-CD3 (T-cell marker), FITC -labelled anti-CD71 (transferrin receptor), and allophycocyanin (APC)-labelled anti-CD45. The erythrocytes were removed through lysis by addition of FACS Lysing solution. After washing with phosphate-buffered saline (PBS), samples were analyzed within 4 hrs after labeling or fixed with 2% paraformaldehyde. Flow-cytometric analysis was performed on a Becton Dickinson FACS Calibur flow cytometer. Standard forward and side scatter gating combined with CD45 was used to set the lymphocyte gate. Data for at least 10,000 leukocytes per samples were acquired; CellQuest Pro Software (Becton Dickinson) was used for analysis. Phenotypes are expressed as a percentage of positive cells of the given lymphocyte subpopulation.
Statistical analysis
Statistical analysis was made using GraphPad Prism 3.02 software. Differences between the experimental groups and the control group were tested using Student’s t-test. P< 0.05 was considered as statistically significant. A standard linear correlation analysis was used to determine the relationship between apoptosis, CA, and PCD.
Results
Main demographic data of the experimental and control groups are summarized in Tabl 1. The participants aged 22 to 60 years. The time of occupational exposure as viscose industry workers were 21.8 years (ranging from 7 to 34 years) and 17.7 years (ranging from 4 to 34 years) in experimental and control group, respectively. The number of current smokers was slightly elevated among the exposed donors. The present study described the lifestyle characteristics such as gender, smoking status. Considering smoking status, 43.96% were smokers (and 48.78% were nonsmokers.
| Particulars | No of | Percentage | |||
| Samples | (%) | ||||
| Group I | 0-3 | 8 | 19.5 | ||
| (Work Duration in | 4-6 | 13 | 31.7 | ||
| Years) | |||||
| 7-9 | 11 | 26.82 | |||
| 10-12 | 7 | 17.07 | |||
| 13-15 | 2 | 4.87 | |||
| Group II | 20-30 | 11 | 26.82 | ||
| (Year of Residence) | 31-40 | 13 | 31.7 | ||
| 41-50 | 6 | 14.63 | |||
| 51-60 | 5 | 12.19 | |||
| 61-70 | 6 | 14.63 | |||
| Controls | 41 | 100 | |||
| Gender | Male | 26 | 63.41 | ||
| Female | 15 | 36.58 | |||
| Smoking Status | Smokers | 18 | 43.96 | ||
| Non-Smokers | 23 | 48.78 | |||
| Age (mean±SD) | Group I | 31.05 ±2.14 | |||
| Group II | 42 ± 2.47 | ||||
The participants in the experimental group and control group were matched for age and gender and no difference was observed in terms of gender. Totally, 26 males (63.41%) and 15 females (36.58%) participated in the present study. Data on chromosomal aberrations and SCE are depicted in Tabl 2. Circulated lymphocytes of Group I and II were similar to those in the control group. The comparative data on general characters, chromosomal alterations, and SCE/1,000 cells are presented in Table 2. In addition, CA and SCE frequency are also depicted in the same Table based on the CS2 exposure in air and in urine.
| Groups | No. of Subjects | CS2 amount in workplace | CS2 content in urine | Total CA | Total SCE | ||
| mean±SD | mean±SD | (mean±SD) | (mean±SD | ||||
| I | 41 | 1.42±0.004 | 1.82±0.84 | 5.41±2.48 | 13.62±3.46 | ||
| II | 41 | 0.054±0.002 | 1.01±0.46 | 4.08±3.16 | 9.31±2.73 | ||
| III | 41 | 0.021±0.004 | 0.54±0.39 | 1.41±2.04 | 3.62±1.18 |
In total, Group I CS2 exposure was higher (1.42±0.004 (mg/m3)), compared to that in Group II (0.054±0.002 mg/m3) and group control (0.021±0.004 mg/m3). With respect to mean ± SD values, it was found that the total CA (5.41±2.48) and SCE (13.62±3.46) for Group I was found to be higher compared to group (9.31±2.73) and control group (3.62±1.18). The values obtained in Group I were found to be statistically significant when compared to their respective controls. Tabl 3 and 4 depict the mean ± SD values of CA and SCE frequencies for group I and II, respectively.
| Particulars | 0-3 | 4-6 | 7-9 | 10-12 | 13-15 | ||
| Age | 34.42±5.64 | 35.41±3.72 | 38.22±4.82 | 41.16±3.74 | 38.3±6.57 | ||
| Work Duration | 3.18±0.49 | 5.46±1.02 | 8.67±0.09 | 11.36±0.28 | 14.56±8.46 | ||
| CTAs | 1.46±0.87 | 2.57±1.03 | 3.67±0.05 | 5.62±1.18 | 6.08±1.40 | ||
| CSAs | 0.43±0.68 | 1.12±0.9 | 4.42±1.03 | 9.41±1.12 | 7.93±0.76 | ||
| Total | 1.86±0.62 | 3.70±0.84 | 7.40±1.14 | 7.32±0.81 | 8.62±2.36 | ||
| SCE/1,000 Cells | 13.04±3.46 | 16.80±3.39 | 18.06±1.86 | 21.06±3.42 | 19.32±3.82 |
The frequency of total CA found in group I was higher [8.62±2.36 (13-15 yrs)] compared to other age groups [7.32±0.81 (10-12 yrs), 9.46 ± 4.04 (7–9 yrs), 3.70±0.84 (4-6 yrs); 1.86±0.62 (0–3)] (Table 4). In group I, the total SCA was higher in 10-12 years exposure (21.06±3.42) compared to other age groups who were exposed to CS2 [19.32±3.82 (13-15 yrs), 18.06±1.86 (7-9 yrs), 16.80±3.39 (4-6 yrs) and 13.04±3.46 (1-3 yrs)] which were found to be statistically significant when compared to other age groups and their respective controls. For group II, the total CA (6.01±2.10) and SCA (18.26±1.86) frequencies were higher in age group of 61–70 years. The present study showed levels of CA in Group I, II, and controls (Table 4). Furthermore, statistical significance for the frequency of CA expressed in mean ± SD was established using the analysis of variance (ANOVA). Cell cultures were effectively established from the blood samples collected from all the participants. The overall CA and SCA frequencies for CS2 exposures were significantly different (Table 2,3, 4) from those of the controls for both chromatid and chromosome type aberrations (P value is 0.03 by ANOVA).
| Group II | 20-30 | 31-40 | 41-50 | 51-60 | 61-70 | ||
| Age | 36.46±3.28 | 41.1±2.86 | 38.1±1.47 | 39.62±4.68 | 38.21±6.16 | ||
| Year of Residence | 23.74±1.82 | 33.80±2.62 | 46.28±2.30 | 52.63±3.47 | 63.62±4.62 | ||
| CTAs | 3.51±1.68 | 3.01±1.46 | 3.87±1.02 | 6.28±0.56 | 6.02±0.83 | ||
| CSAs | 1.02±0.48 | 0.96±1.04 | 1.87±1.06 | 0.89±1.01 | 2.46±0.96 | ||
| Total | 3.24±0.92 | 4.62±1.53 | 5.74±1.67 | 5.75±1.07 | 6.01±2.10 | ||
| SCE/1000 Cells | 4.96±2.15 | 6.46±1.03 | 13.02±3.71 | 16.28±2.52 | 18.26±1.86 |
The results of the flow-cytometric measurements on the percentage of apoptotic cells, cells in S-phase, and CD71 expression, together with the mean values of LI, VF, and UDS are summarized in Tabl 5. The mean percentage of apoptotic cells was significantly higher in groups I and II (8.57±1.10, 5.04±0.48) in comparison with controls (3.91±0.27). Fascinatingly, the percentage of apoptotic cells was even higher in group I exposed to CS2 only, in comparison with the groups II and III exposed to CS2; however, this difference was not statistically significant (p = 0.33).
| Groups | N | Apoptosis % | S-Phase | CD cells % | LI (PHA) % | VFX10-6 | ||
| I | 41 | 8.57±1.10 | 22.13±1.38 | 1.86±0.31 | 12.12±1.08 | 1.86±0.07 | ||
| II | 41 | 5.04±0.48 | 17.13±1.56 | 1.52±0.58 | 9.60±0.51 | 2.32±1.62 | ||
| III | 41 | 3.91±0.27 | 13.86±1.03 | 1.08±0.27 | 8.14±1.16 | 4.26±1.03 |
The mean percentage of cells in S-phase, measured by flow cytometry, as well as the mean values of LI slightly increased in both groups I and II in comparison with the control participants. There was no significant difference between experimental groups and control group in terms of SCE. The mean percentage of HF/SCE showed a non-significant (p = 0.16) increase among participants in group II, exposed mainly to CS2 . The percentages of PCDs were significantly higher in both experimental (Groups I and II) groups in comparison with the controls (Group III).
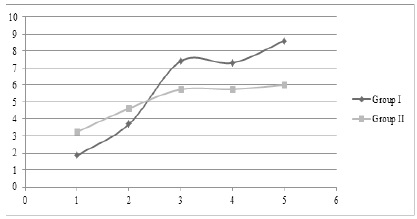
Figure 1 :Graphical Representation of CA Value in Group I and Group II Subjects
Discussion
The results of the present study revealed cytogenetic alterations and induction of apoptosis among viscose factory workers occupationally exposed to CS2. Moreover, the considerable CS2 concentration was always present in the working environments, and the presence of CS2 in the air was always observable by olfactory perception. Safety measures and devices have been introduced in the last few years in these industries, and the employees have used these protective devices during their work. However, none of the workers have worn masks or similar personal safety devices equipped with specific filters for CS2. Therefore, to assess the excess risk among these workers in the second group, we should consider the effects of low dose exposure to organic solvents as a confounding factor and as a cause of bias on the effects of CS2. On the other hand, the first group was almost exclusively exposed to CS2, as they situated nearby these industries.
Furthermore, major effluent water mostly contaminated with CS2. Moreover, some of the industries in our study area release their effluents either on the open land or in surrounding surface water bodies contaminating the soil and surface water ultimately turned into groundwater. Much of the groundwater is unsuitable for irrigation, and hundreds of wells in the region can no longer be used. Based on these earlier reports, we recruited experimental subjects who were known to live for the past 3 decades in and around our study area and were occupationally exposed to CS2 by mode of air and water (drinking).
Mutagenesis is involved in the pathogenesis of many neoplasias. Occupational exposure may contribute to the development of pernicious illnesses through mechanisms that involve genotoxic changes. Continuous efforts have been made to identify genotoxic agents, determine conditions of harmful exposure, and monitor populations that are excessively exposed to these conditions
The present study was designed to assess the genetic damage among viscose plant workers who are occupationally exposed to CS2. CA and SCE is a valuable method for detection of occupational and environmental exposures to genotoxicants, and it can be used as a tool in risk assessment for hazard characterization [29, 30] of various in vitro and in vivo studies [31-33].
An epidemiological study showed a strong correlation between CS2 concentrations from workers in viscose rayon factory and CS2 factory indoor air concentrations up to 64 mg/m3 (20.5 ppm). Ghittori et al., (1998) used personal passive samplers installed in the breathing zones to measure the airborne CS2 levels for 4 hours. Most of the indoor air samples revealed CS2 at levels below the TLV of 31 mg/m3.
Previous studies concluded that urinary CS2 may be a good indicator to estimate the levels of exposure to CS2 in the workplace. Investigating the correlation between urinary concentrations and indoor air levels indicated that a mean level of 15.5 μg/ CS2/L (95% CI 13.8–17.1 μg) was excreted following exposure to CS2 at 31 mg/m3 current occupational exposure limit in viscose industry [34]. Cox et al., (1998) investigated 2-thiothiazolidine-4-carboxylic acid (TTCA) concentrations as a biomarker of CS2 exposure. They concluded that more emphasis should be placed on workplace protection factors rather than just addressing the indoor air CS2 concentrations. Present findings also suggested that urinary concentrations can be a determinant of the workplace protection factor among workers using respirators and even among those who did not wear respirators and adhere to safety precautions in the workplace [35]. Moreover, the present finding depicted that CS2 level was more in the first group of subjects in comparison with subjects of the second and controlgroups.
On the other hand, Jian and Hu (2000) investigated the mechanism of CS2 action on the antioxidative stress mechanisms and measured enzymes linked to oxidative stress, including Cuprozinc-superoxide dismutase (CuZnSOD) and malonyl dialdehyde (MDA) levels in the serum of viscose rayon factory workers and in control subjects. The workers aged between 20 to 41 years and had been exposed to CS2 from 2 to 14 years, with an average exposure of 8 years. They found a dose-response relationship. Serum MDA levels also increased in CS2 exposed workers in both a concentration- and an exposure duration-dependent manner. The enzymes (i.e., CuZnSOD and MDA) may serve as biomarkers for worker’s health surveillance to determine early stages of impairment of the anti-oxidative stress response resulting from CS2 exposure (Jian and Hu, 2000).
Flow-cytometric measurements detected an induction of apoptosis among both experimental and control groups who occupationally exposed to CS2 from viscose factory. Our results are in agreement with the findings of the in vitro experiments by Bao et al., (1996) demonstrating that higher doses of CS2 induce apoptosis. Cell proliferation, as determined by flow-cytometric measurements of the S-phase and expression of CD71 and LI measurement, was not decreased in our study. However, in vitro experiments have shown a decrease in the viability and mitotic activity of cells at CS2 doses inducing apoptosis.
These differences between the findings of the in vitro studies and our results may be attributed, among others, to differences in doses, duration of exposure, and differences in cell types. The slight decrease in apoptosis among workers who aged more than the mean age in the second group and exposed mainly to CS2 may be a consequence of adaptation to CS2 exposure. Mean of CA frequencies were significantly elevated in both groups of pathology personnel, in comparison with the controls. This increase is indicative of exposure to clastogenic agents among viscose industry workers. The mean of CA frequencies also significantly elevated in viscose factory workers in the second group who were partially exposed to CS2. The CA-inducing effect found in our study can be attributed to CS2 as a clastogenic agent. Our findings on the elevated CA frequencies in both groups of workers exposed to a mean ambient air concentration of 0.9mg/m3 CS2 are in good agreement with most of the literature dealing with CS2 induced CA (and/or SCE) among viscose industry workers [7, 9, 10]Cho et al., 2002; [6, 8, 11] Takebayashi et al., 2004; [5].
Moreover in vitro experiments [15] showed that CS2 induces CA in PBL among viscose factory workers. Increased frequencies of CA in PBL were observed in a study by Le and Fu (1996), which is line with our findings, revealing elevated CA level in the PBL of the exposed workers. However, in the second group of the exposed participants aged more than the mean age, a significantly increased SCE was observed, but this increase could probably be due to the effect of smoking. In a study by Bao et al., (1996), no increase in the SCE frequencies in lymphocytes from CS2 exposed viscose factory workers was observed. PCD and PCD/CSG, a disturbance in the process of mitotic division of chromosomes, were significantly increased in both groups, indicating an effect of genotoxic exposure inducing PCDs as suggested by previous studies.
The observation of higher frequencies of CA in the lymphocytes of exposed individuals agrees with the earlier reports by Le and Fu (1996) among viscose rayon workers. These are considered S-dependent alterations, frequently observed due to human chronic exposure to chemical mutagens. In the present study, CA increased with the increase of age and exposure period in the exposed groups. The results of the present study also indicated the role of age in the development of CA observed in PBL of controls.
In the present investigation, a notable CA was observed among the healthy controls. There was a significant difference between experimental and control participants who were occupationally exposed to CS2. In past years, CS2 concentrations in viscose rayon plants averaged about 250 mg/m3; they were subsequently reduced to 50-150 mg/m3 and more recently to below 31 mg/m3 [36].
A report on hypospermia, asthenospermia, and teratospermia in young workers exposed to 40-80 mg/m3 of CS2 confirmed gonadal injury [37]. Le and Fu, (1996) showed that CS2 induced chromosome aberration in human sperm. Numerous epidemiological reports have concluded that the CS2 is toxicant to viscose industry workers [16, 17, 38]. In this study, experimental participants with smoking habits experienced maximum levels of chromosome and SCE alterations when compared to respective controls, demonstrating that CS2 exposure with cigarette smoking has a synergistic effect on inducing genetic damage. Chromosomal aberrations were shown to be good indicators of future risk of cancer [24]. On the other hand, genetic damages are the ultimate causes of cancer because DNA base changes can be mutagenic
The present findings highlighted the importance of investigating the genotoxicity of CS2 among viscose plant workers occupationally exposed to this organic solvent and also revealed that the smoking habit was associated with maximum levels of chromosome and SCE alterations.
The relation between exposure to CS2 and the induction of apoptosis and changes in CA, PCDs and PCD/CSG needs further investigation, and more data from larger groups of CS2 exposed individuals from different industries are also needed to analyze the confounding effects of smoking. Similarly, as in the present study, the investigated viscose factory workers were mostly men, further investigations are needed to collect data from women workers in order to study the possible effect of gender on CS2 induced apoptosis and changes in the cytogenetic end-points.In conclusion, the results of this study demonstrated that occupational exposure to CS2 can induce apoptosis, CA and SCE on circulating fluids, indicating a possible excess cancer risk among exposed subjects. The multiple end-point genotoxicological monitors, developed and run in our laboratory, is able to detect changes in cytogenetic and cell proliferation biomarkers. The results emphasized the importance of personal safeguard at workplaces, with possible occupational exposure. Therefore, the aim of our study was to investigate the genotoxic effects associated with occupational exposure to CS2 by analyzing apoptosis induction, CS, and SCE in circulated blood lymphocytes of workers associated with viscose industry and subjects living in the region surrounding these industries. However, the magnitude of this risk depends on the extent of exposure. Therefore, studies employing indicators for assessing the exposure and biological effects are strongly recommended.
Conflict of intersert
None declared
References
[1]. Fielder RJ SRO. Great Britain. Health and Safety Executive; Great Britain. Health and Safety Commission, 1981. Advisory Committee on Toxic Substances. London: H.M.S.O. Toxicity review, 1981.
[2]. Ruijten MW, Salle HJ, Verberk MM, Muijser H. Special nerve functions and color discrimination in workers with long-term low- level exposure to carbon disulfide. British journal of industrial medicine. 1990;47(9):589-95.
[3]. Ruijten MW, Salle HJ, Verberk MM. Verification of effects on the nervous system of low level occupational exposure to CS2. Br J Ind Med. 1993;50(4):301-7.
[4]. Vanhoorne M, Comhaire F, De Bacquer D. Epidemiological study of the effects of carbon disulfide on male sexuality and reproduction. Arch Environ Health. 1994;49(4):273-8.
[5]. Huang CC, Chu CC, Wu TN, Shih TS, Chu NS. Clinical course in patients with chronic carbon disulfide polyneuropathy. Clinical neurology and neurosurgery. 2002;104(2):115-20.
[6]. Sulsky SI, Hooven FH, Burch MT, Mundt KA. Critical review of the epidemiological literature on the potential cardiovascular effects of occupational carbon disulfide exposure. Int Arch Occup Environ Health. 2002;75(6):365-80.
[7]. Tsai CH LW, Chen CY. Formation of Solid Sulfur by Decomposition of Carbon Disulfide in the Oxygen-Lean Cold Plasma Environment. Ind Eng Chem Res.2002; 41 (6): 1412–1418.
[8]. Tan Xiaodong CG, Peng Xiaoxia, et al. Cross-Sectional Study of Cardiovascular Effects of Carbon Disulfide Among Chinese Workers of a Viscose Factory. Int J Hyg Environ Health.2004; 207(3):217-25.
[9]. Sills RC, Harry GJ, Valentine WM, Morgan DL. Interdisciplinary neurotoxicity inhalation studies: carbon disulfide and carbonyl sulfide research in F344 rats. Toxicol Appl Pharmacol. 2005;207(2 Suppl):245-50.
[10]. Nishiwaki Y, Takebayashi T, O’Uchi T, Nomiyama T, Uemura T, Sakurai H, et al. Six-year observational cohort study of the effect of carbon disulfide on brain MRI in rayon manufacturing workers. Occupational and environmental medicine. 2004;61(3):225-32.
[11]. Korinth G, Goen T, Ulm K, Hardt R, Hubmann M, Drexler H. Cardiovascular function of workers exposed to carbon disulphide. Int Arch Occup Environ Health. 2003;76(1):81-5.
[12]. Liss GM, Finkelstein MM. Mortality among workers exposed to carbon disulfide. Archives of environmental health. 1996;51(3):193-200.
[13]. Swaen GM, Braun C, Slangen JJ. Mortality of Dutch workers exposed to carbon disulfide. Int Arch Occup Environ Health. 1994;66(2):103-10.
[14]. Tang GH, Xuan DF. [Detection of DNA damage induced by carbon disulfide in mice sperm with single-cell gel electrophoresis assay]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2003;21(6):440-3.
[15]. Le JY, Fu XM. Human sperm chromosome analysis--study on human sperm chromosome mutagenesis induced by carbon disulfide. Biomed Environ Sci. 1996;9(1):37-40.
[16]. Wang Q FK, Wu Q. Effects on fertility and menstrual cycle of female workers exposed to carbon disulfide. Chinese J Pub Health.1999;15:215-7.
[17]. Wang YF SY. Investigation on eye injury of workers exposed to CS2. J Lab Clin Med. 2000;17:89.
[18]. Manikantan P B, Sasikala K, et al DNA damage in viscose factory workers occupationally exposed to carbon di-sulfide using buccal cell comet assay. Braz J Oral Sci.2009;8(4):197-200.
[19]. Beauchamp RO, Jr., Bus JS, Popp JA, Boreiko CJ, Goldberg L. A critical review of the literature on carbon disulfide toxicity. Crit Rev Toxicol. 1983;11(3):169-278.
[20]. NF. I. Carbon disulfide. Moscow, Centre for International Projects (GKNT), Scientific reviews of Soviet literature on toxicity and hazards of chemicals, No.1983.
[21]. Albertini RJ, Anderson D, Douglas GR, Hagmar L, Hemminki K, Merlo F, et al. IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans. International Programme on Chemical Safety. Mutat Res. 2000;463(2):111-72.
[22]. Countryman PI, Heddle JA. The production of micronuclei from chromosome aberrations in irradiated cultures of human lymphocytes. Mutation research. 1976;41(2-3):321-32.
[23]. Marzin D. New approaches to estimating the mutagenic potential of chemicals. Cell Biol Toxicol. 1999;15(6):359-65.
[24]. Hagmar L, Brogger A, Hansteen IL, Heim S, Hogstedt B, Knudsen L, et al. Cancer risk in humans predicted by increased levels of chromosomal aberrations in lymphocytes: Nordic study group on the health risk of chromosome damage. Cancer Res. 1994;54(11):2919-22.
[25]. Poirier MC. DNA adducts as exposure biomarkers and indicators of cancer risk. Environmental health perspectives. 1997;105 Suppl 4:907-12.
[26]. Medeiros MG RA, Batoreu MC. Elevated levels of DNA–protein cross-links and micronuclei in peripheral lymphocytes of tannery workers exposed to trivalent chromium. Mutagenesis. 2003;18(1):19–24.
[27]. Jakab MG MJ, Tompa A HPRT mutation frequencies in control human populations in Hungary. Occup and Environ Med. 1996.
[28]. Tompa A, Sapi E. Detection of 6-thioguanine resistance in human peripheral blood lymphocytes (PBL) of industrial workers and lung cancer patients. Mutat Res. 1989;210(2):345-51.
[29]. Moller P. The alkaline comet assay: towards validation in biomonitoring of DNA damaging exposures. Basic Clin Pharmacol Toxicol. 2006;98(4):336-45.
[30]. Dusinska M, Collins AR. The comet assay in human biomonitoring: gene-environment interactions. Mutagenesis. 2008;23(3):191-205.
[31]. Valverde M, del Carmen Lopez M, Lopez I, Sanchez I, Fortoul TI, Ostrosky-Wegman P, et al. DNA damage in leukocytes and buccal and nasal epithelial cells of individuals exposed to air pollution in Mexico City. Environ Mol Mutagen. 1997;30(2):147-52.
[32]. Rojas E, Valverde M, Sordo M, Ostrosky-Wegman P. DNA damage in exfoliated buccal cells of smokers assessed by the single cell gel electrophoresis assay. Mutat Res. 1996;370(2):115-20.
[33]. Fairbairn DW, Olive PL, O'Neill KL. The comet assay: a comprehensive review. Mutat Res. 1995;339(1):37-59.
[34]. Ghittori S, Maestri L, Contardi I, Zadra P, Marraccini P, Imbriani M. Biological monitoring of workers exposed to carbon disulfide (CS2) in a viscose rayon fibers factory. Am J Ind Med. 1998;33(5):478-84.
[35]. Cox C, Hee SS, Tolos WP. Biological monitoring of workers exposed to carbon disulfide. Am J Ind Med. 1998;33(1):48-54.
[36]. Daemen E, van Risseghem M, de Bacquer D, Bulat P, Braeckman L, Vanhoorne M. Preliminary external quality assessment for the biological monitoring of carbon disulfide with urinary 2-thiothiazolidine-4-carboxylic acid. Ann Occup Hyg. 1999;43(2):125-30.
[37]. Lancranjan I, Popescu HI, Klepsch I. Changes of the gonadic function in chronic carbon disulfide poisoning. La Medicina del lavoro. 1969;60(10):566-71.
[38]. Guidotti TL, Hoffman H. Indicators of cardiovascular risk among workers exposed to high intermittent levels of carbon disulfide. Occupational medicine (Oxford, England). 1999;49(8):507-15.
License
Copyright
© ,
Author Details