Natural Killer T Cells (NKT cells) Functions in Malignancies
Download
Abstract
CD1d-restricted natural killer (NKT) cells are unique innate like T lymphocyte that recognize glycolipid antigens. Two major NKT cell subsets, type I and type II, are different in their TCR repertoire and ligand specificity. Up on activation, NKT cells mediated strong and rapid responses through their ability to rapidly produce a large amount of both pro- and anti-inflammatory cytokines. Despite being a small population of αβ T lymphocytes, they can bridge the innate and adaptive arm of immune system through interaction with other immune components. These two subsets of NKT cells play critical apposite roles in anti-tumor immunity. This review focuses on the progress made in understanding the role of NKT cells in tumor immunity and how their activities can be useful in immunotherapeutic strategies.
Introduction
In tumor microenvironment (TME), outcome of anti-tumor immunity is related to the function of infiltrating immune cells and their crosstalk with each other and tumor cells. One regulator of immune response is Natural Killer T (NKT) cells that link the innate and adaptive arms of the immune system [1]. This small innate like T lymphocytes are a heterogeneous lymphoid population that play important modulatory roles in the prevention or induction of various disease including cancer [2]. They were originally defined as a T cell population express both αβ T cell receptor (TCR) and some lineage markers from natural killer (NK) cells such as CD 56/CD161 (humans) and NK1.1 (murine). As all NKT cells do not express NK cell markers, they now describe as the only cells that recognize both exogenous and endogenous lipid antigens presented by non-polymorphic MHC-like molecule CD1d. Indeed, CD1d expression has critical role in thymic development of NKT cells, as CD1d-/- mice are deficient in these cells [3, 4, 5, 6]. In response to stimuli, NKT cells are able to react quickly and secrete simultaneously both pro- and anti-inflammatory cytokines [1, 2]. It has been indicated that these cytokines influence the immune response by affecting other immune cells such as dendritic cells (DC), NK cells, conventional and regulatory T cells and B cells [7, 8].The aims of this review are to provide insight into how NKT cells subsets and their cross-talk with other immune cells and each other to regulate immune responses, and their roles cancer.
NKT cell subsets
The population of NKT cells is a collection of several phenotypically and functionally different subpopulations. There are two main distinct types of NKT cells, type I and type II NKT cells, based on differences between their TCRS, antigen specificity and cytokine profile[3].
The well-defined type I NKT cells otherwise known as invariant NKT (iNKT) cells are the main studied subset of NKT cells. type I NKT cells represent about 1-3% of lymphocytes in blood and lymphatic organs and about over 30% lymphocytes in liver, while in human they comprise only about 0.2 % of T cell population [9]. They express a semi-invariant TCRα chain with rearranged Vα14-Jα18 in mice and Vα24-Jα18 in humans paired with a limited repertoire of TCR-β chain (Vβ2, 7 or 8.2 in mice and Vβ11 in humans). The restricted TCR of type I NKT cells able to recognition both exogenous and endogenous glycolipid antigens with similar structure such as sphingomonas and ehrlichia lipids, lyso-phosphatidilcholine (lyso-PC) and isoglobotrihexosylceramide (iGb3) [10, 11, 12].
Initially type I NKT cells were characterized following recognition of a-galactosylceramide (α-GalCer), originally derived from a marine sponge [13]. Besides, several endogenous mammalian self-lipid have been defined as CD1d ligands that recognized by type I NKT cells such as iGb3 and phosphatidylinositol [14]. Upon activation, type I NKT cells display a broad range of functions relies on their rapid secretion of copious amounts of various, including interferon-γ (IFN-γ), interleukins IL-2, IL-4, IL-9, IL-10, IL-13, IL-17, IL-21, and granulocyte-macrophage colony-stimulating factor (GM-CSF) [14, 15, 16],as well as their interactions with other immune cells [17]. In humans, the majority of type I NKT cells express CD4 which produce both Th1 and Th2 cytokine. The rest are CD4-subset including both CD8+ and CD4-CD8- double negative (DN) population that produce T h1 cytokines [5, 18]. Moreover, type I NKT cells, depending on cytokines and transcription factors profile, can be subdivided into distinct subset including NKT1, NKT2, NKT17 and NKT17 cells that are equivalent to Th1, Th2 and Th17 cells respectively [19, 20]. It has been shown that type I NNKT cells in the presence of TGF-β combined with Rapamycin change to suppressive type I NKT cells that increase L-10 production[21].
The other major subset of NKT cells, named type II or diverse NKT (dNKT) cells, utilize more diverse αβ-TCR repertoire [22]. Unlike type I NKT cells, they do not express the Vα14Jα18 TCRα chain and typically do not recognize α-GalCer. Type II NKT cells are also either CD4+ or CD4−CD8− and produce both Th1 and Th2 cytokines immediately after stimulation. While type II NKT cells are less frequent in mice, they form a major subgroup of the T cells in the bone marrow, liver and gut of humans [1]. It has been demonstrated that, type II NKT cells play a unique role in in different immune responses and able to inhibit tumor rejection [23]. Type II NKT cells are less characterized than type I NKT cells, because unlike type I NKT cells that all population can stimulated by α-GalCer, no antigen is yet know that can stimulate all type II NKT cells. Recently three different methods have been used for studying the function of these cells including: i) the comparison of immune responses in two different NKT cell deficient mice including CD1d-/- mice (deficient in both type I and type II NKT cells) and Jα18-/- mice ( deficient only in type I NKT cell), ii) using sulfatide or tetramer-sulfatide complex in order to stimulate the f
32.01unction of a part of type II NKT cells [24, 25], iii) using 24αβTCR transgenic mice from type II cell clone VIII24 [26].
Interaction between NKT cells and other immune cells
Activation of type I NKT cell could occur directly or indirectly. Direct activation of type I NKT cells started with endocytosis of glycosphingolipids antigens by DCs or others APCs and then presented processing antigens to type I NKT cells through CD1d. This CD1d dependent activation leads to release wide ranges of cytokines (IL-4 and IFN-γ as well as IL-2, IL-5, IL-6, IL-10, IL-17, TNFα and GM-CSF) and also chemokines (RANTES, exotoxin, MIP-1α and MIP-1β) [27, 28, 29]. On the other hand, DCs Could be activated via engagement of their pattern recognition receptor (PRR) as TLR or via inflammasomes as NOD1 and NOD2. These activated DCs cause indirect activation of type I NKT cells through cytokines like IL-12 and IL-18 or by costimulatory molecules like OX40/OX40L interaction [30].
Besides TCR/CD1d interaction, NKT cell subsets could influence much other cell type and orchestrate immune responses via cytokines, chemokines production and surface molecules expression. It has been demonstrated that activated type I NKT cells with α-GalCer result in the modulation of several cell type activities including DCs, macrophages, B cells, NK cells and neutrophils. APC populations are involved in Ag presentation to NKT cells especially in an organ specific manner. Besides, the interaction between APCs and NKT cells is a bidirectional way which can change APC activities in both useful and harmful manner. In a feedback fashion iNKT cells activate antigen-presenting cells (APCs) through CD40-CD40L interaction, and cause DCs to mature and up-regulate co-stimulatory receptors such as CD80 and CD86 [31]. Moreover, activated DCs produce IL-12 that induces more IFN-γ production by NKT cells and plays a critical role together with IFN-γ in the activation of downstream effectors such as NK cells, CD8+ T cells and γδ T cells [32, 33].
Instead of activating cells from both innate and adaptive immune system, NKT cells could enhance tumor immunity via effects on immunosuppressive cells such as MDSCs and neutrophils derived from tumors microenvironment [34, 35]. In both human and mice acute phase protein serum amyloid A-1 (SAA-1) controls plasticity of IL-10 secreting neutrophil. SAA-1 not only induce the differentiation and expansion of suppressive IL-10 producing neutrophils, but also enhanced interaction between neutrophils and type I NKT cells via CD1d and CD40-dependent manner, result in type I NKT cells activation and change neutrophils suppressor activity through reducing IL-10 production [36]. In post-surgical metastasis model, it has been shown that NKT cells activation with α-GalCer loaded DCs decreased metastasis rate and enhanced survival outcome which was associated with reduced the immunosuppressive activity of MDSCs [37].
After presentation of α-GalCer to type I NKT cells, activated DCs produce IFN-γ, IL-12 that result in activation of anti-tumor CTLs. In contrast, activation of non-type I NKT cells via endogenous antigens result in the production of IL-4, IL-13 and TGF-β that impaired the function of CTL and NK cells [38, 39]. Moreover IL-13 enhances production of TGF-β from CD11b+ Gr-1+ MDSC via IL-4/STAT6 signaling pathway [40, 41]. In murine model of breast cancer, on the presence of ex vivo expanded type I NKT cells effected on anti-tumor activity of CTLs by causing these cells more resistant to immunosuppressive activities of MDSCs [42]. It has been demonstrated that vaccination of WT, CD1d-/- and Jα18-/- mice with GM-CSF secreting B16F10 melanoma cells result in the presentation of tumor antigens with recruited tolerance inducing CD8+ CD11c+ DCs only in WT model. These DCs expressed high level of CD1d and macrophage inflammatory protein 2 (MIP-2), a chemokine which recruit type I NKT cells to the splenic marginal zone [34, 43].
Crosstalk between NKT and Treg cells
As noted above, two main subsets of NKT cells can cross regulate one another. Moreover; it seems that these cells can also regulate other regulatory cells such as Treg cells. The majority of investigations on the interaction between NKT and Tregs were evaluated in animal models. It has been shown that bidirectional interplay between NKT and Tregs is required to maintain immune tolerance [44, 45, 46]. However the evidence of a reciprocal NKT and Treg cells interplay in human is so sparse. Recent studies have demonstrated that NKT cells secrete IL-2 and IL-4 that induce Tregs proliferation [47, 48]. In addition, homing of Tregs to the liver can be regulated by NKT cells [49]. Conversely, Tregs can also inhibit NKT cell proliferation and cytokine production in vitro through cell-cell contacts [50]. Accordingly, a better understanding of this cross-talk will be applicable to optimize NKT based therapeutics.
NKT cells in tumor immunity
Despite interaction with other cells, Type I and type II NKT subsets also cross-regulate each other and NKT cell subsets appeared to have a paradoxical role in a wide range of different immune responses. Ambrosino et al showed that in contrast to type I NKT cells that play protective role, type II downregulate tumor immunosurveillance. It has been indicated that in two different murine malignancies, when both type were simultaneously activated, sulfatide activated type II NKT cells suppressed type I NKT cells proliferation and cytokine secretion both In vivo and in vitro conditions. This result suggested a cross regulation between the two NKT cell subsets [51].
Potential role of type I NKT cells in tumor immunity
The role of type I NKT cells in anti-tumor immunity is also reported in several studies in humans and mice. It was highlighted by several studies that the number of type I NKT cells [52, 53], as well their activity and proliferation reduce [54] in peripheral blood of patients with different malignancies.
Direct activation of type I NKT cells
Type I NKT cells can lyse malignance cells directly through perforin/granzyme B, TNF-α or Fas ligand (FasL) mediated cytotoxic pathway (Fig. 1A) [55, 56, 57, 58, 59]. Some tumor cells as B cell lymphoma, myelomonocytic malignancies and small numbers of solid tumors which express CD1d could directly recognized by type I NKT cells [60, 61] and higher level of CD1d expression is associated with lower metastasis incidence [62]. It has been indicated that human type I NKT cells are able to recognize and kill CD1d+ osteosarcoma cells, but not CD1d- osteoblasts cell [63]. In murine model of breast cancer, tumor cells inhibits anti-tumor activities of type I NKT cells and promote metastasis via reduction of CD1d expression on tumor [64].
It is interesting to note that Resent studies has indicated that in chronic lymphocytic leukemia (CLL), high CD1d expression associated with disease progression and impairment in function of type I NKT cells [65, 66].Goini et al. showed that at beginning type I NKT cells postpone disease. Upon disease progration type I NKT cells become functionally impaired which is correlated with high expression of CD1d on CLL cells [67].Goini et al. showed that at beginning type I NKT cells postpone disease. Upon disease progration type I NKT cells become functionally impaired which is correlated with high expression of CD1d on CLL cells [67].
Figure 1 :Type I NKT cell in tumor immunity. A) Type I NKT cells can directly kill tumor cells by recognize lipid antigen presented by cd1d on tumor cell. B) Type I NKT cells can indirectly participate in anti-tumor immunity through rapid cytokines secretion and activating NK cells and CD8+ T lymphocyte after interaction with CD1d expressing APCs.
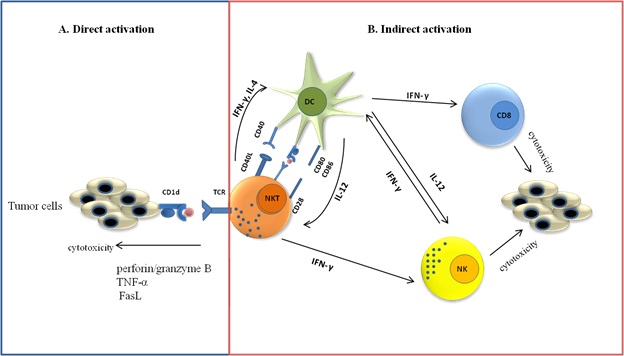
Indirect activation of type I NKT cells
Despite direct activation, type I NKT cells indirectly can participate in anti-tumor immunity in the lack of CD1d expression on tumor cells (Fig. 1B). after interaction with CD1d expressing APCs , activated type I NKT cells can rapidly produce cytokines and activate other immune cells from both innate and adaptive immune responses (NK cells and CD8+ T lymphocyte) [68, 69]. Initially, anti-tumor activities of NKT cells are indicated by α-GalCer as an anti-tumor agent. α-GalCer activated type I NKT cells in a CD1d dependent manner that leads to production of INF-γ. After α-GalCer stimulation, an abundant amount of IFN-γ released that is necessary for tumor protection [70]. Besides, CD28 and CD40L expression on NKT cells play crucial role in anti-tumor immunity through the regulation of Th1 and Th2 functions of Valpha14 NKT cells. α-GalCer-induced IFN-γ production by Vα14 NKT cells is impaired in both CD28- and CD40-deficient mice, but IL-4 production is impaired only in CD28-deficient mice [71]. Smyth et al. demonstrated that IFN-γ secreted by NKT and NK cells is needed for anti-metastatic activity of a-GalCer in both lung and liver metastasis models. Moreover to gain optimal serum IFN-γ and anti-tumor immunity, secretion of IL-12 and IL-18 by DCs is required [72].
Potential role of type II NKT cells in tumor immunity
By contrast to the role of type I NKT cells in improve anti-tumor immunity; type II NKT cells have immunosuppressive effects [36, 73]. CD4+ type II NKT cells produce more IL-4 and IL-13 compared with type I NKT cells. It has been shown that IL-13 is essential for tumor recurrence in 15-12RM, a fibrosarcoma tumor models [23]. IL-13 secretion induced activation of TGF-β secreting CD11b+ Gr-1+ myeloid derived cells that inhibit type I NKT cells or tumor specific CD8+ T cells [40]. A study with subcutaneous CT26, a colon carcinoma cell line, indicated that type I NKT cells play key role to determine balance between the regulatory roles of immunosuppressive Treg cells and type II NKT cells. It has been shown that anti-CD25 in WT mice and CD1d -/- mice reduce tumor burden. But it is not effective in Jα18 -/- mice. Besides, simultaneous blockage with anti-CD1d and anti-CD25 reduce tumor burden in Jα18 -/- mice. It has been suggested that in the presence of type I NKT cells, these cells regulate type II NKT cells activity and Treg cells determined dominant suppressor. By contrast, in the absent of type I NKT cells both type II NKT cells and Treg cells act as suppressive and blockage of both is necessary to remove immune suppression [74].
Although the literature mainly suggested that type I NKT cells have a protective role against tumors based on a Th1 response, in a few studies it has been shown that type I NKT cells can directly prevent anti-tumor immunity depend on their Th2 cytokine production such as IL-13, TGF-β, and suppression of CTL and NK cell activity[75, 76]. Recently it has been shown that type II NKT cells stimulated by CPG secrete IFN-γ, which result in activation of CD8+ T cell and play anti- tumor activity in the melanoma model [77].
NKT cells based immunotherapy
A protective role of type I NKT cells in tumor immunity has been demonstrated in multiple mice malignancy models, including a methylcholanterine (MCA) induced sarcoma, a P53 deficiency model and a TRAMP model, in the absence of exogenous antigen [18, 78, 79]. In these models, tumor growth enhanced in Jα18 -/- mice (deficient in type I NKT cells) and or CD1d -/- mice (deficient in both type I and type II NKT cells) as compared with wild-type (WT) mice. In a model of MCA-induced fibrosarcoma, Jα28 -/- mice lacking type I NKT cells had a greater susceptibility to the disease in comparison with WT mice, and transfer of liver derived NKT cells restored the NKT cell population and improved tumor immunity. It was also shown that not all NKT cells are equally protective and only liver derived CD4- type I NKT cells were protective and cause tumor rejection of MCA-1 sarcomas but when type I NKT cells derived from the thymus or spleen were adoptively transferred, only slight protection was observed [80]. In a phase I clinical trial study, Exley et al. indicated that the transfer of autologous in vitro expanded type I NKT cells are a possible and safe therapy, generating Th1-like responses with antitumor potential in advanced melanoma 81].
Immune adjuvants in order to initiating or promoting host immune response against poorly immunogenic antigens, represent as a potential tool for immunotherapy including those involved in vaccination against cancers. The type I NKT cell and its agonists, such as α-GalCer consider as adjuvant in immunotherapy because of the ability of type I NKT cells to rapidly produce a large amount of cytokines and also activate other immune cells upon stimulation [82]. It is indicated that Injection of α-GalCer or its synthetic analog KRN700, enhance survival in a several of murine malignancy models through induce type I NKT and NK cell response that rejects the tumor [83].
It is demonstrated that, in cancer patients, NKT cells represent a level of hypo responsiveness to α-GalCer administration [84]. Type I NKT cell anergy often result from strong stimulation with glycolipid agonists, particularly after repeated administration [85, 86] which can limit the use of such agonists in some cases that may need repeated doses for optimal effect. In order to avoid induction of anergy in type I NKT cells different strategies can use include administration of α-GalCer pulsed DCs. APC and the root of administration play important role in immune responses. IV injection of α-GalCer pulsed DCs induced strong cytokine production, but its subcutaneous injection did not stimulate effective type I NKT cells response [87]. Recently it has been shown that vaccine contained DCs and tumor cells with α-GalCer induced a strong, long-lasting and specific antitumor immune response in a therapeutic model of B cell lymphoma. This immune response was related to an increase of both Th1 cytokines and IFN-γ secreting type I NKT and T cells [88].
It has been shown that In Em-myc mouse, a model of non-Hodgkin’s B cell lymphomas growth of induced tumors significantly was inhibited by vaccination with irradiated, a-GalCer-loaded lymphoma cells while there was not any evidence about CD8 + T-cell activation or memory cell formation[89]. In contrast, combination immunotherapy with an NKT cell-targeting vaccine along with an agonistic anti-4–1BB antibody resulted in complete clearance of Em-myc lymphomas in over 50% of mice. This result was related to effective generation, differentiation and INF-γ dependent expansion of effector CD8 + T cells [90]. Bae et al, found that in an anti-PD-1-resistant tumor-bearing mice, type I NKT cells stimulated with the synthetic αGalCer can enhance the anti-tumor immunity by renovating the effector function of tumor exhausted CD8 T cells. They suggested that NKT cell stimulated with αGC-loaded APC as an effective therapeutic strategy for the treatment of anti-PD-1-resistant cancer patients [91]. Recently, using chimeric antigen receptors (CARs)-type I NKT cells in Preclinical studies have yielded promising result. CAR-type I NKT cells could eliminate tumor cells by effectively localizing into the tumor sites, and exhibiting specific cytotoxicity activity against tumor cells [92, 93]. in a B-cell lymphoma model, using CD62L+CD19− CAR-engineered NKT cells indicated therapeutic activity [94].
Concluding remark
In this review, we have attempted in the role of NKT cells in cancer immunity. Type I and type II NKT cells form a regulatory axis that in addition to cross talk with each other, they interact with other cells from both arms of innate and adaptive immunity. Despite all progress in our knowledge about activation and functions of NKT cells, many questions still remain unanswered about NKT cell biology and signaling pathway that contribute in activation and regulation of these cells. Moreover, as many studies have been conducted on mice model, more research is needed to translate results to humans’ cases, especially because the frequency and the number of type I NKT cells is significantly lower in human than in mice and it is also more variable between individual. The identification of new type I NKT cell agonists which can promote immune responses without inducing anergy is of high priority. Moreover, better recognition of endogenous self-antigens seems essential and our little information has been limited better understanding of this cell biology.
References
[1]. Arrenberg P, Halder R, Kumar V. Cross-regulation between distinct natural killer T cell subsets influences immune response to self and foreign antigens. J Cell Physiol.2009;218(2):246-50.
[2]. Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest.2004;114(10):1379-88.
[3]. Godfrey DI, et al. NKT cells: what's in a name? .Nat Rev Immunol. 2004;4(3): 231-7.
[4]. Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297-336.
[5]. Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol.2010;11(3):197-206.
[6]. Tuttle KD, Gapin L. Characterization of Thymic Development of Natural Killer T Cell Subsets by Multiparameter Flow Cytometry. Methods Mol Biol. 2018;1799:121-33.
[7]. Galli G, Pittoni P, Tonti E, Malzone C, Uematsu Y, Tortoli M, et al. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci U S A. 2007;104(10):3984-9.
[8]. La Cava A, Van Kaer L, Fu Dong S. CD4+CD25+ Tregs and NKT cells: regulators regulating regulators. Trends Immunol. 2006;27(7):322-7.
[9]. Balato A, Unutmaz D, Gaspari A A.Natural killer T cells: an unconventional T-cell subset with diverse effector and regulatory functions. J Invest Dermatol.2009;129(7):1628-42.
[10]. Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J Exp Med. 1994;180(3):1097-106.
[11]. Zhou D, Mattner J, Cantu C, 3rd, Schrantz N, Yin N, Gao Y, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306(5702):1786-9.
[12]. Brutkiewicz RR. CD1d ligands: the good, the bad, and the ugly. J Immunol. 2006;177(2):769-75.
[13]. Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278(5343):1626-9.
[14]. Gumperz JE, Roy C, Makowska A, Lum D, Sugita M, Podrebarac T, et al. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 2000;12(2):211-21.
[15]. Coquet JM, Chakravarti S, Kyparissoudis K, McNab FW, Pitt LA, McKenzie BS, et al. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc Natl Acad Sci U S A. 2008;105(32):11287-92.
[16]. Watarai H, Sekine-Kondo E, Shigeura T, Motomura Y, Yasuda T, Satoh R, et al. Development and function of invariant natural killer T cells producing T(h)2- and T(h)17-cytokines. PLoS Biol. 2012;10(2):e1001255.
[17]. Brennan P J, Brigl M, Brenner M B. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13(2):101-17.
[18]. Takahashi T, Chiba S, Nieda M, Azuma T, Ishihara S, Shibata Y, et al. Cutting edge: analysis of human V alpha 24+CD8+ NK T cells activated by alpha-galactosylceramide-pulsed monocyte-derived dendritic cells. J Immunol. 2002;168(7):3140-4.
[19]. Coquet J M, et al. Diverse cytokine production by NKT cell subsets and identification of an IL-17–producing CD4(−)NK1.1(−) NKT cell population. Proc Natl Acad Sci U S A. 2008;105(32):11287-11292.
[20]. Lee Y J, et al. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol. 2013;14(11):1146-54.
[21]. Moreira-Teixeira L, et al. Rapamycin combined with TGF-beta converts human invariant NKT cells into suppressive Foxp3+ regulatory cells. J Immunol. 2012;188(2):624-31.
[22]. Arrenberg P, Halder R, Dai Y, Maricic I, Kumar V. Oligoclonality and innate-like features in the TCR repertoire of type II NKT cells reactive to a beta-linked self-glycolipid. Proc Natl Acad Sci U S A. 2010;107(24):10984-9.
[23]. Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1(6):515-20.
[24]. Bedel R, Matsuda JL, Brigl M, White J, Kappler J, Marrack P, et al. Lower TCR repertoire diversity in Traj18-deficient mice. Nat Immunol. 2012;13(8):705-6.
[25]. Jahng A, Maricic I, Aguilera C, Cardell S, Halder RC, Kumar V. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J Exp Med. 2004;199(7):947-57.
[26]. Skold M, Faizunnessa NN, Wang CR, Cardell S. CD1d-specific NK1.1+ T cells with a transgenic variant TCR. J Immunol. 2000;165(1):168-74.
[27]. Lee HJ, Takemoto N, Kurata H, Kamogawa Y, Miyatake S, O'Garra A, et al. GATA-3 induces T helper cell type 2 (Th2) cytokine expression and chromatin remodeling in committed Th1 cells. J Exp Med. 2000;192(1):105-15.
[28]. Mullen A C, et al.Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science.2001;292(5523):1907-10.
[29]. Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198(7):1069-76.
[30]. Kumar V, Delovitch T L. Different subsets of natural killer T cells may vary in their roles in health and disease. Immunology. 2014;142(3):321-36.
[31]. Croudace JE, Curbishley SM, Mura M, Willcox CR, Illarionov PA, Besra GS, et al. Identification of distinct human invariant natural killer T-cell response phenotypes to alpha-galactosylceramide. BMC Immunol. 2008;9:71.
[32]. Terabe M, Berzofsky JA. The immunoregulatory role of type I and type II NKT cells in cancer and other diseases. Cancer Immunol Immunother. 2014;63(3):199-213.
[33]. Paget C, Chow MT, Duret H, Mattarollo SR, Smyth MJ. Role of gammadelta T cells in alpha-galactosylceramide-mediated immunity. J Immunol. 2012;188(8):3928-39.
[34]. De Santo C, Salio M, Masri SH, Lee LY, Dong T, Speak AO, et al. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest. 2008;118(12):4036-48.
[35]. Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138(2):105-15.
[36]. De Santo C, et al. Invariant NKT cells modulate the suppressive activity of Serum Amyloid A-differentiated IL-10-secreting neutrophils. Nat Immunol. 2010;11(11):1039-1046.
[37]. Gebremeskel S, Clattenburg DR, Slauenwhite D, Lobert L, Johnston B. Natural killer T cell activation overcomes immunosuppression to enhance clearance of postsurgical breast cancer metastasis in mice. Oncoimmunology. 2015;4(3):e995562.
[38]. Kwiecinski J, et al. Sulfatide Attenuates Experimental Staphylococcus aureus Sepsis through a CD1d-Dependent Pathway. Infection and Immunity.2013;81(4):1114-1120.
[39]. Duthie MS, Kahn M, White M, Kapur RP, Kahn SJ. Critical proinflammatory and anti-inflammatory functions of different subsets of CD1d-restricted natural killer T cells during Trypanosoma cruzi infection. Infect Immun. 2005;73(1):181-92.
[40]. Terabe M, et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198(11):1741-52.
[41]. Parekh V V, et al. Activated invariant NKT cells control central nervous system autoimmunity in a mechanism that involves myeloid-derived suppressor cells. J Immunol. 2013;190(5): 1948-1960.
[42]. Pang Y, et al. Transforming growth factor β signaling in myeloid cells is required for tumor metastasis. Cancer discovery. 2013;3(8):936-951
[43]. Faunce DE, Sonoda KH, Stein-Streilein J. MIP-2 recruits NKT cells to the spleen during tolerance induction. J Immunol. 2001;166(1):313-21.
[44]. Ly D, Mi QS, Hussain S, Delovitch TL. Protection from type 1 diabetes by invariant NK T cells requires the activity of CD4+CD25+ regulatory T cells. J Immunol. 2006;177(6):3695-704.
[45]. Liu R, La Cava A, Bai XF, Jee Y, Price M, Campagnolo DI, et al. Cooperation of invariant NKT cells and CD4+CD25+ T regulatory cells in the prevention of autoimmune myasthenia. J Immunol. 2005;175(12):7898-904.
[46]. Hongo D, et al. Interactions between NKT cells and Tregs are required for tolerance to combined bone marrow and organ transplants. Blood. 2012;119(6):1581-1589.
[47]. Jiang S, Game DS, Davies D, Lombardi G, Lechler RI. Activated CD1d-restricted natural killer T cells secrete IL-2: innate help for CD4+CD25+ regulatory T cells? Eur J Immunol. 2005;35(4):1193-200.
[48]. Pillai A B, et al. Host natural killer T cells induce an interleukin-4–dependent expansion of donor CD4(+)CD25(+)Foxp3(+) T regulatory cells that protects against graft-versus-host disease. Blood. 2009;113(18):4458-4467.
[49]. Santodomingo-Garzon T, Han J, Le T, Yang Y, Swain MG. Natural killer T cells regulate the homing of chemokine CXC receptor 3-positive regulatory T cells to the liver in mice. Hepatology. 2009;49(4):1267-76.
[50]. Azuma T, Takahashi T, Kunisato A, Kitamura T, Hirai H. Human CD4+ CD25+ regulatory T cells suppress NKT cell functions. Cancer Res. 2003;63(15):4516-20.
[51]. Ambrosino E, et al. Cross-regulation between type I and type II NKT cells in regulating tumor immunity: a new immunoregulatory axis. J Immunol. 2007;179(8):5126-36.
[52]. Tahir SM, Cheng O, Shaulov A, Koezuka Y, Bubley GJ, Wilson SB, et al. Loss of IFN-gamma production by invariant NK T cells in advanced cancer. J Immunol. 2001;167(7):4046-50.
[53]. Yoneda K, Morii T, Nieda M, Tsukaguchi N, Amano I, Tanaka H, et al. The peripheral blood Valpha24+ NKT cell numbers decrease in patients with haematopoietic malignancy. Leuk Res. 2005;29(2):147-52.
[54]. Dhodapkar MV, Geller MD, Chang DH, Shimizu K, Fujii S, Dhodapkar KM, et al. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J Exp Med. 2003;197(12):1667-76.
[55]. Bassiri H, Das R, Guan P, Barrett DM, Brennan PJ, Banerjee PP, et al. iNKT cell cytotoxic responses control T-lymphoma growth in vitro and in vivo. Cancer Immunol Res. 2014;2(1):59-69.
[56]. Coquet J M, et al. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. 2007;178(5):2827-34.
[57]. Wingender G, et al. Antigen-specific cytotoxicity by invariant NKT cells in vivo is CD95/CD178 dependent and is correlated with antigenic potency. J Immunol.2010;185(5):2721-2729.
[58]. Hagihara M, Gansuvd B, Ueda Y, Tsuchiya T, Masui A, Tazume K, et al. Killing activity of human umbilical cord blood-derived TCRValpha24(+) NKT cells against normal and malignant hematological cells in vitro: a comparative study with NK cells or OKT3 activated T lymphocytes or with adult peripheral blood NKT cells. Cancer Immunol Immunother. 2002;51(1):1-8.
[59]. Metelitsa LS, Weinberg KI, Emanuel PD, Seeger RC. Expression of CD1d by myelomonocytic leukemias provides a target for cytotoxic NKT cells. Leukemia. 2003;17(6):1068-77.
[60]. Metelitsa LS. Anti-tumor potential of type-I NKT cells against CD1d-positive and CD1d-negative tumors in humans. Clin Immunol. 2011;140(2):119-29.
[61]. Nowak M, et al. Defective NKT cell activation by CD1d+ TRAMP prostate tumor cells is corrected by interleukin-12 with alpha-galactosylceramide. PLoS ONE.2010;5(6):e11311.
[62]. Spanoudakis E, Hu M, Naresh K, Terpos E, Melo V, Reid A, et al. Regulation of multiple myeloma survival and progression by CD1d. Blood. 2009;113(11):2498-507.
[63]. Fallarini S, Paoletti T, Orsi Battaglini N, Lombardi G. Invariant NKT cells increase drug-induced osteosarcoma cell death. Br J Pharmacol. 2012;167(7):1533-49.
[64]. Bellone M, Ceccon M, Grioni M, Jachetti E, Calcinotto A, Napolitano A, et al. iNKT cells control mouse spontaneous carcinoma independently of tumor-specific cytotoxic T cells. PLoS One. 2010;5(1):e8646.
[65]. Anastasiadis A, et al.CD1d expression as a prognostic marker for chronic lymphocytic leukemia. Leuk Lymphoma.2014;55(2):320-5.
[66]. Bojarska-Junak A, et al. CD1d expression is higher in chronic lymphocytic leukemia patients with unfavorable prognosis. Leuk Res. 2014;38(4): 435-42.
[67]. Gorini F, Azzimonti L, Delfanti G, Scarfo L, Scielzo C, Bertilaccio MT, et al. Invariant NKT cells contribute to chronic lymphocytic leukemia surveillance and prognosis. Blood. 2017;129(26):3440-51.
[68]. Parekh VV, Lalani S, Van Kaer L. The in vivo response of invariant natural killer T cells to glycolipid antigens. Int Rev Immunol. 2007;26(1-2):31-48.
[69]. Metelitsa LS, Naidenko OV, Kant A, Wu HW, Loza MJ, Perussia B, et al. Human NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. J Immunol. 2001;167(6):3114-22.
[70]. Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood. 2001;97(1):192-7.
[71]. Crowe NY, Smyth MJ, Godfrey DI. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med. 2002;196(1):119-27.
[72]. Smyth MJ, et al.Sequential production of interferon-gamma by NK1.1(+) T cells and natural killer cells is essential for the antimetastatic effect of alpha-galactosylceramide. Blood. 2002;99(4):1259-66.
[73]. Izhak L, et al.Delicate balance among three types of T cells in concurrent regulation of tumor immunity. Cancer research.2013;73(5):1514-1523.
[74]. Terabe M, Berzofsky JA. Immunoregulatory T cells in tumor immunity. Curr Opin Immunol. 2004;16(2):157-62.
[75]. Renukaradhya GJ, Sriram V, Du W, Gervay-Hague J, Van Kaer L, Brutkiewicz RR. Inhibition of antitumor immunity by invariant natural killer T cells in a T-cell lymphoma model in vivo. Int J Cancer. 2006;118(12):3045-53.
[76]. Bjordahl, R.L., et al., iNKT Cells Suppress the CD8(+) T Cell Response to a Murine Burkitt’s-Like B Cell Lymphoma. PLoS ONE.2012;7(8):e42635.
[77]. Zhao J, Bagchi S, Wang CR. Type II natural killer T cells foster the antitumor activity of CpG-oligodeoxynucleotides. Oncoimmunology. 2014;3:e28977.
[78]. Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M, et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191(4):661-8.
[79]. Swann JB, Uldrich AP, van Dommelen S, Sharkey J, Murray WK, Godfrey DI, et al. Type I natural killer T cells suppress tumors caused by p53 loss in mice. Blood. 2009;113(25):6382-5.
[80]. Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202(9):1279-88.
[81]. Exley MA, et al.Adoptive Transfer of Invariant NKT Cells as Immunotherapy for Advanced Melanoma: a Phase 1 Clinical Trial. Clin Cancer Res, 2017;23(14):3510-3519.
[82]. Hayakawa Y, et al.Critical contribution of IFN-gamma and NK cells, but not perforin-mediated cytotoxicity, to anti-metastatic effect of alpha-galactosylceramide. Eur J Immunol. 2001;31(6):1720-7.
[83]. Shimizu K, Goto A, Fukui M, Taniguchi M, Fujii S. Tumor cells loaded with alpha-galactosylceramide induce innate NKT and NK cell-dependent resistance to tumor implantation in mice. J Immunol. 2007;178(5):2853-61.
[84]. Yanagisawa K, Exley MA, Jiang X, Ohkochi N, Taniguchi M, Seino K. Hyporesponsiveness to natural killer T-cell ligand alpha-galactosylceramide in cancer-bearing state mediated by CD11b+ Gr-1+ cells producing nitric oxide. Cancer Res. 2006;66(23):11441-6.
[85]. Sullivan BA,Kronenberg M.Activation or anergy: NKT cells are stunned by α-galactosylceramide. Journal of Clinical Investigation.2005;115(9):2328-2329.
[86]. Parekh VV, Wilson MT, Olivares-Villagomez D, Singh AK, Wu L, Wang CR, et al. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115(9):2572-83.
[87]. Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-gamma-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat Immunol. 2002;3(9):867-74.
[88]. Escriba-Garcia L, Alvarez-Fernandez C, Tellez-Gabriel M, Sierra J, Briones J. Dendritic cells combined with tumor cells and alpha-galactosylceramide induce a potent, therapeutic and NK-cell dependent antitumor immunity in B cell lymphoma. J Transl Med. 2017;15(1):115.
[89]. Mattarollo SR, West AC, Steegh K, Duret H, Paget C, Martin B, et al. NKT cell adjuvant-based tumor vaccine for treatment of myc oncogene-driven mouse B-cell lymphoma. Blood. 2012;120(15):3019-29.
[90]. Kobayashi T, et al.NKT cell-targeted vaccination plus anti-4–1BB antibody generates persistent CD8 T cell immunity against B cell lymphoma. Oncoimmunology. 2015;4(3):e990793.
[91]. Bae EA, Seo H, Kim BS, Choi J, Jeon I, Shin KS, et al. Activation of NKT Cells in an Anti-PD-1-Resistant Tumor Model Enhances Antitumor Immunity by Reinvigorating Exhausted CD8 T Cells. Cancer research. 2018;78(18):5315-26.
[92]. Heczey A, Liu D, Tian G, Courtney AN, Wei J, Marinova E, et al. Invariant NKT cells with chimeric antigen receptor provide a novel platform for safe and effective cancer immunotherapy. Blood. 2014;124(18):2824-33.
[93]. Simon B, Wiesinger M, Marz J, Wistuba-Hamprecht K, Weide B, Schuler-Thurner B, et al. The Generation of CAR-Transfected Natural Killer T Cells for the Immunotherapy of Melanoma. Int J Mol Sci. 2018;19(8).
[94]. Tian G, et al. CD62L(+) NKT cells have prolonged persistence and antitumor activity in vivo. J Clin Invest.2016.;126(6): 2341-23.
License
Copyright
© ,
Author Details