Chrysin Sensitizes Human Lung Cancer Cells to Tumour Necrosis Factor Related Apoptosis-Inducing Ligand (TRAIL) Mediated Apoptosis
Download
Abstract
Background: Lung cancer is the primary cause of cancer deaths worldwide. Thus, the requisite for more coherent methods to lung cancer therapy is needed.
Purpose: Chrysin (5, 7-dihydroxyflavone) is a naturally occurring flavonoid having a wide range of pharmacological properties and is commonly found in fruits, vegetables, honey and propolis. In our study, we have hypothesized that chrysin would have anticancer activity on L132 lung cancer cell line.
Methods: The cytotoxic effects were assessed by MTT and NRU assay. DAPI was used to evaluate the cell death. The pro- or anti-apoptotic proteins were detected by Western Blot assay, and, besides, mRNA expression was analysed with RT-PCR. In silico study of chrysin was performed to identify suitable inhibitors against the protein function.
Results: Results indicated that chrysin enhanced the inhibitory effects of TRAIL (Tumour Necrosis Factor Related Apoptosis-Inducing Ligand) in comparison to TNF-α (tumour necrosis factor) on cell viability in L132 lung cancer cells and altered nuclear morphology of cells was observed in DAPI (4’,6-diamidino-2-phenylindole) staining after 48 hrs treatment. Treatment with chrysin enhances TRAIL-induced apoptosis by increasing the expression of apoptosis-related proteins including caspase-3, 8, 9 and Bax, whereas the expression of Bcl-2 was decreased. Chrysin was docked with caspase-3, 8, 9, Bax, and Bcl-2 proteins to identify suitable inhibitors against the protein function.
Conclusion: We concluded that chrysin sensitizes lung cancer cells to TRAIL-induced apoptosis and may be considered for future studies as a promising therapeutic candidate for human lung cancer.
Introduction
Lung cancer is considered as a big killer among cancer diseases, which signifies a serious risk to human health [1]. Thus, it is essential to explore novel targeted compounds against lung cancer [2] Scagliotti et al., 2008). The flavonoids are plant phenolic compounds and have biochemical and pharmacological properties viz., antibacterial, antiviral, anti-inflammatory, antiallergic, antithrombotic, anti-mutagenic and antineoplastic [3]. Epidemiological studies further validated the significance of flavonoids with a reduced risk of cardiovascular ailment and different types of cancers together with breast, colon, lung, pancreas, oral and prostate both in vitro and in vivo [4].
Chrysin (5, 7-dihydroxyflavone) is a natural flavonoid present in many plant extracts, honey, and propolis. It possesses numerous biological and pharmacological properties including antioxidant, apoptotic, anticancer, anti-inflammatory, etc [5]. Recent studies have revealed that chrysin regulates key molecules involved in inflammation, cancer, and aging [6]. It has also been reported that chrysin induces apoptosis in cancer cells, making it a possible candidate as an anticancer agent [7]. It has been demonstrated that chrysin inhibits the growth of Hela cells via apoptosis and inactivation of AKT in cancer cell lines [8]. It also considerably sensitizes TNF-α induced apoptosis in several human cancer cells [9].
It might be possible that a combinatorial effect of a chemotherapeutic drug with TRAIL or TNF-α is required to achieve efficient cell death clinically. Currently, much exploration in the field of research is being carried on the appraisal of non-toxic and competent plant-based products against cancer because of their constructive outcomes in vivo and in vitro.
In the present study, we have demonstrated that chrysin synergistically enhances the upregulation of gene expressions of caspase-3, 8, 9, Bax, and downregulation of the expression of Bcl-2 in TRAIL-resistant lung cancer cells to TRAIL-induced apoptosis. Besides TRAIL, TNF-α was also thought to be a potent anticancer agent due to its cytotoxicity against several tumour cell lines. However, the clinical use of TNF-α is limited because of its systemic toxicity. The reason behind this is largely due to the activation of the pro-inflammatory NFκB (Nuclear factor Kappa-B) family transcription factors [10][11][12]. TRAIL and TNF-α individually did not show the significant change in the expression of apoptotic and anti-apoptotic proteins, however, in combination with TRAIL, chrysin showed a significant change of expression in apoptotic and anti-apoptotic protein. Additionally, chrysin was docked with caspase-3, 8, 9, Bax and Bcl-2 to identify suitable inhibitors against the protein function. Thus, our findings raise the possibility that combined use of chrysin and TRAIL could be a candidate therapy for the treatment of lung cancer.
Materials and Methods
Procurement of chemicals
Chrysin was purchased from Sigma–Aldrich (USA). DMEM (Dulbecco’s modified Eagle’s medium) powder, Neutral Red, MTT (3-(4,5-dimethylthiazole-2-yl)-2,5 diphenyl tetrazolium bromide) and 0.25% trypsin and 0.02% EDTA mixture were purchased from Himedia (India). Fetal bovine serum (FBS) was from Gibco (USA). All the other chemicals and reagents were purchased from Merck and were of molecular biology grade. Chrysin was dissolved in DMSO and stored at 4º C.
Preparation of cell culture medium
DMEM with 2 mM L-glutamine and glucose 4.5 gm/L supplied as dry powder was dissolved in 1L Milli-Q water and supplemented with 25mM NaHCO3, 10mM HEPES, thereafter, 1% (v/v) antibiotics and antimycotic was added. After that, the pH was adjusted to 7.4 using 1N NaCl or HCl, and the medium was filtered sterilize (0.22µm). Incubating the medium at 37 ºC for 72 h routinely checked the sterility and then the medium was stored at 4ºC. Finally, the complete culture medium was prepared by the addition of Fetal Bovine Serum 10% (v/v) for maintaining cell lines.
Procurement cell lines and maintenance
Lung cancer cell lines were procured from NCCS Pune, India. L-132 was cultured and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM), supplemented with 10% Fetal Bovine Serum and 1% antibiotics solution containing penicillin, streptomycin, and amphotericin with 25mM sodium bicarbonate and 10mM HEPES in a humidified atmosphere of 5% CO2 at 37 °C in culture dishes/flasks. The stock culture was maintained in the exponential growth phase by passaging as monolayer culture using in 0.02% EDTA. The dislodged cells were suspended in complete medium and reseeded routinely.
Cytotoxicity assay
The cytotoxic effect was assessed in lung cancer cell exposed to different concentrations of chrysin by the MTT assay. 3-(4,5-dimethyl-2-yl)-2,5-diphynyl tetrazolium bromide (MTT) is a metabolic substrate, which is reduced by the mitochondrial succinate dehydrogenase enzyme and forms formazan crystal. Cells were seeded overnight at the number of 1 × 104 per well and then incubated with various concentration of chrysin for 48h. At the end of the treatment, the medium was removed, and cells were incubated with 20 µl of MTT (5mg/mL in PBS) in fresh medium (50 µL) for 4h in CO2 incubator. After four hours formazan crystal, formed by mitochondrial reduction of MTT were solubilized in DMSO (Dimethyl sulfoxide) (150 µL/well) and the absorbance was read at 570nm after 10 min incubation on the iMark Microplate Reader (Bio-Rad, USA). Percent of cytotoxicity was expressed as IC50 [13][14].
Neutral red dye uptake assay
Neutral red (3-amino-m-dimethylamino-2-methylphenazine hydrochloride) assay determined the accumulation of the neutral red dye in the lysosomes of viable, uninjured cells. The Chrysin was incubated with cells for 48 h. Neutral red dye (100 µg/mL) was dissolved in serum-free medium (DMEM). The pH of the neutral red solution was adjusted in all the experiments to 6.35 with the addition of KH2PO4 (1M). Ten microlitres of neutral red were incubated with the cells for 1 h. After that, washed with phosphate buffer saline (PBS) and added 1mL of elution medium (EtOH/AcCOOH, 50%/1%) followed by gentle shaking for 10 min so that complete dissolution was achieved. The absorbance was taken at 540 nm using iMark Microplate Reader (Bio-Rad, USA). Percent cytotoxicity was expressed as IC50 [15].
DAPI Staining
Cell nuclear morphology was evaluated by fluorescence microscopy following DAPI staining. L132 cells were treated with chrysin and in combination with TRAIL for 48h. The cells were washed with PBS (pH 7.4), fixed with ice-cold 70% ethanol and resuspended in DAPI, and incubated for 15 min at 37°C wrapped in aluminium foil. The cells were then washed with PBS and examined under Nikon Eclipse fluorescence microscope (Nikon Instruments Inc., NY, and USA [16].
In-silico study of chrysin
To understand the mechanism of chrysin towards apoptotic/anti-apoptotic gene, molecular docking was carried out for the chrysin using Auto Dock 4.2 [17]. Lamarckian Genetic Algorithm was used to carry out the docking analysis. The docking simulations end with multiple runs and cluster analysis of ligands were performed with their corresponding docked energy. The binding sites for these molecules were selected based on the ligand-binding pocket of the templates [18]. Docking solutions with ligands all-atom RMSDs within 2.0 A of each other were clustered together and ranked by the lowest energy representative. Finally, the obtained top-posed docking conformations were subjected to post-docking energy minimization on DS 3.5. The resultant structure files were analyzed using PyMOL visualization programs [19].
Western Blot
Western blot analysis was carried out according to [20] using cytosolic as well as nuclear fractions of human lung cancer cells L132 treated with selected concentrations of chrysin alone and in combination with TRAIL for 48h. Protein concentration was determined by using Bradford reagent and lysates were resolved on 15% sodium dodecyl sulfate (SDS) polyacrylamide gels. The proteins were then electrotransferred onto a nitrocellulose membrane (Sigma, St. Louis, MO, USA). After blocking with 5% non-fat milk in Tris-buffered saline (TBS, 0.1 M, pH 7.4), blots were subjected to various primary antibody incubations with caspase-3, 8, 9, Bax and Bcl-2 (R & D System, USA) at 4 °C overnight. Protein abundance of β-actin served as a control for protein loading for cytosolic and nuclear fractions, respectively. Membranes were incubated with secondary antibody concerning primary antibody and diluted at appropriate dilution in 1% BSA, for 1 h at room temperature. After each step, blots were washed thrice with Tris-buffer saline-Tween 20 (TBST). Protein bands were detected by the enhanced chemiluminescence method (ECL, Bio-Rad, Hercules, CA, USA). The protein expression pattern was obtained by normalizing the density to that of β-actin for cytosolic and nuclear fractions.
Quantitative Real-Time PCR
Total RNA was isolated from untreated and treated L132 cells with selected concentrations of the chrysin alone and in combination with TRAIL for 48h using TRI reagent (Sigma, St. Louis, MO, USA). Complementary DNA (cDNA) was synthesized using the easy cDNA synthesize kit (Bio-Rad, USA). The reaction PCR was performed in a final volume of 20μL containing 10μL Taq premix, 2μL cDNA, 2μL primer (forward and reverse) and water. RT-PCR was started with an initial cycle of reverse transcription at 95 °C for 4 min followed by 35 cycles of denaturation at 94°C for the 30s, annealing (58°C for 30s), and extension (72°C for 30s). The primer sequences used for Cas-3, 8, 9, Fas, Bcl2 and Bax and β-actin were designed for L132 cells are as follows: Following amplification, the PCR products were electrophoresed in a 2% agarose gel.
| S.No | GENES | SEQUENCES |
| 1 | Caspase3 | Forward 5'-AATACCAGTGGAGGCCGACTT-3'; Reverse 5'-TCAAGCTTGTCGGCATACTGTT-3'. |
| 2 | Caspase8 | Forward 5'-CATCCAGTCACTTTGCCAGA-3'; Reverse 5'-GCATCTGTTTCCCCATGTTT-3'. |
| 3 | Caspase9 | Forward 5'-TTCCCAGGTTTTGTTTCCTG-3'; Reverse 5'-CCTTTCACCGAAACAGCATT-3'. |
| 4 | BcL2 | Forward 5'-CTGCACCTGACGCCCTTCACC-3'; Reverse 5'-CACATGACCCCACCGAACTCAAAGA-3'. |
| 5 | Bax | Forward 5'-GAGCTGCAGAGGATGATTGC-3'; Reverse 5'-CCGGGAGCGGCTGTTGGGCT3'. |
| 6 | 𝛽-actin | Forward 5'- AAATCGTGCGTGACATTAA-3; Reverse 5'- CTCGTCATACTCCTGCTTG-3. |
Statistical analysis
Data were analyzed using Microsoft Excel (Microsoft, Redmond, USA). Results are expressed as mean ± standard deviation. The difference between each experimental group and the control group was analyzed with Student's t-test. Probability values of less than 0.05 were considered statistically significant.
Results
Chrysin sensitizes lung cancer cells for TRAIL-induced cytotoxicity but not for TNF-α
The cytotoxic effect was assessed by the MTT and NRU assay in L132, which were exposed to different concentrations of chrysin. Results indicate that chrysin alone is not inducing cytotoxicity in lung cancer cells even at higher concentration (Figure. 1).
Figure 1. Cytotoxic effect of chrysin on lung cancer cell (L132) treated with different concentrations at 48hrs by MTT and NRU assay. Values were expressed as mean ± SD, and the experiment was performed in triplicate (p<0.05).
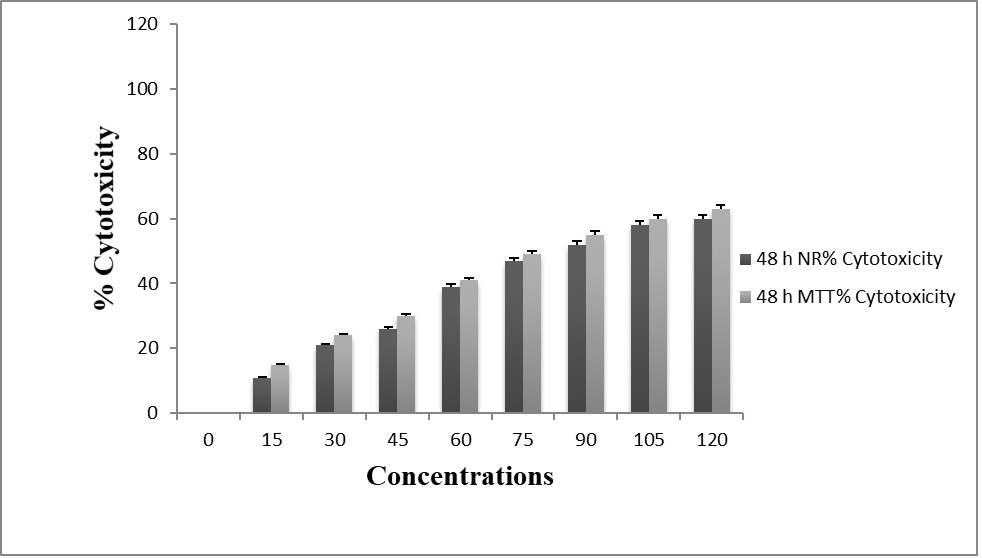
Also, treatment with TRAIL and TNF-α alone (50-100 ng/mL) also did not show any significant effect on cell viability on L132 cell line (Figure 2).
Figure 2. Cytotoxic effect of Trail and TNF-α on on lung cancer cell (L132) treated with different concentrations at 48hrs by MTT assay. The experiment was performed in triplicate (p<0.05).
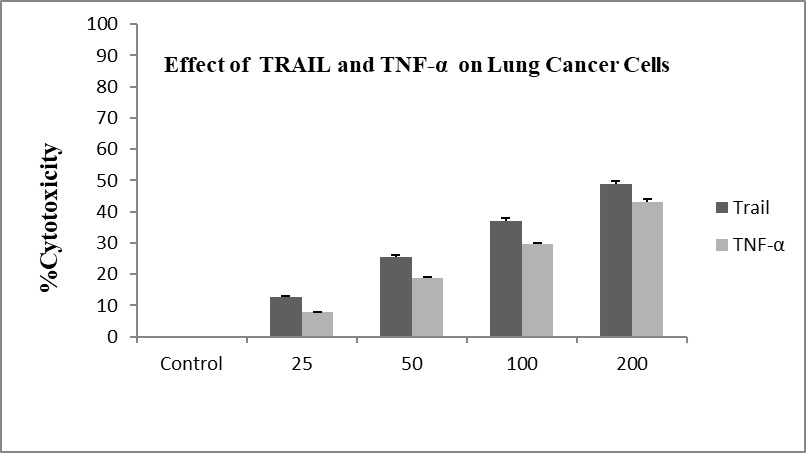
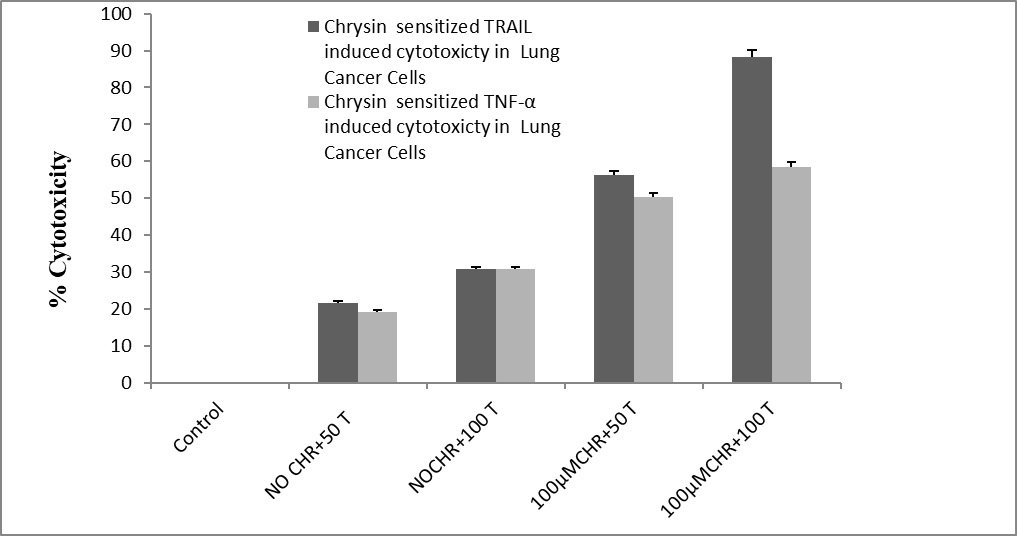
However, we found that cytotoxic effects of chrysin were increased when we treated the cancer cells in combination with TRAIL whereas, treatment in combination with TNF-α did not show any significant toxicity on lung cancer cell line as shown in figure (3). Chrysin enhanced the inhibitory effects of TRAIL on cell viability in L132 lung cancer cells within 48h dose-dependently.
Figure 3. Chrysin sensitized Trail and TNF-α cytotoxicity on on lung cancer cell (L132) treated with different concentrations at 48hrs by MTT assay. The experiment was performed in triplicate (p<0.05).
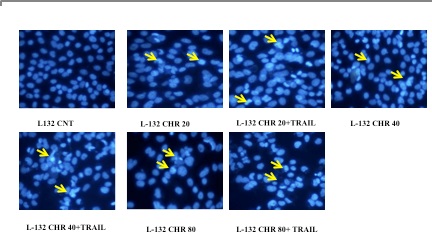
Chrysin induced cell apoptosis through DAPI staining
Cell death was confirmed through the fluorescence microscopic study. Results showed that the cells treated with chrysin and in combination with TRAIL 48h showed altered nuclear morphology. Control cells showed normal/regular morphology, however in chrysin and TRAIL treated cells; nuclear morphology seems to have more nuclear condensation, nuclear blebbing, nuclear fragmentation and overall morphological changes as shown in figure (4).
Figure 4. Microscopic appearance of chrysin and its combination with Trail treated lung cancer cells (L132). Fluorescence microscope photographs of L132 cells treated for 48 hrs and stained with DAPI (magnification 40x).
Expression of apoptotic marker proteins by western blotting
The expression of apoptotic and anti-apoptotic proteins was confirmed through the western blot analysis by using protein-specific monoclonal antibody. We found that chrysin treated cells showed upregulation of apoptotic marker proteins viz; Bax, Caspases-3, 8 and 9 and down-regulation of the anti-apoptotic protein, Bcl-2 when treated in combination with TRAIL than treated alone, as shown in figure (5). Our data show that treatment with chrysin enhances TRAIL-induced apoptosis by increasing the expression of apoptosis-related proteins including caspase-3, 8, 9 and Bax, whereas by decreasing the expression of Bcl-2. However, we did not find any significant effect on the expression of these genes when we treated the cells with chrysin and TNF-α in combination (data not shown).
Figure 5. Immunoblotting showing the effect of chrysin and its combination with TRAIL-induced caspase-3,caspase-9, caspase-8, Bax and Bcl-2 expression in human lung cancer cells.
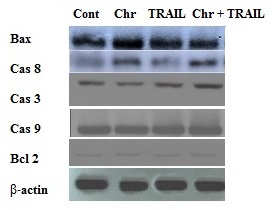
Chrysin augments TRAIL-induced apoptosis through activation of caspases and Bcl-2 family members
We performed quantitative real-time PCR to analyze the extrinsic and intrinsic apoptotic pathways in chrysin, TRAIL-induced apoptosis individually and in combination with TRAIL. We found that the genes viz., caspase-3, 8, 9, Bax are upregulated whereas Bcl-2 was found to be downregulated in chrysin, TRAIL individually and in combination with TRAIL in L132 as shown in figure (6).
Figure 6. Quantitative Real-Time PCR showing the effect of chrysin on TRAIL-induced caspase-3,caspase-9, caspase-8, Bax and Bcl-2 expression in human lung cancer cells.
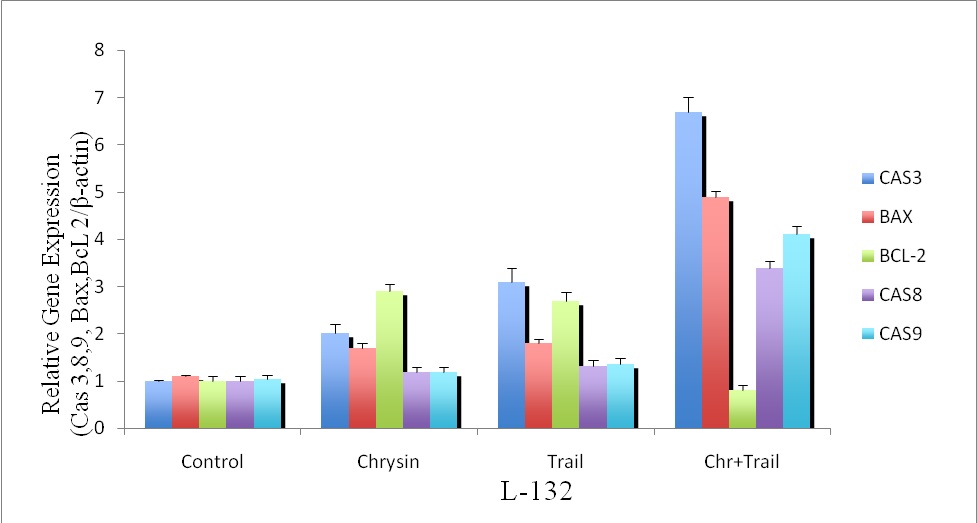
In silico study of chrysin on apoptotic and anti-apoptotic proteins
Molecular docking of chrysin with Bax, Bcl-2, caspase-3, caspase-8, and caspase-9 was performed through the latest version of Vina tool. Chrysin bind to the apoptotic and anti-apoptotic proteins via different amino acids. The detail of chrysin binding with amino acids and their position is summarized in table 1 and figure (7).
| Compound | Protein | Binding Amino Acids and Position |
| Chrysin | Bcl-2 | Asparagine-102 |
| Arginine-105 | ||
| Tyrosin- 67 | ||
| Bax | Glycine-26 | |
| Glutamine-73 | ||
| Caspase 9 | Glutamine-154 | |
| Glutamic Acid-297 | ||
| Caspase 3 | Methionine-39 | |
| Tyrosine-37 and 276 | ||
| Caspase 8 | Isoleucine-154 | |
| Lysine-409 |
Figure 7. A-E showed the complex structures of dock proteins with chrysin.
Discussion
Cancer is a complex disease; abnormal intracellular signal transduction system is connected to the occurrence and progression of cancer cells. Chemotherapy is one of the basic approaches to the treatment of cancer. Conversely, the chemotherapeutic drugs currently used for treating different types of cancer have adverse side effects. Thus, recent research is primarily focused on herbal plants that have been studied for being nontoxic and for the treatment and prevention of cancer [21].
The anti-cancer property of chrysin has been described earlier. The effect of chrysin on cell viability elucidated that chrysin possesses potent in vitro anticancer activity (Bhadra et al., 2012). The results obtained from the MTT and NRU assays indicated that lung cancer line (L132) showed time and dose-dependent viability loss by the addition of chrysin. Chrysin has been reported to induce apoptosis in a numerous cancer cell lines, as well as HeLa, cervical cancer cells [22], U937, HL-60, and L1210 leukemia cells [23][24][25], OE33 oesophageal adenocarcinoma cells [26] and KYSE-510 oesophageal squamous carcinoma cells [27]. In the present study, we attempted to further address the anti-cancer potential of chrysin by evaluating the sensitization effect of chrysin on TNF-α and TRAIL-induced apoptotic cell death and the molecular mechanisms involved.
According to the studies conducted by [28] it has been evaluated that TRAIL selectively kills various types of cancer cells without affecting normal cells, but TRAIL resistance has been recurrently observed in cancerous cells. It was demonstrated previously that chrysin sensitizes A549 and HeLa human cancer cell lines to TRAIL-induced apoptosis.
TRAIL is a potent inducer of apoptosis in cancerous cells. Several studies demonstrated that most of the cancerous cells are resistant to TRAIL-induced death, but combinatorial approaches based on TRAIL and different chemotherapeutic compounds like small-molecule inhibitors, drugs, and natural compounds, have been developed to overwhelm the resistance of cancer cells to TRAIL [29]. Therefore, to evade tumour cell resistance to TNF and TRAIL, combinatorial therapies are desirable.
Our results demonstrated that TRAIL individually did not show a significant change in the expression of apoptotic and anti-apoptotic proteins, however, in combination with TRAIL, chrysin showed a significant change of expression in apoptotic and anti-apoptotic protein. In earlier studies, it was reported that chrysin promotes TRAIL-induced caspase activation in CNE1 nasopharyngeal cancer cells [30]. Activation of caspases plays a significant role in a programmed cell death generated by different stimuli. Caspase-8 activates caspase- the 9-mediated apoptotic pathway through cleavage of Bid and then activate caspase-3 by cleavage of procaspase-3 [31]. Chrysin sensitizes tumour cells to TRAIL-induced apoptosis in our study as well. Chrysin exhibits a strong cytotoxic effect in combination with TRAIL on lung cancer cells. TRAIL-mediated apoptotic pathways could be a target of the anticancer efficacy of chrysin in lung cancer cells. We found that combined treatment of chrysin with TRAIL enhances the upregulation of caspase-3, 8 9 and Bax and downregulation of Bcl-2 in treated cells. The results indicate that chrysin in combination with TRAIL is a potentially upregulated and downregulated the expression of genes. Real-time PCR showed that chrysin upregulated the gene expression of caspase-3, 8, 9, Bax and downregulated the expression of Bcl-2 individually and in combination with TRAIL in L132 cells. Chrysin was docked with caspase-3, 8, 9, Bax and Bcl-2 to identify suitable inhibitors against the protein function. Thus, chrysin interacts with the target can be used as a potent inhibitor to block the action of protein viz., caspase-3, 8, 9, Bax and Bcl-2. Outcomes from our study thus demonstrated a novel function of chrysin and augmented the significance of chrysin as a useful chemotherapeutic compound.
In conclusion, our results revealed that combinatorial treatment of chrysin with TRAIL considerably augments apoptosis in lung cancer cells via Bcl-2 and caspases-dependent apoptotic pathway. Further investigation is needed to dichotomize the link among the above mechanisms. Hence, these results propose that combinatorial treatment of chrysin with TRAIL could be an effective strategy for human lung cancer therapy.
Acknowledgements
The authors would like to acknowledge the University Grants Commission (India) DS Kothari Fellowship (Grant Number: 4-2/2006 (BSR)/BL/13-14/0190) for providing financial assistance.
Competing Interests
Authors declare that they have no competing interests.
References
- Activation of endoplasmic reticulum stress and the extrinsic apoptotic pathway in human lung cancer cells by the new synthetic flavonoid, LZ-205 Zhang Yi, Xu Xuefen, Li Wei, Miao Hanchi, Huang Shaoliang, Zhou Yuxin, Sun Yang, Li Zhiyu, Guo Qinglong, Zhao Li. Oncotarget.2016;7(52). CrossRef
- Phase III Study Comparing Cisplatin Plus Gemcitabine With Cisplatin Plus Pemetrexed in Chemotherapy-Naive Patients With Advanced-Stage Non–Small-Cell Lung Cancer Scagliotti Giorgio Vittorio, Parikh Purvish, von Pawel Joachim, Biesma Bonne, Vansteenkiste Johan, Manegold Christian, Serwatowski Piotr, Gatzemeier Ulrich, Digumarti Raghunadharao, Zukin Mauro, Lee Jin S., Mellemgaard Anders, Park Keunchil, Patil Shehkar, Rolski Janusz, Goksel Tuncay, de Marinis Filippo, Simms Lorinda, Sugarman Katherine P., Gandara David. Journal of Clinical Oncology.2008;26(21). CrossRef
- Rutin ameliorates cyclophosphamide induced oxidative stress and inflammation in Wistar rats: Role of NFκB/MAPK pathway Nafees Sana, Rashid Summya, Ali Nemat, Hasan Syed Kazim, Sultana Sarwat. Chemico-Biological Interactions.2015;231. CrossRef
- Inhibition of precancerous lesions development in kidneys by chrysin via regulating hyperproliferation, inflammation and apoptosis at pre clinical stage Rashid Summya, Nafees Sana, Vafa Abul, Afzal Shekh Muhammad, Ali Nemat, Rehman Muneeb U., Hasan Syed Kazim, Siddiqi Aisha, Barnwal Preeti, Majed Ferial, Sultana Sarwat. Archives of Biochemistry and Biophysics.2016;606. CrossRef
- Influence of chrysin on hepatic marker enzymes and lipid profile against d-galactosamine-induced hepatotoxicity rats Pushpavalli Ganesan, Veeramani Chinnadurai, Pugalendi Kodukkur Viswanathan. Food and Chemical Toxicology.2010;48(6). CrossRef
- Chrysin, a natural flavone, improves murine inflammatory bowel diseases Shin Eun Kyung, Kwon Hyuck-Se, Kim Yoon Hee, Shin Hyun-Kyung, Kim Jin-Kyung. Biochemical and Biophysical Research Communications.2009;381(4). CrossRef
- Anti-tumor activity evaluation of novel chrysin–organogermanium(IV) complex in MCF-7 cells Yang Fen, Jin Hua, Pi Jiang, Jiang Jin-huan, Liu Li, Bai Hai-hua, Yang Pei-hui, Cai Ji-Ye. Bioorganic & Medicinal Chemistry Letters.2013;23(20). CrossRef
- Preventive effects of chrysin on the development of azoxymethane-induced colonic aberrant crypt foci in rats Miyamoto Shingo, Kohno Hiroyuki, Suzuki Rikako, Sugie Shigeyuki, Murakami Akira, Ohigashi Hajime, Tanaka Takuji. Oncology Reports.2006. CrossRef
- Chrysin sensitizes tumor necrosis factor-α-induced apoptosis in human tumor cells via suppression of nuclear factor-kappaB Li Xin, Huang Qing, Ong Choon-Nam, Yang Xing-Fen, Shen Han-Ming. Cancer Letters.2010;293(1). CrossRef
- A phase I trial of intravenously-administered recombinant tumor necrosis factor-alpha in cancer patients. Feinberg B, Kurzrock R, Talpaz M, Blick M, Saks S, Gutterman J U. Journal of Clinical Oncology.1988;6(8). CrossRef
- NF-κB at the crossroads of life and death Karin Michael, Lin Anning. Nature Immunology.2002;3(3). CrossRef
- Signalling pathways of the TNF superfamily: a double-edged sword Aggarwal Bharat B.. Nature Reviews Immunology.2003;3(9). CrossRef
- Paraquat-Induced Ultrastructural Changes and DNA Damage in the Nervous System Is Mediated via Oxidative-Stress-Induced Cytotoxicity in Drosophila melanogaster Mehdi Syed Hassan, Qamar Ayesha. Toxicological Sciences.2013;134(2). CrossRef
- Malathion induced cell injury and cell death in the nervous system via oxidative stress induced cytotoxicity in Drosophila melanogaster SH M, A Q, Zafaryab M ea. Int J Sci Res.2017;6(12):447-450.
- Relief of Oxidative Stress Using Curcumin and Glutathione Functionalized ZnO Nanoparticles in HEK-293 Cell Line Kumar Amit, Zafaryab Md., Umar Ahmad, Rizvi M. M. A., Ansari H. Z. A. Fouad, Ansari S. G.. Journal of Biomedical Nanotechnology.2015;11(11). CrossRef
- Characterization and anti-proliferative activity of curcumin loaded chitosan nanoparticles in cervical cancer Khan Md. Asad, Zafaryab Md., Mehdi Syed Hassan, Ahmad Irfan, Rizvi M. Moshahid A.. International Journal of Biological Macromolecules.2016;93. CrossRef
- Monocyclicβ-lactam and unexpected oxazinone formation: synthesis, crystal structure, docking studies and antibacterial evaluation Aneja Babita, Irfan Mohammad, Hassan Md. Imtaiyaz, Prakash Amresh, Yadava Umesh, Daniliuc Constantin G., Zafaryab Md., Rizvi M. Moshahid A., Azam Amir, Abid Mohammad. Journal of Enzyme Inhibition and Medicinal Chemistry.2015. CrossRef
- In Silico Docking studies of Aldose Reductase Inhibitory activity of selected Flavonoids Muthuswamy U AC, Kuppusamy A, et al . Int J Drug Develop Res.2012;4(3):328-334.
- Virtual Screening for HIV Protease Inhibitors: A Comparison of AutoDock 4 and Vina Chang Max W., Ayeni Christian, Breuer Sebastian, Torbett Bruce E.. PLoS ONE.2010;5(8). CrossRef
- Cysteinyl leukotriene receptor antagonist regulates vascular permeability by reducing vascular endothelial growth factor expression Lee Kyung S., Kim So R., Park Hee S., Jin Gong Y., Lee Yong C.. Journal of Allergy and Clinical Immunology.2004;114(5). CrossRef
- Role of Caspases, Bax and Bcl-2 in Chrysin-Induced Apoptosis in the A549 Human Lung Adenocarcinoma Epithelial Cells Samarghandian Saeed, Nezhad Mohsen, Mohammadi Gholamreza. Anti-Cancer Agents in Medicinal Chemistry.2014;14(6). CrossRef
- Chrysin and its phosphate ester inhibit cell proliferation and induce apoptosis in Hela cells Zhang Ting, Chen Xiaolan, Qu Lingbo, Wu Jinglan, Cui Ran, Zhao Yufen. Bioorganic & Medicinal Chemistry.2004;12(23). CrossRef
- Flavonoids Induce Apoptosis in Human Leukemia U937 Cells Through Caspase- and Caspase-Calpain-Dependent Pathways Monasterio Alberto, Urdaci Maria C., Pinchuk Irina V., Lopez-Moratalla Natalia, Martinez-Irujo Juan J.. Nutrition and Cancer.2004;50(1). CrossRef
- Chrysin-induced apoptosis is mediated through caspase activation and Akt inactivation in U937 leukemia cells Woo Kyung Jin, Jeong Yong-Jin, Park Jong-Wook, Kwon Taeg Kyu. Biochemical and Biophysical Research Communications.2004;325(4). CrossRef
- Effects of flavonoids on cisplatin-induced apoptosis of HL-60 and L1210 leukemia cells Čipák Luboš, Rauko Peter, Miadoková Eva, Čipáková Ingrid, Novotný Ladislav. Leukemia Research.2003;27(1). CrossRef
- Flavones and flavonols exert cytotoxic effects on a human oesophageal adenocarcinoma cell line (OE33) by causing G2/M arrest and inducing apoptosis Zhang Qiang, Zhao Xin-Huai, Wang Zhu-Jun. Food and Chemical Toxicology.2008;46(6). CrossRef
- Cytotoxicity of flavones and flavonols to a human esophageal squamous cell carcinoma cell line (KYSE-510) by induction of G2/M arrest and apoptosis Zhang Qiang, Zhao Xin-Huai, Wang Zhu-Jun. Toxicology in Vitro.2009;23(5). CrossRef
- Chrysin overcomes TRAIL resistance of cancer cells through Mcl-1 downregulation by inhibiting STAT3 phosphorylation LIRDPRAPAMONGKOL KRIENGSAK, SAKURAI HIROAKI, ABDELHAMED SHERIF, YOKOYAMA SATORU, ATHIKOMKULCHAI SIRIVAN, VIRIYAROJ AMORNRAT, AWALE SURESH, RUCHIRAWAT SOMSAK, SVASTI JISNUSON, SAIKI IKUO. International Journal of Oncology.2013;43(1). CrossRef
- Combination therapy with TRAIL: Recent developments and potential pitfalls Voelkel-Johnson Christina. Cancer Biology & Therapy.2009;8(1). CrossRef
- Chrysin promotes tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) induced apoptosis in human cancer cell lines Li Xin, Wang Jian-Ning, Huang Jun-Ming, Xiong Xi-Kun, Chen Mei-Fen, Ong Choon-Nam, Shen Han-Ming, Yang Xing-Fen. Toxicology in Vitro.2011;25(3). CrossRef
- Lithium enhances TRAIL-induced apoptosis in human lung carcinoma A549 cells Lan Yan, Liu Xiufeng, Zhang Rong, Wang Kai, Wang Yao, Hua Zi-Chun. BioMetals.2013;26(2). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2019
Author Details