Expression of Villin associated with Epithelial to Mesenchymal Transition in patients with gastric cancer relating to their clinical and morphological specifications
Download
Abstract
Aim and Background: Gastric cancer is the fourth most common cancer in the world and the second leading cause of cancer-related deaths. The metastatic invasive cells of tumor tissue are the main cause of mortality. Numerous biological phenomena are involved in organizing the metastatic process. The Epithelial to Mesenchymal Transition is one of the major mechanisms modulating malignant phenotypes by gastric epithelial cells. Specific cell signals are responsible for epithelial or mesenchymal maintenance of the cells in the tissue. These signals are evaluated by measuring the expression of epithelial and mesenchymal biomarkers in that tissue. Villin is an actin-binding protein mainly expressed in the brush border of epithelium which preserves the shape of the cell and its adhesion to the tissue. The aim of the present research is to study the expression of Villin in the cells as a feasible epithelial biomarker in order to evaluate the cross-sectional situation of the cells.
Materials and Methods: 38 patients with gastric cancer that were admitted to the Cancer Institute of Imam Khomeini in a period of 6 months were chosen randomly. two samples were collected from each individual; one from the tumoral tissue and one from normal margin of the tumorous tissue. These samples were evaluated after obtaining informed consent from the patients. RNA was extracted from the samples and used as template for cDNA synthesis. The Villin expression was then measured through Real-Time PCR and statistical data according to tissue type and different grades were collected.
Results: The expression of Villin in tumor tissue of the patients with gastric cancer was significantly lower than the normal tissue.
Conclusion: As it appears decreased expression of Villin can act as an effective factor toward loss of epithelial nature of the cell and occurring Epithelial to Mesenchymal Transition followed by metastasis.
Introduction
Cancer is the second leading cause of death globally [1]. Gastric cancer is developed in the lining of the stomach due to uncontrolled division of mucosa cells [2]. Infection with Helicobacter Pylori is the major cause responsible for more than 60% of developed gastric cancers [3-4]. In spite of decreasing incidence and mortality over last decades, gastric cancer is still the fourth most common cancer and the second related to cancer deaths worldwide.
Gastric cancer occurs two times as often in males as in females [5]. Older people are mostly affected by gastric cancer. Average age of diagnosis is 69. The probability of developing stomach cancer in lifetime is about 1 in 111 which changes by risk factors including sexuality, common bacterial infections, smoking and obesity [6].
The progression of cancer is commonly evaluated by two parameters called Grade and Stage [7]. In order to grade gastric cancer, doctors attribute numbers 1 to 4 to the tumor tissue in which lesser numbers mean more differentiation and less desire of spreading through the neighboring tissues [8]. Whereas, staging a cancer means the spreading distance of tumor which is attributed to numbers from 0 to 4. Lesser numbers mean that the tumor has not spread distant to the neighboring tissues yet [9].
Gastric cancer is often diagnosed when it has metastasized and this is the reason of its poor prognosis [10]. During metastasis, some tumor cells are detached from their primary site and achieve the ability to penetrate to vessels and migrate through the bloodstream in a process called lymphatic or hematogenous spread. Then these abnormal cells could exit from the vessels and re-penetrate to another tissue and begin proliferating there forming a new tumor. This mechanism is thought to be the major reason of cancer-related deaths [11].
Numeral biological phenomena are involved in organizing metastasis. One of the major mechanisms is Epithelial to Mesenchymal Transition (EMT). EMT is an evolutionarily conserved process which occurs during organogenesis and morphogenesis for shaping embryos [12-13]. In addition, this mechanism occurs during wound healing process where cells gain the ability to migrate across the wound bed to restore the epidermal barrier [14-15]. EMT could be organized in abnormal cancerous epithelial cells and lead to malignant phenotypes [16].
Cytoskeleton is an intracellular network consisting of actin, microtubules and inter-mediate filaments which is present in cytoplasm of eukaryotes [17-18]. The cytoskeleton is a contractive network and involves in many different cell signaling pathways such as cell division and migration by reforming the cell [19]. Villin is an actin-binding protein which associates with actin in order to form brush border of epithelium. Villin counts as an epithelial marker and its downregulation would cause the cell to undergo epithelial to mesenchymal transition followed by metastasis [20].
With duo attention to wide incidence of gastric cancer and also involvement of Villin which is being affected in EMT phenomenon leading to metastasis, the present research is conducted to measure alterations of Villin expression as an epithelial marker.
Materials and Methods
In a cross-sectional study, 76 samples belonging to 38 patients with gastric cancer who were admitted to Cancer Institute of Imam Khomeini Hospital in a period of 6 months beginning from September 2016 were randomly chosen. Samples were saved in Iran National Tumor Bank (INTB). All of these samples were confirmed by a pathologist in order to evaluate tumor grade. Samples were entered the study after obtaining informed consent from the patients. Two samples were collected from each individual: one from the tumoral tissue and one from normal margin of the tumorous tissue. Clinical characteristics of patients are shown in Table 1.
| No. | Sex | Tumor Size (cm) | Grade |
| 1 | Female | 10 | I |
| 2 | Male | 4 | I |
| 3 | Male | 13 | III |
| 4 | Male | 9 | III |
| 5 | Male | 7 | II |
| 6 | Male | 5 | II |
| 7 | Male | 3 | III |
| 8 | Male | 3 | I |
| 9 | Male | 4 | I |
| 10 | Male | 5.5 | III |
| 11 | Male | 3 | II |
| 12 | Male | 2.1 | II |
| 13 | Male | 3 | II |
| 14 | Female | 3.5 | III |
| 15 | Male | 14 | III |
| 16 | Male | 6 | III |
| 17 | Male | 5 | II |
| 18 | Male | 4.5 | II |
| 19 | Female | 8 | II |
| 20 | Male | 10 | III |
| 21 | Female | 6 | I |
| 22 | Female | 2 | II |
| 23 | Male | 2 | II |
| 24 | Male | 1.5 | IV |
| 25 | Male | 3 | X |
| 26 | Male | 3.5 | III |
| 27 | Male | 7.5 | III |
| 28 | Male | 11 | III |
| 29 | Male | 10 | III |
| 30 | Male | 2.5 | III |
| 31 | Male | 5 | II |
| 32 | Male | 16 | X |
| 33 | Male | 8 | II |
| 34 | Male | 4 | III |
| 35 | Female | 5.5 | X |
| 36 | Male | 9 | II |
| 37 | Male | N/A | X |
| 38 | Male | N/A | X |
First total RNA was extracted from tumorous and normal tissues using TriPure (Roche – Germany) and the purity of extracted RNA was quantified with spectrophotometer (NanoDrop 1000 Thermo Fisher Scientific – USA). Extracted RNA was used as sample for cDNA synthesis using PrimeScript (Takara Bio – USA). The integrity of synthesized cDNA was confirmed by PCR with GAPDH primer and electrophoresis. Villin reference sequence was obtained from GenBank database (NCBI – USA) and was used as template in order to design forward and reverse Villin primers by Primer 3 software. designed primers had appropriate Tm and GC ratio, avoided secondary structures, cross homology. The same protocol was done for GAPDH as the housekeeping gene as well (Table 2). Primers were synthesized by Takapou Zist company (Iran).
| Target Gene | Forward Primer | Reverse Primer |
| Villin | 5'- GGCCAGCCAAGATGAAATTA -3' | 5'- CTCAAAGGCCTTGGTGTTGT -3' |
| GAPDH | 5'- GAAGGTGAAGGTCGGAGTCA -3' | 5'- AATGAAGGGGTCATTGATGG -3' |
Then the synthesized primers were mixed with SYBR Green Quantitative RT-PCR Kit (Merck – Germany) and Real-Time PCR (Rotor-Gene Q, QIAGEN – USA) was run for 40 cycles and the expression of Villin was quantified during replication. Real-Time PCR setup parameters are shown in Table 3.
| Step | T (˚C) | Duration (min) |
| Primary Preparation | 95 | 10 |
| Step 1: Denaturation | 95 | 0.25 |
| Step 2: Annealing | 55 | 0.5 |
| Step 3: Extension | 72 | 0.5 |
REST software (developed by QIAGEN – USA) was used to process yielded data from Real-Time PCR and then Cycle Thresholds (Ct), ΔCt, ΔΔCt and Fold Change (2-ΔΔCt ) were calculated. The data were processed in SPSS software using 2-tailed Independent T-test (P≤0.05) and significance of correlation between tumorous and normal tissues and different tumoral grades was evaluated.
Results
Demographic data of patients including sexuality, tumor size and tumor grade are shown in Table 4. There was no statistically significant correlation between Villin expression changes and sexuality.
| Specifications | Number | Frequency (%) |
| Sex | ||
| Female | 6 | 15.8 |
| Male | 32 | 84.2 |
| Tumor Size | ||
| < 3 cm | 7 | 18.4 |
| ≥ 3 cm | 31 | 81.6 |
| Tumor Grade | ||
| X, I | 10 | 26.3 |
| II | 13 | 34.2 |
| III | 14 | 36.8 |
| IV | 1 | 2.7 |
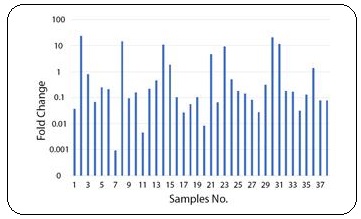
The comparison between samples showed that Villin expression was upregulated in 8 and downregulated in 28 patients. The rest 2 patients didn’t show significant changes. Also, there was statistically significant correlation (P=0.001) between tumoral and normal tissues of the patients (Figure 1).
Figure 1: Villin Expression in Patients.
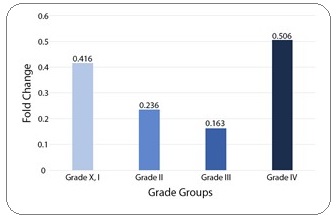
Comparison of Villin expression between tumor grade groups showed no significant correlation (Figure 2).
Figure 2: Mean Comparison of Villin Expression between Tumor Grade Groups.
Discussion
In most cancers, primary tumor is not fetal itself but its metastasis to distant tissues and uncontrolled proliferation. Metastasis and cell invasion are regulated by numeral phenomena. Actin filaments rearrangement followed by Epithelial to Mesenchymal Transition is a major biological mechanism of metastasis. Villin is an actin binding protein which associates with numeral cell mechanisms and facilitates growth, differentiation, division and motility of the cell [21]. In fact, Villin molecules play an important role in adhesion of the cell to each other and the basal layer and avoid cell migration [22-23]. The present research is a relative quantification study and the results may differ than other studies.
According to similar studies, Villin protein is expressed in all intestinal cells and the expression level increases parallel to differentiation [24-25]. In addition, in another research studying on a cancerous intestinal cell line with unstable microsatellites, it showed poorly differentiated cells followed by significantly decreased Villin expression [26]. Generally, it appears that more differentiation results in significantly higher survival rate [27]. Overall, Epithelial to Mesenchymal Transition is one of crucial mechanisms during morphogenesis and transformation of primary tumors into invasive malignancies [28] and evaluation of Villin expression changes can act as a beneficial clinical biomarker in diagnosis, prognosis and treatment [29].
In conclusion, in the present study, we found a statistically significant decreased amount of Villin protein in cancerous tissue versus normal tissue which suggests that Villin expression changes can be a good biomarker to evaluate cross-sectional situation of the tissue and a predicting factor in Epithelial to Mesenchymal Transition followed by metastasis.
References
- Molecular Markers for Breast Cancer: Prediction on Tumor Behavior Banin Hirata Bruna Karina, Oda Julie Massayo Maeda, Losi Guembarovski Roberta, Ariza Carolina Batista, Oliveira Carlos Eduardo Coral de, Watanabe Maria Angelica Ehara. Disease Markers.2014;2014. CrossRef
- Stomach (Gastric) Cancer. NCI. Archived from the original on 4 July 2014. Retrieved 1 July 2014. Available at: https:// www.cancer.gov/types/stomach .
- Sim, F. McKee, M., (2011). Issues in public health (2nd ed.). Maidenhead: Open University Press. p. 74. ISBN 978-0-335-24422-5 .
- Stewart B.W, (2014).World Cancer Report. World Health Organization. 2014. pp. Chapter 5.4. ISBN 9283204298 .
- Epidemiology of Stomach Cancer Brenner H, Rothenbacher D, Arndt V. Methods in Molecular Biology.2009;472:467-477. CrossRef
- The American Cancer Society medical and editorial content team: Stomach Cancer, Last Medical Review: May 20, 2014 Last Revised: January 6, 2017. Available at: https://www. cancer.org/content/dam/CRC/PDF/Public/8838.00.pdf .
- Histopathologic indicators of breast cancer biology: insights from population mammographic screening Webster L R, Bilous A M, Willis L, Byth K, Burgemeister F C, Salisbury E L C, Clarke C L, Balleine R L. British Journal of Cancer.2005;92(8). CrossRef
- (1979) Typing, Grading, and Staging of Gastric Carcinoma. In: Herfarth C.H., Schlag P.M. (eds) Gastric Cancer. Springer, Berlin, Heidelberg Hermanek P. .
- American Joint Committee on Cancer. Stomach Cancer. In: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2017: 117–121. ISBN 978-3-319-40617-6 Amin M.B. .
- “Stomach Cancer: Causes, Symptoms, and Treatments.” Medical News Today Nordqvist C. MediLexicon, Intl., 15 Sep. 2016. Web..
- A Core Invasiveness Gene Signature Reflects Epithelial-to-Mesenchymal Transition but Not Metastatic Potential in Breast Cancer Cell Lines and Tissue Samples Marsan Melike, Van den Eynden Gert, Limame Ridha, Neven Patrick, Hauspy Jan, Van Dam Peter A., Vergote Ignace, Dirix Luc Y., Vermeulen Peter B., Van Laere Steven J.. PLoS ONE.2014;9(2). CrossRef
- The basics of epithelial-mesenchymal transition Kalluri Raghu, Weinberg Robert A.. Journal of Clinical Investigation.2009;119(6). CrossRef
- Epithelial-mesenchymal transitions: insights from development Lim J., Thiery J. P.. Development.2012;139(19). CrossRef
- Interactions of the Extracellular Matrix and Progenitor Cells in Cutaneous Wound Healing Volk Susan W., Iqbal Syed Amir, Bayat Ardeshir. Advances in Wound Care.2013;2(6). CrossRef
- Epithelial-mesenchymal transition in tissue repair and fibrosis Stone Rivka C., Pastar Irena, Ojeh Nkemcho, Chen Vivien, Liu Sophia, Garzon Karen I., Tomic-Canic Marjana. Cell and Tissue Research.2016;365(3). CrossRef
- The Epithelial-to-Mesenchymal Transition in Cancer Roche Joëlle. Cancers.2018;10(2). CrossRef
- (2015). Becker’s World of the Cell (8th ed.). New York: Pearson. pp. 422–446. ISBN 978013399939-6 Hardin J, Bertoni G, Kleinsmith LJ . .
- (2015). Human Anatomy (4th ed.). New York: McGraw Hill Education. p. 29. ISBN 978-0-07-352573-0 McKinley M, Dean O’Loughlin V, Pennefather O’Brien E, Harris R . .
- Cell mechanics and the cytoskeleton Fletcher Daniel A., Mullins R. Dyche. Nature.2010;463(7280). CrossRef
- Villin Function in the Organization of the Actin Cytoskeleton Friederich Evelyne, Vancompernolle Katia, Louvard Daniel, Vandekerckhove Joël. Journal of Biological Chemistry.1999;274(38). CrossRef
- Focus on ADF/Cofilin: Beyond Actin Cytoskeletal Regulation Tsai Cheng-Han, Lee Yi-Jang. ISRN Cell Biology.2012;2012. CrossRef
- Breast cancer growth and metastasis: interplay between cancer stem cells, embryonic signaling pathways and epithelial-to-mesenchymal transition Takebe Naoko, Warren Ronald Q, Ivy S Percy. Breast Cancer Research.2011;13(3). CrossRef
- Interplay of distinct growth factors during epithelial–mesenchymal transition of cancer progenitor cells and molecular targeting as novel cancer therapies Mimeault M., Batra S.K.. Annals of Oncology.2007;18(10). CrossRef
- Changes in villin synthesis and subcellular distribution during intestinal differentiation of HT29-18 clones Dudouet B, Robine S, Huet C, Sahuquillo-Merino C, Blair L, Coudrier E, Louvard D. The Journal of Cell Biology.1987;105(1). CrossRef
- Cdx1 promotes differentiation in a rat intestinal epithelial cell line Soubeyran Philippe, André Frédéric, Lissitzky Jean-Claude, Mallo Gustavo Vidal, Moucadel Virginie, Roccabianca Monique, Rechreche Hocine, Marvaldi Jacques, Dikic Ivan, Dagorn Jean-Charles, Iovanna Juan Lucio. Gastroenterology.1999;117(6). CrossRef
- Villin Expression Is Frequently Lost in Poorly Differentiated Colon Cancer Arango Diego, Al-Obaidi Sheren, Williams David S., Dopeso Higinio, Mazzolini Rocco, Corner Georgia, Byun Do-Sun, Carr Azadeh A., Murone Carmel, Tögel Lars, Zeps Nikolajs, Aaltonen Lauri A., Iacopetta Barry, Mariadason John M.. The American Journal of Pathology.2012;180(4). CrossRef
- Loss of Villin Immunoexpression in Colorectal Carcinoma Is Associated with Poor Differentiation and Survival Al-Maghrabi Jaudah, Gomaa Wafaey, Buhmeida Abdelbaset, Al-Qahtani Mohmmad, Al-Ahwal Mahmoud. ISRN Gastroenterology.2013;2013. CrossRef
- Epithelial-Mesenchymal Transitions Kang Yibin, Massagué Joan. Cell.2004;118(3). CrossRef
- Gene Expression Profiling of Gastric Cancer Marimuthu A, K.C. Jacob H, Jakharia A, Subbannayya Y, et al . J Proteomics Bioinform.2011;4(4):74-82.
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2020
Author Details