Pretreatment Absolute Neutrophil-to-Lymphocyte Ratio (NLR) Predict the Risk for Febrile Neutropenia in the First Cycle Adjuvant Chemotherapy for Breast Cancer
Download
Abstract
Background: Chemotherapy-induced febrile neutropenia (FN) is a condition affecting mortality and morbidity. The records show that absolute neutrophil-to-lymphocyte ratio (NLR) is associated with the cancer prognosis and reflects the immune response system on the infection. It can be used as an independent prognostic biomarker and predictive marker in patients with chronic inflammatory diseases, cardiovascular diseases, or malignancies. Therefore, we have been conducted on using absolute NLR to predict FN in a patient with breast cancer who has adjuvant chemotherapy.
Materials and Methods: The authors retrospectively evaluated the pretreatment absolute NLR of patients with early stage breast cancer who had adjuvant chemotherapy. Then, the relationship to FN was analyzed by using multivariate logistic regression analysis.
Results: We conducted a retrospective analysis of 339 patients where 21 patients had developed FN (6.19%). The multivariate logistic regression analysis results indicated that the pretreatment absolute NLR cut-off point equal to or greater than 2.4 was a significant independent predictive biomarker of the chemotherapy-induced FN (odds ratio = 2.810, 95%,; CI 1.061 - 7.442; p = 0.038). The predictive performance of the high level of absolute NLR was an acceptable discrimination [AUC= 0.7626 (95% and CI 0.650 - 0.875)]. Furthermore, a calibration curve and the Hosmer-Lemeshow test to assess the accuracy of the predictive model showed a goodness of fit for a logistic predictive model (Hosmer-Lemeshow chi2 = 2.50; p = 0.645).
Conclusions: Pretreatment absolute NLR would be a useful predictive biomarker for febrile neutropenia after the first cycle of adjuvant chemotherapy for breast cancer that would be simple and easy to integrate in daily practice without extra costs.
Introduction
Breast cancer is the most commonly found disease in Thai females. According to the National Cancer Institute of Thailand, there were 40.8% new patients in 2018 [1]. Breast cancer treatment with adjuvant chemotherapy plays an important role and its precautionary side effects is febrile neutropenia (FN) although the treatment is the standard chemotherapy regimen, which is not dose-dense chemotherapy [2-3]. FN is mostly found on the first cycle of chemotherapy and is a significant condition, as it increases mortality [4-7]. Recent information illustrated that FN caused 5-20% of mortality [8-9].
Theis et al. [10] found that there were various patient factors affecting FN after the adjuvant chemotherapy.
These factors included being female, aged over 65 years, cancer type, disease stage, low albumin, elevated bilirubin, low creatinine clearance, infection before chemotherapy, and number and type of chemotherapy drugs. However, such factors did not directly reflect the granulocyte reservoir or stem cell pool of the bone marrow, which the pretreatment hematological parameters were the white blood cell count [11], platelet count [12], absolute neutrophil count (ANC) [13-14], absolute lymphocyte count (ALC) [15-16], and absolute monocyte count (AMC) [17-19] that were hypothesized to reflect the patients’ predisposition to FN.
Some studies applied the clinical predictive model by using the pretreatment hematological parameters to predict the FN [13-14-19]. It was found that there were some data that could be used to predict the FN of the patient with cancer in some chemotherapy regimens [13-14]. However, it could not be practical after the validation [19].
Furthermore, a number of studies utilized the absolute neutrophil-to-lymphocyte ratio (NLR) for the prognosis; such as, chronic inflammatory disease, cardiovascular disease and cancer [20-26]. It was discovered that the high cut-off NLR was related to the poor prognosis since the NLR indicated the balance of the inflammatory pathway and anti-immune function and the cut-off of the NLR was unclear [27]. Azab et al. [28] applied NLR > 3.3 as the independent significant predictor to the mortality in patients with chemotherapy. Moreover, Dirican et al. [29] used NLR where four was the independent prognostic factor to the disease free survival (DFS) and overall survival (OS) whereas Krenn-Piko et al. [30] used NLR >3 as the independent risk factor related to the poor DFS. However, it was unable to predict the OS.
In addition, Howard et al. [31] examined the NLR in patients with cancer and discovered that baseline NLR varied with age, gender, race, disease stage, and type of cancer. Thus, in order to apply the NLR, the type of cancer of the population should be studied.
For this reason, this research studied the pretreatment NLR to predict the FN in patients with breast cancer who had adjuvant chemotherapy.
Materials and Methods
The information of the patients with early stage breast cancer during 2016-2019 was collected from the database of the Division of Medical Oncology, Buddhasothorn Hospital, Chachoengsao, Thailand. Exclusion criteria included 1) stage IV breast cancer, 2) a history of other cancers, 3) unavailable essential data, 4) a history of anemia or other hematological disorders, 5) renal and hepatic impairment, 6) the first chemotherapy cycle was not administered at this hospital, and 7)a prophylactic use of granulocyte-colony stimulating factor (G-CSF). The sample size was calculated from the baseline incidence and population variance at a probability of a type-I error of 5% and probability of a type-II error of 20%. Consequently, a size of 238 samples was acquired.
FN was defined as a temperature higher than 38.5°C and an ANC higher than 0.5×109/L, or higher than 1.0×109/L and expected to fall below 0.5×109/L.
Pretreatment NLR and FN after the first cycle of chemotherapy was examined using an explanatory model multivariate (adjusted) and the effects logistic regression analysis. Then, the area under the ROC curve of the NLR used to predict FN and the appropriate cut-off of the pretreatment absolute NLR was investigated.
This study was approved by the Institutional Review Broad of Buddhasothorn Hospital.
Results
From the information of the 339 patients, the average age was 49.74 years. There were four regimens of adjuvant chemotherapy, which were the cyclophosphamide, methotrexate, fluorouracil (CMF) regimen, fluorouracil, adriamycin, cyclophosphamide (FAC) regimen, adriamycin, cyclophosphamide (AC) regimen, and paclitaxel, cyclophosphamide (TC) regimen. The FN at the first cycle of chemotherapy of each regimen is shown in Table 1.
| Regimen | No febrile Neutropenia | Febrile Neutropenia |
| Number (%) | Number (%) | |
| CMF | 40 (100) | 0 (0) |
| FAC | 163 (97.60) | 4 (2.40) |
| AC | 113 (87.60) | 16 (12.40) |
| TC | 2 (66.67) | 1 (33.33) |
It was discovered that there were 21 patients with FN. From the basic factors of both the patients with and without FN which included age, body surface area (BSA), pretreatment ANC, pretreatment ALC, post-treatment ANC, and pretreatment NLR, the post-treatment ANC was the only one different factor with statistical significance, p=0.002 (Table 2).
| Factors | No febrile Neutropenia N=318 | Febrile Neutropenia N=21 | |||
| mean | ±SD | mean | ±SD | p-value | |
| Age | 49.739 | 10.749 | 49.81 | 10.75 | 0.977 |
| BSA | 1.5885 | 0.152 | 1.572 | 0.118 | 0.623 |
| Pre ANC | 4501.459 | 1053.2 | 4733.333 | 1196.383 | 0.333 |
| Pre ALC | 2185.327 | 383.905 | 2061.905 | 414.097 | 0.156 |
| Post ANC | 1399.047 | 1763.598 | 215.143 | 147.589 | 0.002 |
| Pre NLR | 2.121 | 0.624 | 2.352 | 0.616 | 0.101 |
When analyzing the pretreatment absolute NLR, which was related to FN, there was the risk of FN at 1.693 times (cOR = 1.693; 95% CI 0.898- 3.190; p = 0.103) (Table 3).
| Risk Factors | Crude Odds Ratio (cOR) | 95% Confidence Interval | p-value |
| pre NLR | 1.693 | 0.898- 3.190 | 0.103 |
However, the confounding effects which were those patients aged over 60 years old (elderly), low BSA (< 1.4 m2) and chemotherapy regimens had not yet been adjusted. Such factors affected the FN in patients who had chemotherapy. Moreover, the pretreatment absolute NLR to be applied to the clinical practice should have the appropriate cut-off point in order to predict the FN.
Then, the cut-off point of the pretreatment absolute NLR to predict the FN was considered (Figure 1).
Figure 1: The Sensitivity and Specificity of Each Cut-off Point Value of Pretreatment Absolute NLR.
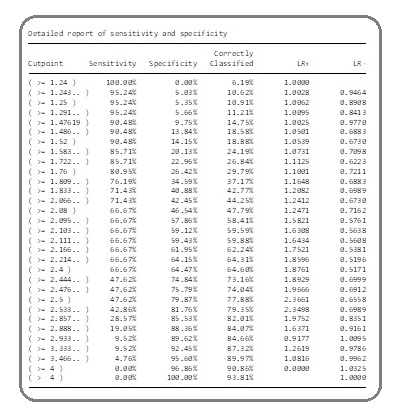
This showed that the cut-off point > 2.4 contained 66.67% of sensitivity (95%CI 43.0% - 85.4%) and 64.47% of specificity (95% CI 58.9% - 69.7%), which was the optimal point because of the highest value of sensitivity and specificity. In addition, at the cut-off point >2.4, the positive predictive value (PPV) was 11.0% (95% CI 6.2% - 17.8%), negative predictive value (NPV) was 96.7% (95% CI 93.3% - 98.7%),positive likelihood ratio (LR+) was 1.88 (95% CI 1.34 - 2.63), and the negative likelihood ratio (LR-) was 0.52 (95% CI 0.28 - 0.95) (Table 4).
| 95% Confidence Interval | ||
| Sensitivity | 66.70% | 43.0% - 85.4% |
| Specificity | 64.50% | 58.9% - 69.7% |
| Positive predictive value | 11.00% | 6.2% - 17.8% |
| Negative predictive value | 96.70% | 93.3% - 98.7% |
| Likelihood ratio (+) | 1.88 | 1.34 - 2.63 |
| Likelihood ratio (-) | 0.52 | 0.28 - 0.95 |
However, the obtained predictability had not adjusted the confounding effects.
Therefore, when analyzing the pretreatment absolute NLR at the cut-off point > 2.4 and the relationship to the FN by adjusting the confounding effects with the multivariate logistic regression analysis, it was found that the pretreatment absolute NLR > 2.4 had the risk of FN at 2.810 times with statistical significance (aOR = 2.810; 95% CI 1.061 - 7.442; p = 0.038) (Table 5).
| Risk Factors | Adjusted Odds Ratio (aOR) | 95% Confidence Interval | p-value |
| pre NLR cut-off point > 2.4 | 2.81 | 1.061 - 7.442 | 0.038 |
| elderly | 0.338 | 0.043 - 2.686 | 0.305 |
| lowBSA | 0.552 | 0.068 - 4.505 | 0.579 |
| CMF | 1 | (Reference category) | |
| FAC | 0.018 | 0.001 - 0.493 | 0.017 |
| AC | 0.089 | 0.004 - 2.195 | 0.139 |
| TC | 1 | (Reference category) |
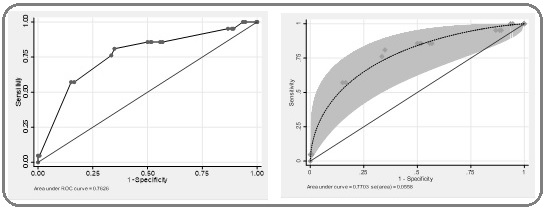
Additionally, for the overall test accuracy of predicting the FN when using the > 2.4 and adjusting the confounding effects, the area under the receiver operating characteristic curve (ROC curve) was 0.7626 (95% CI 0.650 - 0.875) (Figure 2).
Figure 2: (A; Left, ROC curve) Displaying the Area under the Receiver Operating Characteristic Curve (ROC curve) of the Pretreatment Absolute NLR when using the Cut-off Point > 2.4 to Predict the FN after Adjusting the Effects of the Confounders, and AUC, 0.7626 (95% CI 0.650 - 0.875). (B; right, the fitted ROC curve and simultaneous confidence bands).
A logistic regression model is a way to predict the probability of FN based on the values of the pretreatment absolute NLR. Therefore, it is important to be able to assess the accuracy of a predictive model. Thus, the calibration plot was created to qualitatively compare the model’s predicted probability of an event to the empirical probability (Figure 3).
Figure 3: The Calibration Plot Showing the Expected Probabilities (x) Against the Observed Probabilities (y) of the Use of the Pretreatment Absolute NLR >2.4 to Predict the FN. The Hosmer-Lemeshow was chi2 = 2.50, p= 0.645.
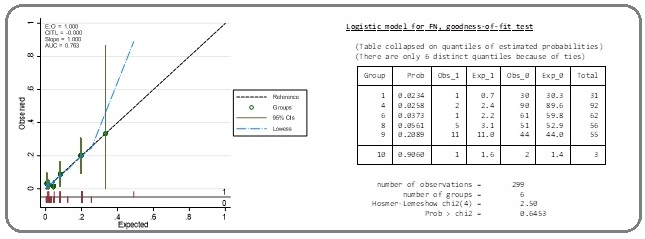
This illustrated that the obtained calibration curve from the expected probabilities (spike plot) and observed probabilities (Lowess smoother) was close to the diagonal reference line. When testing the model performances with the Hosmer–Lemeshow test, the Hosmer-Lemeshow was chi2 = 2.50 and p = 0.645.
Discussion
After adjusting the confounding effects, the pretreatment absolute NLR at the cut-off point > 2.4 was significantly correlated with the development of FN in the first cycle of the adjuvant chemotherapy (odds ratio = 2.810; 95% CI 1.061 - 7.442; p = 0.038).
When applying the ROC curve to examine the overall test accuracy of the FN prediction, AUC = 0.7626 (95% CI 0.650 - 0.875), which was the acceptable discrimination. Moreover, the results of using a calibration curve along with the Hosmer–Lemeshow test to assess the predictive model performances indicated that there was a goodness of fit for a logistic predictive model (Hosmer-Lemeshow chi2 = 2.50, p = 0.645).
Recently, there were research studies [13-14] that applied pretreatment hematological parameters to predict FN in the first cycle of chemotherapy for breast cancer; this was the Jenkins’ model, which combined ANC to ALC where the patients were classified into a low-risk and high-risk group to predict the FN in the patients with breast cancer who had the FEC regimen or TAC regimen. In addition, the study of Chen et al. [19] that validated Jenkins’ model indicated that it could not be applied to his population. As a result, he developed the predictive mode that helped to classify the patients in order to predict the FN from the chemotherapy treatment by using ANC, ALC and AMC (Table 6).
| Jenkins’s Model (FEC regimen) | Jenkins’s Model (TAC Regimen Plus G-CSF prophylaxis) | Chen’s Model | This Study (pretreatment absolute NLR cut-off point > 2.4) | |
| Number | 741 | 263 | 428 | 339 |
| FN rate | 7.15% | 11.79% | 12.80% | 6.19% |
| FN in cycle 1 high risk group | 21% | 23.80% | 23.10% | 11.02% |
| FN in cycle 1 low risk group | 6.03% | 4.55% | 10.10% | 3.30% |
| P value | 0.002 | < 0.001 | < 0.01 | 0.038 |
| sensitivity | 13.21% | 31% | 38.20% | 66.70% |
| specificity | 95.25% | 94% | 81.20% | 64.50% |
| PPV | 21.21% | 24% | 23.10% | 11.00% |
| NPV | 91.89% | 95% | 89.10% | 96.70% |
| AUC | NA | NA | 0.58-0.6 | 0.7626 |
*FN, febrile neutropenia; FEC, fluorouracil/epirubicin/cyclophosphamide; TAC, docetaxel/adriamycin/cyclophosphamide; PPV, positive predictive value; NPV, negative predictive value; G-CSF, granulocyte-colony stimulating factor; AUC, area under curve, NA, not available data.
Currently, the information about the genetic risk factors affecting the FN from early stage breast cancer presented by Pfeil et al. [32] showed that apart from the clinical risk factors, genetic factors had the impact on the prediction of FN, which involved homozygous carriers of the rs4148350 variant T-allele in MRP1 (odds ratio = 6.7; 95% CI 1.04-43.17), the higher alanine aminotransferase (odds ratio = 1.02; 95% CI 1.01-1.03]), the carriers of the rs246221 variant C-allele in MRP1 (odds ratio = 2.0; 95% CI 1.03-3.86), and the rs351855 variant C-allele in FGFR4 (odds ratio = 2.48; 95% CI 1.13-5.44).
Consequently, the use of pretreatment hematological parameters solely to predict the FN might have less accuracy. Nevertheless, examination of genetic risk factors in the clinical practice was not widely proceeded and the cost-effectiveness was questionable. Thus, the clinical risk factors and pretreatment hematological parameters to predict the FN was vital.
From the predictive model, ANC, ALC or AMC was utilized to classify the patients into the high risk and low risk group of FN; however, there was no use of NLR to predict the FN; the patients with neutropenia from having chemotherapy might not have FN. Chemotherapy induced FN might be related to infection during neutropenia. Recent studies[4-13-14-19-33-37] found that the low pretreatment ANC, ALC, AMC affected the neutropenia and FN positively. This was because neutropenia increased the risk of infection, which might result in FN. On the other hand, NLR reflected the balance between the inflammation pathway activity and anti-immune function. The previous research discovered that the higher NLR was concerned with the poor cancer prognosis and inflammation [20-31-38-39].
The study of Kaushik et al. [40] also reported that the elevated levels of NLR could diagnose and predict the early sepsis and late sepsis by using the cut-off point NLR > 3.3 with AUC= 0.911 at the early sepsis phase, and > 8.3 with AUC = 0.732 at the late sepsis phase. This concurred with the research of Jager et al. [41], which illustrated that NLR was the predictive marker of bacteremia and was more efficient than the conventional marker in the emergency unit at the cut-off point NLR > 10 with AUC = 0.73.
Therefore, the condition of FN, which would be related to the infection in neutropenia that compromised the immune systems using the high absolute NLR obtained from the high levels of neutrophil count associated with the severe inflammation or infection along with the lymphocytopenia indicated that the compromising reflect immune response system was likely one of the predictive markers of chemotherapy induced FN.
The research illustrated that pretreatment absolute NLR could be a useful predictive biomarker for FN after the first cycle of adjuvant chemotherapy for breast cancer, which was simple and easy to integrate in daily practice and without extra costs so to prevent FN in patients with a high risk and minimize the mortality and morbidity.
References
- National cancer institute of Thailand, Hospital-based cancer registry 2018 2019;(34):19-20.
- Delivering adjuvant chemotherapy to women with early-stage breast carcinoma Link Brian K., Budd G. Thomas, Scott Shane, Dickman Elliot, Paul David, Lawless Grant, Lee Martin W., Fridman Moshe, Ford Jon, Carter William B.. Cancer.2001;92(6). CrossRef
- Baseline and early lymphopenia predict for the risk of febrile neutropenia after chemotherapy Ray-Coquard I, Borg C, Bachelot Th, Sebban C, Philip I, Clapisson G, Le Cesne A, Biron P, Chauvin F, Blay J Y. British Journal of Cancer.2003;88(2). CrossRef
- First cycle risk of severe and febrile neutropenia in cancer patients receiving systemic chemotherapy: results from a prospective nationwide study Crawford J, Wolff DA, Culakova E, Poniewierski MS, Selby C, Dale DC, Lyman GH. Blood.2004;104:607-608.
- Patterns of chemotherapy-associated toxicity and supportive care in US oncology practice: a nationwide prospective cohort study Culakova Eva, Thota Ramya, Poniewierski Marek S., Kuderer Nicole M., Wogu Adane F., Dale David C., Crawford Jeffrey, Lyman Gary H.. Cancer Medicine.2014;3(2). CrossRef
- Management of Breast Cancer Patients with Chemotherapy-Induced Neutropenia or Febrile Neutropenia Fontanella Caterina, Bolzonello Silvia, Lederer Bianca, Aprile Giuseppe. Breast Care.2014;9(4). CrossRef
- Incidence of febrile neutropenia during adjuvant chemotherapy for breast cancer: a prospective study Rayson D., Lutes S., Sellon M., Colwell B., Dorreen M., Drucker A., Jeyakumar A., Snow S., Younis T.. Current Oncology.2012;19(3). CrossRef
- Empirical antibiotic monotherapy for febrile neutropenia: systematic review and meta-analysis of randomized controlled trials Paul Mical, Yahav Dafna, Fraser Abigail, Leibovici Leonard. Journal of Antimicrobial Chemotherapy.2005;57(2). CrossRef
- Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients Kuderer Nicole M., Dale David C., Crawford Jeffrey, Cosler Leon E., Lyman Gary H.. Cancer.2006;106(10). CrossRef
- Development and Validation of a Risk Score for Febrile Neutropenia After Chemotherapy in Patients With Cancer: The FENCE Score Aagaard Theis, Roen Ashley, Reekie Joanne, Daugaard Gedske, Brown Peter de Nully, Specht Lena, Sengeløv Henrik, Mocroft Amanda, Lundgren Jens, Helleberg Marie. JNCI Cancer Spectrum.2018;2(4). CrossRef
- Predicting individual risk of neutropenic complications in patients receiving cancer chemotherapy Lyman Gary H., Kuderer Nicole M., Crawford Jeffrey, Wolff Debra A., Culakova Eva, Poniewierski Marek S., Dale David C.. Cancer.2010;117(9). CrossRef
- A general chemotherapy myelotoxicity score to predict febrile neutropenia in hematological malignancies Moreau M., Klastersky J., Schwarzbold A., Muanza F., Georgala A., Aoun M., Loizidou A., Barette M., Costantini S., Delmelle M., Dubreucq L., Vekemans M., Ferrant A., Bron D., Paesmans M.. Annals of Oncology.2009;20(3). CrossRef
- Pretreatment haematological laboratory values predict for excessive myelosuppression in patients receiving adjuvant FEC chemotherapy for breast cancer Jenkins P., Freeman S.. Annals of Oncology.2009;20(1). CrossRef
- Validation of a predictive model that identifies patients at high risk of developing febrile neutropaenia following chemotherapy for breast cancer Jenkins P., Scaife J., Freeman S.. Annals of Oncology.2012;23(7). CrossRef
- Early lymphopenia after cytotoxic chemotherapy as a risk factor for febrile neutropenia. Blay J Y, Chauvin F, Le Cesne A, Anglaret B, Bouhour D, Lasset C, Freyer G, Philip T, Biron P. Journal of Clinical Oncology.1996;14(2). CrossRef
- Early lymphopenia as a risk factor for chemotherapy-induced febrile neutropenia Choi Chul Won, Sung Hwa Jung, Park Kyong Hwa, Yoon So Young, Kim Seok Jin, Oh Sang Cheul, Seo Jae Hong, Kim Byung Soo, Shin Sang Won, Kim Yeul Hong, Kim Jun Suk. American Journal of Hematology.2003;73(4). CrossRef
- Development and validation of a prediction model for the risk of developing febrile neutropenia in the first cycle of chemotherapy among elderly patients with breast, lung, colorectal, and prostate cancer Hosmer Wylie, Malin Jennifer, Wong Mitchell. Supportive Care in Cancer.2010;19(3). CrossRef
- Early Monocytopenia After Chemotherapy as a Risk Factor for Neutropenia Kondo Mutsumi, Oshita Fumihiro, Kato Yuji, Yamada Kouzo, Nomura Ikuo, Noda Kazumasa. American Journal of Clinical Oncology.1999;22(1). CrossRef
- Clinical Predictive Models for Chemotherapy-Induced Febrile Neutropenia in Breast Cancer Patients: A Validation Study Chen Kai, Zhang Xiaolan, Deng Heran, Zhu Liling, Su Fengxi, Jia Weijuan, Deng Xiaogeng. PLoS ONE.2014;9(6). CrossRef
- Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-Analysis Templeton Arnoud J., McNamara Mairéad G., Šeruga Boštjan, Vera-Badillo Francisco E., Aneja Priya, Ocaña Alberto, Leibowitz-Amit Raya, Sonpavde Guru, Knox Jennifer J., Tran Ben, Tannock Ian F., Amir Eitan. JNCI: Journal of the National Cancer Institute.2014;106(6). CrossRef
- Post-treatment neutrophil-to-lymphocyte ratio predicts for overall survival in brain metastases treated with stereotactic radiosurgery Chowdhary Mudit, Switchenko Jeffrey M., Press Robert H., Jhaveri Jaymin, Buchwald Zachary S., Blumenfeld Philip A., Marwaha Gaurav, Diaz Aidnag, Wang Dian, Abrams Ross A., Olson Jeffrey J., Shu Hui-Kuo G., Curran Walter J., Patel Kirtesh R.. Journal of Neuro-Oncology.2018;139(3). CrossRef
- Prognostic Significance of Neutrophil Lymphocyte Ratio in Patients with Gastric Cancer: A Meta-Analysis Zhang Xi, Zhang Wei, Feng Li-jin. PLoS ONE.2014;9(11). CrossRef
- Neutrophil Lymphocyte Ratio and Cardiovascular Disease Risk: A Systematic Review and Meta-Analysis Angkananard Teeranan, Anothaisintawee Thunyarat, McEvoy Mark, Attia John, Thakkinstian Ammarin. BioMed Research International.2018;2018. CrossRef
- Pretreatment levels of peripheral neutrophils and lymphocytes as independent prognostic factors in patients with nasopharyngeal carcinoma He Jian-Rong, Shen Guo-Ping, Ren Ze-Fang, Qin Han, Cui Cui, Zhang Ying, Zeng Yi-Xin, Jia Wei-Hua. Head & Neck.2012;34(12). CrossRef
- The systemic inflammation-based neutrophil–lymphocyte ratio: Experience in patients with cancer Guthrie Graeme J.K., Charles Kellie A., Roxburgh Campbell S.D., Horgan Paul G., McMillan Donald C., Clarke Stephen J.. Critical Reviews in Oncology/Hematology.2013;88(1). CrossRef
- Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis Ethier Josee-Lyne, Desautels Danielle, Templeton Arnoud, Shah Prakesh S., Amir Eitan. Breast Cancer Research.2017;19(1). CrossRef
- The neutrophil-to-lymphocyte ratio: a narrative review Socorro Faria Sara, Fernandes Jr Paulo César, Barbosa Silva Marcelo José, Lima Vladmir C, Fontes Wagner, Freitas-Junior Ruffo, Eterovic Agda Karina, Forget Patrice. ecancermedicalscience.2016;10. CrossRef
- Usefulness of the Neutrophil-to-Lymphocyte Ratio in Predicting Short- and Long-Term Mortality in Breast Cancer Patients Azab Basem, Bhatt Vijaya R., Phookan Jaya, Murukutla Srujitha, Kohn Nina, Terjanian Terenig, Widmann Warren D.. Annals of Surgical Oncology.2011;19(1). CrossRef
- Do the derived neutrophil to lymphocyte ratio and the neutrophil to lymphocyte ratio predict prognosis in breast cancer? Dirican Ahmet, Kucukzeybek Betul Bolat, Alacacioglu Ahmet, Kucukzeybek Yuksel, Erten Cigdem, Varol Umut, Somali Isil, Demir Lutfiye, Bayoglu Ibrahim Vedat, Yildiz Yasar, Akyol Murat, Koyuncu Betul, Coban Eyup, Ulger Eda, Unay Fulya Cakalagaoglu, Tarhan Mustafa Oktay. International Journal of Clinical Oncology.2014;20(1). CrossRef
- The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients Krenn-Pilko S, Langsenlehner U, Thurner E-M, Stojakovic T, Pichler M, Gerger A, Kapp K S, Langsenlehner T. British Journal of Cancer.2014;110(10). CrossRef
- Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer Howard Rachel, Kanetsky Peter A., Egan Kathleen M.. Scientific Reports.2019;9(1). CrossRef
- Multivariable regression analysis of febrile neutropenia occurrence in early breast cancer patients receiving chemotherapy assessing patient-related, chemotherapy-related and genetic risk factors Pfeil Alena M, Vulsteke Christof, Paridaens Robert, Dieudonné Anne-Sophie, Pettengell Ruth, Hatse Sigrid, Neven Patrick, Lambrechts Diether, Szucs Thomas D, Schwenkglenks Matthias, Wildiers Hans. BMC Cancer.2014;14(1). CrossRef
- Pretreatment monocyte counts and neutrophil counts predict the risk for febrile neutropenia in patients undergoing TPF chemotherapy for head and neck squamous cell carcinoma Shimanuki Marie, Imanishi Yorihisa, Sato Yoichiro, Nakahara Nana, Totsuka Daisuke, Sato Emiri, Iguchi Sena, Sato Yasuo, Soma Keiko, Araki Yasutomo, Shigetomi Seiji, Yoshida Satoko, Uno Kosuke, Ogawa Yusuke, Tominaga Takehiro, Ikari Yuichi, Nagayama Junko, Endo Ayako, Miura Koshiro, Tomioka Takuya, Ozawa Hiroyuki, Ogawa Kaoru. Oncotarget.2018;9(27). CrossRef
- Impact of Pretreatment Neutrophil Count on Chemotherapy Administration and Toxicity in Dogs with Lymphoma Treated with CHOP Chemotherapy Fournier Q., Serra J.-C., Handel I., Lawrence J.. Journal of Veterinary Internal Medicine.2017;32(1). CrossRef
- Risk Models for Predicting Chemotherapy‐Induced Neutropenia Lyman Gary H., Lyman Christopher H., Agboola Olayemi. The Oncologist.2005;10(6). CrossRef
- Incidence and risk factors of febrile neutropenia in patients with non-Hodgkin B-cell lymphoma receiving R-CHOP in a single center in Japan Yokoyama Masahiro, Kusano Yoshiharu, Takahashi Anna, Inoue Norihito, Ueda Kyoko, Nishimura Noriko, Mishima Yuko, Terui Yasuhito, Nukada Tomoyuki, Nomura Takanobu, Hatake Kiyohiko. Supportive Care in Cancer.2017;25(11). CrossRef
- Prediction of adverse events during intensive induction chemotherapy for acute myeloid leukemia or high-grade myelodysplastic syndromes Buckley Sarah A., Othus Megan, Vainstein Vladimir, Abkowitz Janis L., Estey Elihu H., Walter Roland B.. American Journal of Hematology.2014;89(4). CrossRef
- Neutrophil/lymphocyte ratio and its association with survival after complete resection in non–small cell lung cancer Sarraf Khaled M., Belcher Elizabeth, Raevsky Evgeny, Nicholson Andrew G., Goldstraw Peter, Lim Eric. The Journal of Thoracic and Cardiovascular Surgery.2009;137(2). CrossRef
- Early Blood Biomarkers to Improve Sepsis/Bacteremia Diagnostics in Pediatric Emergency Settings Tamelytė , Vaičekauskienė , Dagys , Lapinskas , Jankauskaitė . Medicina.2019;55(4). CrossRef
- Diagnostic and Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Early and Late Phase of Sepsis Gupta Monika, Sharma Madhu, Jain Neetu, Sinha Nitin, Kaushik Rajnish, Jash Debraj, Chaudhry Aditya. Indian Journal of Critical Care Medicine.2018;22(9). CrossRef
- Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit de Jager Cornelis PC, van Wijk Paul TL, Mathoera Rejiv B, de Jongh-Leuvenink Jacqueline, van der Poll Tom, Wever Peter C. Critical Care.2010;14(5). CrossRef
License
Copyright
© ,
Author Details