CD30 Expression and Its Correlation with Clinicopathologic Features in Indonesian Diffuse Large B-Cell Lymphoma
Download
Abstract
Background: Diffuse large B-cell lymphoma (DLBCL) is the most common subtype (68.2%) of B cells Non Hodgkin Lymphoma in Indonesia. This tumor heterogeneity is characterized by a variety of clinical conditions, morphology, genetic profiles, therapeutic response, prognosis and survival. Recent studies have shown that CD30 immunohistochemical staining also plays an important role in determining the therapy and prognosis of DLBCL disease. CD30 can also be expressed in DLBCL in approximately 9.5-40%. However, CD30 expression and clinicopathological characteristics of Indonesian DLBCL remain unknown. This study aimed to determine the prevalence of CD30 expression and its correlation with clinicopathological characteristics of Indonesian DLBCL patients.
Methods: During a study period of four years, a total of 104 FFPE of DLBCL cases were collected from Anatomical Pathology Department, Sardjito Hospital, Special Region of Yogyakarta, Indonesia. CD30 expression was studied using immunohistochemical techniques (Mouse monoclonal antibody MoAb CD30 cell marque Ber-H2). Correlations between positive CD30 immunoreactivity and clinicopathological characteristics in DLBCL patients were statistically analyzed using chi-square tests.
Result: Positivity rate of CD30 expression in 104 DLBCL samples was 13.5% (14/104) using cutoff value of 0% while using a 20% cutoff, it was 1.9% (2/104). Statistical associations of positive CD30 expression and clinicopathological characteristics (age, sex, Ann Arbor stage, extranodal involvement and morphological variants) were not significant (p > 0.05).
Conclusions: The prevalence of positive CD30 expression in Indonesian DLBCL patients is 13.5%. There was no statistically significant associations between positive CD30 expression and clinicopathological characteristics.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of Non-Hodgkin Lymphoma (NHL) comprising about 30% - 40% cases globally with a high percentage in developing countries [1]. DLBCL is the most common histological subtype (68.2%) of B cells NHL in Indonesia [2]. DLBCL is an aggressive tumor and many cases are diagnosed at an advanced stage. In general, patients show very rapid tumor growth either in single or multiple, nodal or extranodal locations. This tumor heterogeneity is characterized by a variety of clinical conditions, morphology, genetic profiles, therapeutic response, prognosis and survival [3]. The International Prognostic Index (IPI) including age, Ann Arbor stage, serum lactate dehydrogenase (LDH) levels, extranodal involvement and the status of the Eastern Cooperative Oncology Group (ECOG) performance are prognostic factors for DLBCL [4]. Histopathological examination showed various morphologies of DLBCL including centroblastic, immunoblastic and anaplastic subtypes in which centroblastic subtypes were the most common subtypes and have a better prognosis and survival than other subtypes [5].
CD30 antigens are members of the transmembrane cytokine receptor superfamily 120-kd tumor necrosis factor (TNF) that are identified as cell surface antigens in Hodgkin Lymphoma (Reed-Sternberg cells) [6]. CD30 can also be expressed in various types of T cells and B cells NHL. CD30 is a marker of activation induced in vitro by mitogenic signals and under normal conditions expressed in several B cell and T cell immunoblasts in parafollicular regions and peripheral germinal centers in healthy individuals, as well as being involved in regulation of cell proliferation, activation, differentiation, including controlling cell survival or death by apoptosis or cytotoxicity [7]. The limited distribution of CD30 expression makes CD30 an ideal target for monoclonal antibody therapy in lymphoma patients with positive CD30.
Brentuximab vedotin or SGN-35, monoclonal anti-CD30 which has been approved by U.S. The Food and Drug Administration (FDA) responds to relapsed Hodgkin lymphoma (HL) and Anaplastic Large Cell Lymphoma (ALCL) [8-9]. SGN-35 as a new therapy target continues to be developed in various types of lymphomas with CD30 positive [6-10-11]. Recent studies have shown that CD30 immunohistochemical (IHC) staining also plays an important role in determining the therapy and prognosis of DLBCL disease.
Dr. Sardjito Hospital, Special Region of Yogyakarta, is a referral hospital for patients all over Indonesia. Studies and data related to CD30 expression and clinicopathological characteristics (age, sex, Ann Arbor stage, extranodal involvement and morphological variants) are considerably limited in Indonesia. Therefore, it is important to conduct a study to investigate the association of CD30 immunoreactivity with clinicopathological characteristics including age, sex, Ann Arbor stage, extranodal involvement and morphological variants in Indonesian DLBCL patients.
Materials and Methods
Study Subjects
This study used a retrospective, non-experimental, observational analytic design with a cross-sectional approach to detect CD30 expression with IHC examination methods and analyzed the correlations between positive CD30 expression with clinicopathological characteristics including age, sex, Ann Arbor stage, extranodal involvement and morphological variants in Indonesian DLBCL patients.
The subjects of this study were patients diagnosed with large cell type NHL at Dr. Sardjito Hospital, Special Region of Yogyakarta, Indonesia and the diagnosis was confirmed by histopathological examination and positive CD20 IHC between January 2015 to December 2018.
All glass slides were retrieved, reviewed and reclassified as DLBCL by 2 independent pathologists, then classified according to their morphology. Eventually, there were 104 samples obtained.
CD30 IHC examination was performed on all samples. The study was conducted in the Anatomical Pathology Laboratory, Sardjito hospital, Special Region of Yogyakarta, Indonesia. Clinical data of the patients were obtained from medical records at the Medical Records Installation, Dr. Sardjito Hospital, Special Region of Yogyakarta, Indonesia.
Immunohistochemistry (IHC)
Detection of CD30 expression in this study was performed using the IHC method. This examination aimed to determine the expression of CD30 antigen/ protein. The formalin-fixed paraffin embedded (FFPE) blocks that met inclusion criteria were then cut into a 4 micron sections. The sections were then incubated, deparaffinized and rehydrated. Antigen retrieval was performed using Starr Track detection kit protocol. Sample sections were stained using monoclonal anti-CD30 antibodies (Mouse monoclonal antibody MoAb CD30 cell marque Ber-H2) to identify CD30 immunoreactivity. Immunoreactive cells were visualized using diaminobenzidine (DAB) chromogen. Lymph node tissue from a confirmed Hodgkin lymphoma case was used as positive control. All glass slides were examined by a pathologist.
CD30 expression assessment was performed using 0% and 20% cutoffs which had been used in several previous studies [12-13]. CD30 positivity was expressed in cytoplasmic membrane and Golgi (paranuclear dot like). CD30 positive percentage was calculated utilizing a total of 200 cells in 5 well-preserved areas (positively stained tumor cells) with various staining intensities calculated in percent units (%) [14].
Statistical Analysis
Correlations between CD30 immunoreactivity and clinicopathological characteristics including age, sex, Ann Arbor stage, extranodal involvement and morphological variants in DLBCL patients were statistically analyzed using Chi-square tests. Statistical associations were considered significant if the p-value < 0.05. Statistical analyses were performed using IBM SPSS version 20 (IBM Corp., Chicago).
Results
Patients’ Characteristics
Based on the inclusion and exclusion criteria, 104 tissue samples of patients with DLBCL were included in this study. Male patients were 63 (60.6%) and female patients were 41 (39.4%). Based on age category, there were 45 (43.3%) patients ≥ 60 years and 59 (56.7%) patients < 60 years. Data concerning Ann Arbor stage showed 83 (79.8%) patients were in the early stage (stage I-II) and 21 (20.2%) patients were in the advanced stage (stage III-IV). Based on extranodal involvement, 88 (84.6%) patients with extranodal involvement < 2 and 16 (15.4%) patients with extranodal involvement ≥ 2 (Table 1).
| Clinical Characteristics | Frequency (%) (n=104) | |
| Sex | ||
| Male | 63 (60.6%) | |
| Female | 41 (39.4%) | |
| Age | ||
| ≥ 60 years old | 45 (43.3%) | |
| < 60 years old | 59 (56.7%) | |
| Ann Arbor stage | ||
| I-II | 83 (79.8%) | |
| III-IV | 21 (20.2%) | |
| Extranodal involvement | ||
| ≥ 2 | 16 (15.4%) | |
| < 2 | 88 (84.6%) | |
| Morphology variants | ||
| Centroblastic | 93 (89.4%) | |
| Immunoblastic | 10 (9.6%) | |
| Anaplastic | 1 (1.0%) |
*n, number of cases
Slides classified as DLBCL based on the WHO classification system and positive CD20 examination were then reviewed by two independent pathologists to determine morphological variants as centroblastic, immunoblastic or anaplastic. Centroblastic morphological variants were identified in 93 patients (89.4%), immunoblastic variants were identified in 10 patients (9.6%) and anaplastic variants were identified in 1 patient (1%).
CD30 Expression and Clinicopathological Characteristics Correlation
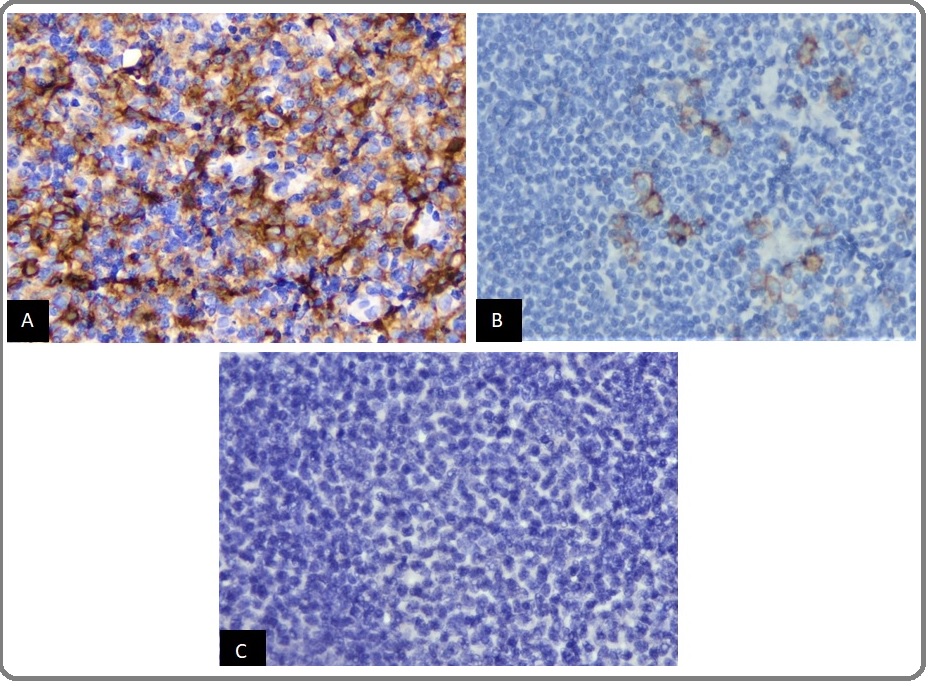
CD30 expression criteria were calculated based on positivity percentages with the cutoffs of > 0% and > 20% [12-13]. Of the 104 tissue samples examined, 90 patients (86.5%) were negative, 14 patients (13.5%) were positive with cutoff > 0%, and 2 patients (1.9%) were positive with cutoff > 20% (Figure 1, A-C) (Table 2).
Figure 1: CD30 Expression in DLBCL. (A) DLBCL with Expression of CD30 in > 20% of Tumor Cells (CD30 400x), (B) DLBCL with Expression of CD30 in > 0% of Tumor Cells (CD30 400x), and (C) DLBCL with no Expression of CD30 by Tumor Cells (CD30 400x).
| CD30 expressions | Frequency (%) (n=104) | |
| Negative | 90 (86.5%) | |
| Positive | > 0% | 14 (13.5%) |
| > 20% | 2 (1.9%) |
*n, number of cases
Correlations between positive CD30 expression (cutoff > 0%) with clinicopathological characteristics (age, sex, Ann Arbor stage, extranodal involvement and morphological variants) were not statistically significant (p > 0.05) (Table 3).
| CD30 | P values | |||
| Positive > 0% (n/%) | Negative (n/%) | |||
| (n=14) | (n=90) | |||
| Sex | ||||
| Male | 9 (14.3) | 54 (85.7) | 0.76 | |
| Female | 5 (12.2) | 36 (87.8) | ||
| Age | ||||
| ≥ 60 years old | 7 (15.6) | 38 (84.4) | 0.585 | |
| < 60 years old | 7 (11.9) | 52 (88.1) | ||
| Ann Arbor stage | ||||
| I-II | 12 (14.5) | 71 (85.5) | 0.73 | |
| III-IV | 2 (9.5) | 19 (90.5) | ||
| Extranodal involvement | ||||
| ≥ 2 | 3 (18.8) | 13 (81.3) | 0.448 | |
| < 2 | 11 (12.5) | 77 (87.5) | ||
| Morphology variants | ||||
| Centroblastic | 12 (12.9) | 81 (87.1) | 0.641 | |
| Others | 2 (18.2) | 9 (81.8) |
*n, number of cases
In this study, there were 2 cases with > 20% positive CD30 expression. The first case, a 57-year-old male with a lump in the right axillary region and left inguinal lymph nodes enlargement (Ann Arbor stage III) with centroblastic morphological variants. CD10 expression was negative, BCL6 and MUM1 were positive (Figure 2, A-D).
Figure 2: (A) DLBCL with Expression of CD30 in > 20% of Tumor Cells (CD30 400x), (B) Negative Expression of CD10 (CD10 400x), (C) Positive Expression of BCL6 (BCL6 400x), and (D) Positive Expression of MUM1 (MUM1 400x).
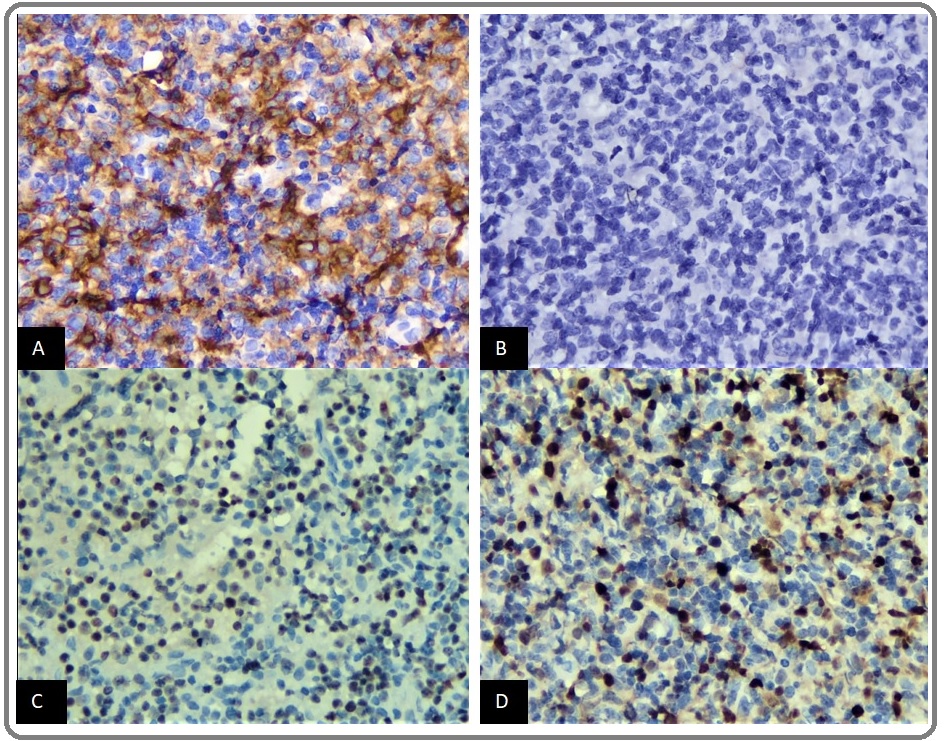
Based on Hans algorithm, the case is compatible with activated B-cell like (ABC) subtype. This patient had never started chemotherapy with 7 months overall survival (OS). The second case, a 58-year-old male with a lump in bilateral inguinal lymph nodes (Ann Arbor stage II) and immunoblastic morphological variants. CD10 and BCL 6 expressions were negative. Based on Hans algorithm, this case is compatible with ABC subtype. This patient received R-CHOP therapy with 3 months OS after completion of treatment.
Discussion
This study involved a case by case review of 104 patients with DLBCL. The study revealed that Indonesian DLBCL cases are more common in men than women (60.6%) with an average age of 57 years. According to previous epidemiology study of NHL cases from academic medical centers in Indonesia involving 164 samples, a similar result also revealed that NHL B cells with the most common histological subtype is DLBCL (68.2%) and are more common in men (55%) with an average patient age of 51 years-old [2]. The incidence of lymphoma is more common in men and this is explained in the phenomenon of the immune system in women. Other mechanisms show that the hormone estrogen has an anti-proliferation effect on lymphoid cells via β estrogen receptor signaling [15].
In recent years, CD30 expression has been detected in DLBCL cases [16]. Several previous studies have shown that CD30 is expressed in DLBCL for 9.5-40% and depending on the cutoff used, difficulty in standardizing IHC staining methods, as well as regional and ethnic differences [12-17], in 14-25% [18]. CD30 is reported to be a useful predictor of good clinical outcome in DLBCL. Patients with CD 30 positive DLBCL show a better development of survival compared to patients with CD30 negative [16].
The International DLBCL Rituximab-CHOP Consortium Study Program (IDCP) study reported a prevalence of positive CD30 expression of 14% of 903 de novo DLBCL cases using a 20% cutoff [8]. Campuzano-Zaluaga (2014) obtained 21% of 167 de novo DLBCL cases with positive CD30 expression ≥ 20%. The study was a retrospective study with predominantly Latin American populations and included patients with EBV infection (27.5%) with unknown human immunodeficiency virus (HIV) status [19]. Another study from British Columbia with the inclusion criteria of HIV negative patients showed 11% of cases with positive CD30 expression ≥ 20% and 25% of cases with positive CD30 expression > 0%. Xu (2017) obtained 12% of DLBCL with expressed CD30 using > 0% cutoff and 25% with ≥ 20% cutoff [20]. Gong (2015) obtained 12% of cases of de novo DLBCL with positive CD30 expression > 0% and 9.5% with positive CD30 expression ≥ 20% [16]. In this study, there were 14 (13.5%) cases from 104 tissue samples of patients with DLBCL with positive CD30 expression > 0% and 2 (1.9%) cases with positive CD30 expression > 20%. The relatively low percentage of positive CD30 expression in this study may be attributed to differences in demographic factors and variability in sampling handling strategies. This lower result might reflect differences in underlying tumor biology from a diverse ethnic population [4-13-16]. CD30 expression assessment is closely related to pre-analytical phase including sample handling and IHC staining processes [21-23]. The pre-analytic process is most likely to give false positive or negative results [24-25].
Assessment of CD30 expression with > 0% cutoff in DLBCL cases is also strongly influenced by tumor microenvironment (TME) factors. Generally, a DLBCL specimen consists of approximately 60-80% tumor cell components. TME factors in DLBCL include natural killer cells (NK) (± 20%), dendritic cells (± 15%), macrophages (± 15%), CD4 + T cells (± 10%) and CD8 + T cells (< 5%) [26-27]. NK cells were positively stained with CD58 IHC staining. Dendritic cells are positively identified with S100. Macrophages can play a role in tumor cells killing but may also contribute to tumor growth, invasion and progression by influencing immunosuppression and synthesizing angiogenic factors such as vascular endothelial growth factor (VEGF), Interleukin 8 (IL-8), TNF-alpha, metalloprotease and fibroblast growth factor 1 (FGF-1). Macrophages are positively identified with CD68/CD163 staining [28]. CD30 expression in B lymphocytes (activated immunoblast cells in the peripheral germinal center and parafollicular region) is limited, which can co-expression together with acute activation markers [29].
The present study also analyzed the correlations between positive CD30 expression and clinicopathological characteristics (sex, age, Ann Arbor stage, extranodal involvement and DLBCL morphological variants). No significant statistical associations were found between positive CD30 expression (using either a 0% or 20% cutoff) and clinicopathological characteristics. Slack (2014) also examined CD30 expression in 308 cases of de novo DLBCL using a positive threshold of CD30 expression > 0% and > 20% and there was also no statistically significant relationship between positive CD30 expression and clinicopathological characteristics [13].
In our study, there were 2 cases with > 20% positive CD30 expression and based on the Hans algorithm, both of them were compatible with ABC subtype. Salas (2016) also obtained 70% positive CD30 expression in ABC subtype from 40 cases positive CD30 DLBCL [30]. Gandhi (2013) obtained 23% positive CD30 expression in ABC DLBCL higher than 9.5% in GCB DLBCL [31]. CD30 expression by IHC is associated with higher expression levels of CD30 mRNA [32]. ABC DLBCL has more frequent and higher CD30 mRNA expression than GCB DLBCL [14-32-33].
Positive CD30 expression status in DLBCL patients may aid management strategies using brentuximab vedotin as an alternative therapy and is associated with improved therapy response and prolonged patient survival [13]. Positive CD30 expression indicates better outcomes in both GCB and non-GCB subtypes because there is increased gene encoding that blocks NF-ĸB activation and lymphocyte cell survival, decreases B cell receptor signal regulation and B cell proliferation with cytokine and stromal signals [8].
In our present study, it was concluded that the prevalence of DLBCL with positive CD30 expression > 0% was 13.5% and positive CD30 expression > 20% was 1.9%. There was no statistically significant association between CD30 immunoreactivity (either with 0% or 20% cutoff) and clinicopathological characteristics (age, sex, Ann Arbor stage, extranodal involvement and morphological variants).
Acknowledgements
The authors are grateful to Agustina Supriyanti, Datik Ratih Wijayanti, Yakub Setyawan, Sumantri, Ika Fi’ila Sari, MD for their excellent technical assistance. The authors declare no conflict of interest.
Funding Statement
This work was supported by the Grant for Research fund, Dr. Sardjito Hospital in 2018 (Grant No: HK.02.03/ XI.2/19045/2018)
References
- Diffuse large B-cell lymphoma: 10�years' real-world clinical experience with rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisolone Horvat Matej, Zadnik Vesna, Južnič Šetina Tanja, Boltežar Lučka, Pahole Goličnik Jana, Novaković Srdjan, Jezeršek Novaković Barbara. Oncology Letters.2018. CrossRef
- Multicentre Epidemiology and Survival Study of B Cell Non Hodgkin Lymphoma Patients In Indonesia Reksodiputro Ary Harryanto. Journal of Blood Disorders & Transfusion.2015;06(02). CrossRef
- Diffuse large B-cell lymphoma Li Shaoying, Young Ken H., Medeiros L. Jeffrey. Pathology.2018;50(1). CrossRef
- CD30, another useful predictor of survival in DLBCL? Chan Wing C.. Blood.2013;121(14). CrossRef
- Clear cell variant of diffuse large B-cell lymphoma: a case report and review of the literature Xue Y, Wang Q, He X. Int J Clin Exp Pathol.2015;8:7594-7599.
- Brentuximab Vedotin (SGN-35) Katz J., Janik J. E., Younes A.. Clinical Cancer Research.2011;17(20). CrossRef
- CD30-CD30 Ligand Interaction in Primary Cutaneous CD30+T-Cell Lymphomas: A Clue to the Pathophysiology of Clinical Regression Mori M., Manuelli C., Pimpinelli N., Mavilia C., Maggi E., Santucci M., Bianchi B., Cappugi P., Giannotti B., Kadin M.E.. Blood.1999;94(9). CrossRef
- CD30 expression defines a novel subgroup of diffuse large B-cell lymphoma with favorable prognosis and distinct gene expression signature: a report from the International DLBCL Rituximab-CHOP Consortium Program Study Hu Shimin, Xu-Monette Zijun Y., Balasubramanyam Aarthi, Manyam Ganiraju C., Visco Carlo, Tzankov Alexander, Liu Wei-min, Miranda Roberto N., Zhang Li, Montes-Moreno Santiago, Dybkær Karen, Chiu April, Orazi Attilio, Zu Youli, Bhagat Govind, Richards Kristy L., Hsi Eric D., Choi William W. L., Han van Krieken J., Huang Qin, Huh Jooryung, Ai Weiyun, Ponzoni Maurilio, Ferreri Andrés J. M., Zhao Xiaoying, Winter Jane N., Zhang Mingzhi, Li Ling, Møller Michael B., Piris Miguel A., Li Yong, Go Ronald S., Wu Lin, Medeiros L. Jeffrey, Young Ken H.. Blood.2013;121(14). CrossRef
- Brentuximab Vedotin and Diffuse Large B-cell Lymphoma Neha Gupta Shipra Gandhi. Journal of Hematology & Thromboembolic Diseases.2015;03(01). CrossRef
- Brentuximab vedotin: clinical updates and practical guidance Yi Jun Ho, Kim Seok Jin, Kim Won Seog. Blood Research.2017;52(4). CrossRef
- A Phase II Study of Brentuximab Vedotin for Relapsed or Refractory CD30-Positive Non-Hodgkin Lymphomas Other Than Anaplastic Large Cell Lymphoma Park S, Kim SJ, Hong JY, Yoon DH, Kim JS, Kang HJ, et al . Blood.2017;130:4077. CrossRef
- CD30 expression and its correlation with MYC and BCL2 in de novo diffuse large B-cell lymphoma Gong Qi-Xing, Wang Zhen, Liu Chong, Li Xiao, Lu Ting-Xun, Liang Jin-Hua, Xu Wei, Li Jian-Yong, Zhang Zhi-Hong. Journal of Clinical Pathology.2018;71(9). CrossRef
- CD30 expression inde novodiffuse large B-cell lymphoma: a population-based study from British Columbia Slack Graham W., Steidl Christian, Sehn Laurie H., Gascoyne Randy D.. British Journal of Haematology.2014;167(5). CrossRef
- The Expression of CD30 Based on Immunohistochemistry Predicts Inferior Outcome in Patients with Diffuse Large B-Cell Lymphoma Hao Xiaoxiao, Wei Xiaolei, Huang Fen, Wei Yongqiang, Zeng Hong, Xu Linwei, Zhou Qinjun, Feng Ru. PLOS ONE.2015;10(5). CrossRef
- Does Gender Matter in Non-Hodgkin Lymphoma? Differences in Epidemiology, Clinical Behavior, and Therapy Horesh Nurit, Horowitz Netanel A.. Rambam Maimonides Medical Journal.2014;5(4). CrossRef
- Prevalence and clinicopathologic features of CD30-positive de novo diffuse large B-cell lymphoma in Chinese patients: A retrospective study of 232 cases Gong QX, Lu TX, Liu C, Wang Z, Liang JH, Xu W, et al . Int J Clin Exp Pathol.2015;8:15825-15835.
- CD30 and CD30-Targeted Therapies in Hodgkin Lymphoma and Other B cell Lymphomas Bhatt Geetika, Maddocks Kami, Christian Beth. Current Hematologic Malignancy Reports.2016;11(6). CrossRef
- Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression Jacobsen Eric D., Sharman Jeff P., Oki Yasuhiro, Advani Ranjana H., Winter Jane N., Bello Celeste M., Spitzer Gary, Palanca-Wessels Maria Corinna, Kennedy Dana A., Levine Pamela, Yang Jing, Bartlett Nancy L.. Blood.2015;125(9). CrossRef
- Differential CD30 expression in adult T-cell leukemia-lymphoma subtypes Campuzano-Zuluaga Germán, Pimentel Agustin, Chapman-Fredricks Jennifer R, Ramos Juan. Retrovirology.2014;11(Suppl 1). CrossRef
- CD30 expression and prognostic significance in R-EPOCH–treated patients with diffuse large B-cell lymphoma Xu Jie, Oki Yasuhiro, Saksena Annapurna, Desai Parth, Lin Pei, Tang Guilin, Yin C. Cameron, You M. James, Thakral Beenu, Medeiros L. Jeffrey, Li Shaoying. Human Pathology.2017;60. CrossRef
- Targeting CD30 in Malignant Tissues: Challenges in Detection and Clinical Applications Wasik Mariusz A., Jimenez Gretchen S., Weisenburger Dennis D.. Pathobiology.2013;80(5). CrossRef
- Interobserver variation in CD30 immunohistochemistry interpretation; consequences for patient selection for targeted treatment Koens Lianne, van de Ven Peter M, Hijmering Nathalie J, Kersten Marie J, Diepstra Arjan, Chamuleau Martine, de Jong Daphne. Histopathology.2018;73(3). CrossRef
- Immunohistochemistry for Pathologists: Protocols, Pitfalls, and Tips Kim So-Woon, Roh Jin, Park Chan-Sik. Journal of Pathology and Translational Medicine.2016;50(6). CrossRef
- Recommendations for Improved Standardization of Immunohistochemistry Goldstein Neal S., Hewitt Stephen M., Taylor Clive R., Yaziji Hadi, Hicks David G.. Applied Immunohistochemistry & Molecular Morphology.2007;15(2). CrossRef
- Diagnostic Immunohistochemistry: What Can Go Wrong and How to Prevent It Gown Allen M.. Archives of Pathology & Laboratory Medicine.2016;140(9). CrossRef
- Targeting the Immune Microenvironment in Lymphomas of B-Cell Origin: From Biology to Clinical Application Mulder , Wahlin , Österborg , Palma . Cancers.2019;11(7). CrossRef
- The tumour microenvironment in B cell lymphomas Scott David W., Gascoyne Randy D.. Nature Reviews Cancer.2014;14(8). CrossRef
- Tumor Microenvironment in Diffuse Large B-Cell Lymphoma: Role and Prognosis Cioroianu Alexandra Ioana, Stinga Patricia Irina, Sticlaru Liana, Cioplea Mirela Daniela, Nichita Luciana, Popp Cristiana, Staniceanu Florica. Analytical Cellular Pathology.2019;2019. CrossRef
- Understanding CD30 biology and therapeutic targeting: a historical perspective providing insight into future directions van der Weyden C A, Pileri S A, Feldman A L, Whisstock J, Prince H M. Blood Cancer Journal.2017;7(9). CrossRef
- CD30 Expression in Diffuse Large B-Cell Lymphoma (DLBCL) Correlates with Non-GCB Subtype but Does Not Have Prognostic Impact in Patients Treated with First Line R-CHOP/R-CHOP-like Salas Maria Queralt, Climent Fina, Domingo Domenech Eva, Mercadal Santiago, Paredes Viviana, Oliveira Ana Carla, Aguilera Carmen, de la Banda Esmeralda, Lucas Anna, Garcia Nadia, Baca Cristina, Fernandez de Sevilla Alberto, Sureda Anna, Gonzalez Barca Eva, Riasol Maria Lutgarda. Blood.2016;128(22). CrossRef
- Distinct CD30 Expression Patterns In Germinal Center B-Cell (GCB) and Non-GCB Diffuse Large B-Cell Lymphoma (DLBCL) Gandhi Shipra, Neppalli Vishala T., Deeb George, Czuczman Myron S., Hernandez-Ilizaliturri Francisco J. Blood.2013;122(21). CrossRef
- CD30 Expression in Diffuse Large B-Cell Lymphoma Slack Graham W., Steidl Christian, Sehn Laurie H., Gascoyne Randy D.. Blood.2012;120(21). CrossRef
- Frequency and extent of CD30 expression in diffuse large B-cell lymphoma and its relation to clinical and biologic factors: a retrospective study of 167 cases Campuzano-Zuluaga Germán, Cioffi-Lavina Maureen, Lossos Izidore S., Chapman-Fredricks Jennifer R.. Leukemia & Lymphoma.2013;54(11). CrossRef
License
Copyright
© ,
Author Details