Anti-mutagenic Activity of Oxycarotenoid-rich Extracts Isolated from Coriandrum Sativum and Murraya koenigii
Download
Abstract
Introduction: Edible and medicinal plants contain active principles that can act as antimutagens, and hence their intake may be useful for human cancer prevention. Green leafy vegetables are important sources of carotenoids which possess antioxidant, anti-inflammatory and antimutagenic properties.
Objective: To study the antimutagenic activity of oxycarotenoid-rich extracts isolated from Coriandrum sativum (coriander leaves) and Murraya koenigii (curry leaves).
Methods: Oxycarotenoid-rich extracts isolated from Coriandrum sativum (coriander leaves) and Murraya koenigii (curry leaves) were investigated for antimutagenic activity in vitro by Ames test using Salmonella typhimurium strains TA 98 and TA 1535. Mutagens used were, nitro-o- phenylenediamine (NPD) (20μg/plate) and N-methyl- N-nitro-N-nitrosoguanidine (MNNG) (1μg/ plate).
Results: The results revealed that oxycarotenoid-rich extracts isolated from Coriandrum sativum (coriander leaves) and Murraya koenigii (curry leaves) administered at doses of 1.0 mg, 2.5 mg and 5 mg per plate significantly inhibited mutagenicity induced by NPD and MNNG.
Conclusion: These findings suggest that oxycarotenoid- rich extracts isolated from Coriandrum sativum (coriander leaves) and Murraya koenigii (curry leaves) have antimutagenic properties.
Introduction
Edible plants are commonly used as natural food, tea and herbal medicine [1]. Most of these plants have not been screened for their anti-mutagenic activities. Thus, the information regarding the anti-mutagenic activity of these plants will be useful to validate their traditional uses and to establishing anti-mutagenic data bases [2]. Antimutagens isolated from edible and medicinal plants are useful because they may prevent human cancer, and have no toxic effects on living organisms [3]. Herbal drugs, containing active principles are currently developed to protect against attack of free radicals on DNA and its outcome such as aging and cancer [4]. Several useful drugs have been developed from components discovered from medicinal plants, and this forms an important source of new pharmaceuticals [5]. Developing countries are utilising medicinal plants in healthcare systems for primary health care needs. The use of medicinal plants is highly widespread because of their easy access, claimed therapeutic efficacy based on local knowledge and expertise amongst the local communities as well as the affordability [6]. Plants contain many metabolites with various bioactivities including antioxidant, anti-inflammatory and anticancer activities [7]. Bioactive compounds of plant origin are found to be antioxidants and are shown to have possible health effects, mainly due to their anti- oxidative properties [8]. Many mutagens and carcinogens act by producing reactive oxygen species (ROS), which are harmful to living systems.
Reactive oxygen species induce oxidative damage to cell structures and biomolecules such as lipids, nucleic acids and proteins [8]. DNA mutation is an important step in carcinogenesis. Elevated levels of oxidative DNA changes have been noted in many tumours, strongly implicating such damage in the aetiology of cancer [9]. The prevention of oxidative damage to DNA is important to limit mutagenesis, cytostasis, and cytotoxicity. It may contribute to prevention of mutation-related diseases [10-11]. A common factor in the pathogenesis of chronic degenerative diseases is the involvement of oxidative stress. Plant compounds may reduce oxidative stress, thereby reducing the risk of diseases [12].
Carotenoids form a part of natural pigments which have been found to lessen the risk of many chronic diseases such as cancer, heart disease, and certain eye diseases [13]. The bioavailability of carotenoids is affected by a number of factors such as food matrix, processing conditions, and fat content [14]. The bioavailability of lutein and zeaxanthin is greater in heat-processed than from unprocessed/raw vegetables [15]. Lutein and zeaxanthin are also more (from 50% to 100%) bioaccessible from fruits than from dark green vegetables [16].
Upon intestinal absorption as micelles, lutein and zeaxanthin are incorporated in chylomicrons, together with other dietary lipids [17]. Chylomicrons are rapidly remodeled by lipoprotein lipase in peripheral tissues and then enter as chylomicron remnants into the bloodstream [18]. The resulting chylomicron remnants containing carotenoids are then transferred to the liver, where they can be either stored or re-secreted into the circulation in association with lipoproteins [18]. In the fasting blood, lutein (> 50%) and zeaxanthin (> 40%) are predominantly transported by HDL [19]. The serum concentration of lutein ranges from 0.1–1.44 μmol/L, and from 0.07–0.17 μmol/L for zeaxanthin, the large variability being due to the wide difference in carotenoids intake among individuals [20].
The specific affinity of lutein and zeaxanthin to HDL regulate their post-hepatic tissue distribution via HDL receptor mediated uptake. In humans, the highest levels of these pigments are reached in the macula of the eye where their concentrations range between 0.1 and 1 mM [21]. Shyam et al (2017) have found that all three human SR-B proteins and CD36 are capable of binding the macular xanthophyll carotenoids, zeaxanthin, lutein, and its metabolite meso-zeaxanthin [22]. A cross-sectional study by Renzi et al. (2012) observed that serum lutein and zeaxanthin and lipoprotein concentrations are significantly related and changing lipoprotein levels may impact levels of these retinal xanthophylls. Johnson et al (2016) reported that lutein concentrations are related to levels of steroidogenic acute regulatory domain-3 (StARD3) protein in human brain [23]. A role of StARD3 as a lutein-binding protein was first identified in the macula lutea of primates [24] together with glutathione S-transferase-1 (GSTP1) as a zeaxanthin-binding protein [25]. StARD3 along GSTP1 provide numerous binding sites for lutein and zeaxanthin respectively, that account for the unique distribution and stability of carotenoids found in the primate macula lutea. It is possible that CD36 interacts with StARD3 as a surfaces receptor to mediate carotenoid uptake into the macula [24]. These data prove the involvement of HDL transport and StARD3 in the uptake of lutein by the brain, and may partially explain the preferential accumulation of the xanthophylls in the central nervous system.
Two enzymes - β-carotene-15,15′-oxygenase (BCO1) and β-carotene-9′,10′-oxygenase (BCO2) - mediate the cleavage of carotenoids at specific sites of the polyene chain [26]. These two enzymes have different substrate specificity for various carotenoids, different cellular localization and perform different functions within the cell [27]. Specifically BCO2 localized in the mitochondria has been proposed to prevent toxic accumulation of these pigments in the tissues [28]. Xanthophylls can serve as substrate for BCO2 giving rise to a number of 3-hydroxy metabolites depending on the site and number of cleavages [29]. These hydroxyl-metabolites may be excreted through urine after conjugation.
Ravikrishnan et al.,(2011) observed that administration (gavage) of lutein/zeaxanthin concentrate at dose levels of 0, 4, 40 and 400 mg/kg bw/day for 90-days in Wistar rats did not result in any toxicity- related changes in clinical observations, ophthalmic examinations, body weights, body weight gains, feed consumption, and organ weights. No toxicity findings were noted in urinalysis, hematology or clinical biochemistry parameters at the end of the treatment or recovery period and terminal necropsy also did not reveal any treatment- related gross or histopathology findings. The results of mutagenicity testing in Salmonella typhimurium did not reveal any genotoxicity. The no-observed-adverse-effect level (NOAEL) for lutein/zeaxanthin concentrate was determined as 400mg/kg bw/day, the highest dose tested.
The Salmonella typhimurium assay (Ames test) is a widely accepted as a short term assay for identifying substances that can produce genetic damage that leads to gene mutations [31-32].
In the present study, an effort has been made to analysing the antimutagenic activity of oxycarotenoid (lutein and zeaxanthin) rich extracts isolated from Coriandrum sativum (coriander leaves) and Murraya koenigii (curry leaves).
Materials and Methods
Chemicals
4-nitro-O-phenylene diamine (NPD) and N-methyl- N-nitro-N-nitrosoguanidine (MNNG) was purchased from Sigma Chemicals; glucose-6-phosphate, L-histidine, D-biotin purchased from Sisco, Mumbai, and other chemicals employed in these studies were of laboratory reagent grade.
Preparation of oxycarotenoid- rich extracts
The Preparation of crude extract from the dried leaves of Coriandrum sativum and Murraya koenigii and the isolation of oxycarotenoid- rich extracts were done as reported earlier [33]. After confirmation of rich presence of oxycarotenoids in the extracts by HPLC, fraction 3 (hexane/ acetone 80/20 fraction) showing maximum concentration of lutein [beta,beta-Carotene-3, 3’-diol, (3R, 3’R)] and zeaxanthin [beta, epsilon-Carotene-3, 3’-diol, (3R, 3’R,6’S)] were selected for the study (Figure 1a).
Figure 1a.
Test material
Oxycarotenoid-rich fraction of Coriandrum sativum and Murraya koenigii extracts prepared was used as test material. The extracts were in the form of dark brown to yellowish brown viscous liquid. The extracts prepared from Coriandrum sativum (coriander leaves) are mentioned as coriander leaf extract (COLE) and the extract prepared from Murraya koenigii (curry leaves) as curry leaf extract (CULE) using 5% Dimethyl sulfoxide (DMSO) as the vehicle. The test item forms good suspension with 5% DMSO.
Bacterial strain
The strains used in this study, histidine requiring strain of Salmonella typhimurium TA 98 and TA1535 was purchased from Institute of Microbial Technology, India. The strain TA 98 was constructed to detect mutagens causing frame shift mutation and TA1535 was constructed to detect base-pair substitution. The strains were incubated in nutrient broth for 12 hr at 37oC and stored at -70oC as frozen permanents in presence of 9% DMSO. To carry out the study, fresh cultures were prepared by inoculating 40 μl of the frozen permanents in 5 ml of nutrient broth for 12 hr at 37oC.
Mutagens
The mutagens NPD and MNNG were dissolved in dimethyl sulphoxide.
Toxicity Assay
Oxy-carotenoid-rich extracts isolated from Coriandrum sativum and Murraya koenigii up to a concentration 10 mg/plate did not show any toxicity to Salmonella typhimurium strains as compared to the number of spontaneous revertants formed in control and DMSO control plates (data not shown).
Anti-mutagenic assay
Anti-mutagenic assay of the oxycarotenoid-rich extracts isolated from Criandrum sativum and Murraya koenigii was performed by standard plate incorporation method as per Ames test [34] in compliance with OECD guidelines. The extracts were added at the concentration of 1 mg, 2.5 mg and 5 mg to 2ml of the top agar containing 0.5 mM histidine-biotin along with 0.1 ml direct acting mutagens. The mixture was layered on minimal agar plates and the plates were incubated at 37oC for 48 hr.
The plates with mutagen alone acted as control; plates without the test sample as spontaneous revertants and plates with mutagen and DMSO as the vehicle control were also incubated. After the incubation period colony counter was used to count the revertant colonies developed on the plates. The extracts were tested against the mutagen 4-nitro-o-phenylene diamine (NPD) 20 μg/plate in strains TA 98 and N-methyl-N-nitro-N-nitrosoguanidine (MNNG) 1 μg/plate in strains TA 1535.
Percentage inhibition of mutagens was calculated as:
% Inhibition = (R1-SR)-(R2-SR) X100/(R1-SR)
Where R1 is the number of revertants in presence of mutagen alone
R2 is the number of revertants in presence of extracts SR is the spontaneous revertants
Results
The test items are found to be nontoxic at a maximum concentration of 10 mg/plate through preliminary toxicity determination. The oxycarotenoid extracts isolated from Coriandrum sativum (coriander leaves) and Murraya koenigii (curry leaves) tested at concentrations of 1 mg, 2.5 mg and 5 mg/plate showed significant anti-mutagenicity towards the mutagens MNNG and NPD but it was more pronounced in TA1535 (Figure 1 and 2).
Figure 1: Percentage Inhibition of Antimutagenic Activity in Oxycarotenoid-rich Extract of Coriandrum sativum (coriander leaves).
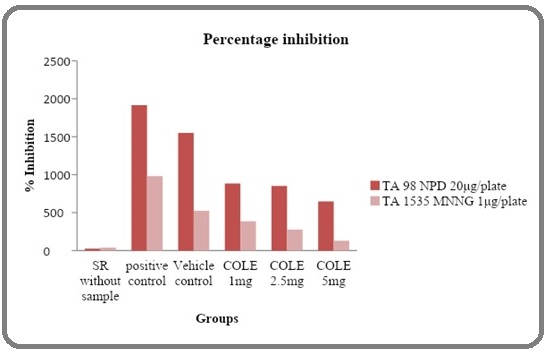
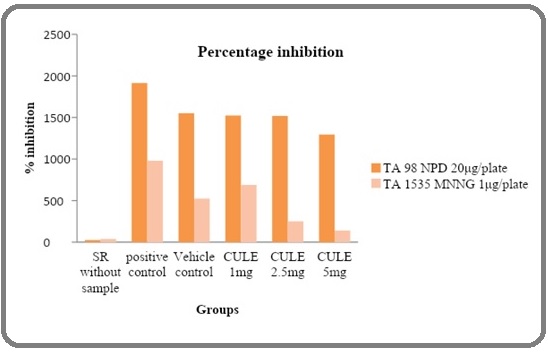
Figure 2: Percentage Inhibition of Antimutagenic Activity in Oxycarotenoid-rich Extract of Murraya Koenigii (curry leaves).
The revertant colonies in each plate was counted. The decline in the number of revertant colonies in the extracts treated plates indicated its antimutagenic potential. The oxycarotenoid extracts isolated from the Coriandrum sativum at concentration of 5 mg/plate showed inhibition of 89.2% in mutagenicity induced by MNNG on strains TA 1535 and exhibited 32.8% inhibition in mutagenicity induced by NPD on strains TA 98 (Table 1).
| Treatment | TA 98 | TA 1535 | %Inhibition | |
| Concentration of COLE in mg/plate | NPD 20μg/plate | MNNG 1μg/plate | TA 98 | TA 1535 |
| SR without sample | 25.66±0.58 | 38.7±2.83 | ||
| positive control | 1915.7±81.5 | 980.5±8.48 | ||
| Vehicle control | 1550.9±47.88 | 524.33±36.86 | ||
| COLE 1mg | 882.6±50.29 | 386.3±24.65 | 54.66022 | 63.09195 |
| COLE 2.5mg | 851.1±53.11 | 276.5±14.14 | 56.32685 | 74.75048 |
| COLE 5mg | 646.7±54.19 | 130±7.34 | 67.14144 | 90.3058 |
*COLE, Oxycarotenoid- rich extract of Coriandrum sativum (coriander leaves); SR, Spontaneous revertants; TA 98 and TA 1535 –Bacterial strains; Positive controls, For TA 98, NPD(4-Nitro-o-phenylene diamine) 20μg/plate; TA 1535, MNNG (N-methyl-N-nitro-N-nitrosoguanidine) 1μg/plate; Vehicle control, Plates with mutagen and dimethyl sulphoxide; Colony counted as mean of 3 plates; Statistically significant than the vehicle control group p<0.05
On the other hand, extracts isolated from Murraya koenigii at concentration of 5 mg/plate showed inhibition of 90.3% in mutagenicity induced by MNNG on strains TA 1535 and exhibited 67.1% inhibition in mutagenicity induced by NPD on strains TA 98 (Table 2).
| Treatment | TA 98 | TA 1535 | %Inhibition | |
| Concentration of CULE in mg/plate | NPD 20μg/plate | MNNG 1μg/plate | TA 98 | TA 1535 |
| SR without sample | 25.66±0.71 | 38.7±3.21 | ||
| Positive control | 1915.7±81.5 | 980.5±8.48 | ||
| Vehicle control | 1550.9±47.88 | 524.33±36.86 | ||
| CULE 1mg | 1524.3±48.78 | 687.9±30 | 20.71 | 31.07 |
| CULE 2.5mg | 1519.3±41 | 250.9±16.31 | 20.97 | 77.47 |
| CULE 5mg | 1295.6±20.67 | 140.3±6.34 | 32.81 | 89.21 |
*CULE, Oxycarotenoid extract of Murraya koenigii (curry leaves); SR, spontaneous revertants; TA 98 and TA 1535:Bacterial strains; Positive controls, For TA 98, NPD (4-Nitro-o-phenylene diamine) 20μg/plate; For TA 1535, MNNG(N-methyl-N-nitro-N-nitrosoguanidine) 1μg/plate; Vehicle control, Plates with mutagen and dimethyl sulphoxide; Colony counted as mean of 3 plates; Test groups statistically significant compared to vehicle control group p<0.05
Discussion
Green leafy vegetables are shown to increase the antioxidant capacity of the plasma and reduce the risk of diseases such as cancer, heart diseases, and stroke [35]. The reason of this beneficial effect of antioxidants from plants could be their protective effects by counter acting reactive oxygen species [36]. The natural pigments, carotenoids, have been found to lessen the risk of many chronic diseases such as cancer, heart disease, and certain eye diseases [13]. Carotenoid-rich diets from fruits and vegetables have been associated with a decreased risk of lung, stomach and prostate cancers [37]. Lutein and zeaxanthin are antioxidant carotenoids found in many fruits, vegetables and flowers as well as in dark leafy vegetables [33].
Oxycarotenoid (lutein and zeaxanthin) rich extracts form Coriandrum sativum and Murraya koenigii showed significant antimutagenicity against direct acting mutagens TA 98 and TA1535 and this antimutagenic activity may be due to the antioxidant property.
In conclusion, this study showed that the oxycarotenoid- rich extracts of Coriandrum sativum and Murraya koenigii possessed significant antimutagenic properties and hence the inclusion of these plants in the diet may have beneficial effects on human health. A plant extract indicating antimutagenicity may not necessarily be an anticarcinogen; however, it can be an indication of a possible candidate for such purpose.
Acknowledgements
The authors acknowledge the support of Little Flower Medical Research Centre, Kerala and Care Kerala Ltd, Kerala.
References
- Antioxidant activity of wild edible plants in the Black Sea Region of Turkey Özen Tevfik. Grasas y Aceites.2009;61(1). CrossRef
- Antioxidant, mutagenic, and antimutagenic activities of Tragopogon longirostis var. longirostis, an edible wild plant in Turkey Sarac Nurdan. Indian Journal of Pharmacology.2015;47(4). CrossRef
- Antioxidant and antimutagenic activities of pomegranate peel extracts Negi P.S., Jayaprakasha G.K., Jena B.S.. Food Chemistry.2003;80(3). CrossRef
- Mutagenic and antimutagenic activities of Mitragynaspeciosa Korth extract using Ames test Ghazali R, Abdullah R, Ramli N, Rajab NF, Ahmad-Kamal MS, Yahya NA. J Med Plant Res.2011;5:1345-1348.
- Natural Products as Sources of New Drugs over the Period 1981−2002 Newman David J., Cragg Gordon M., Snader Kenneth M.. Journal of Natural Products.2003;66(7). CrossRef
- International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans. Volume 82.Some traditional herbal medicines, some mycotoxins, naphthalene and styrene WHO . Lyon: IARC Press.2002;82.
- Antioxidant and antitumor effects of Manda Kim JH, Park MK, Lee JY, Okuda H, Kim S, Hwang WI. Biochem Arch.1998;14:211-219.
- Angiogenesis inhibited by drinking tea Cao Yihai, Cao Renhai. Nature.1999;398(6726). CrossRef
- Reactive oxygen species in human health and disease Castro Laura, Freeman Bruce A. Nutrition.2001;17(2). CrossRef
- Oxidative DNA damage: mechanisms, mutation, and disease Cooke Marcus S., Evans Mark D., Dizdaroglu Miral, Lunec Joseph. The FASEB Journal.2003;17(10). CrossRef
- Dietary antioxidants and cardiovascular disease Blomhoff Rune. Current Opinion in Lipidology.2005;16(1). CrossRef
- Antigenotoxic, antimutagenic and ROS scavenging activities of a Rhoeo discolor ethanolic crude extract González-Avila M, Arriaga-Alba M, de la Garza M, del Carmen HernándezPretelı́n M, Domı́nguez-Ortı́z M.A, Fattel-Fazenda S, Villa-Treviño S. Toxicology in Vitro.2003;17(1). CrossRef
- The Role of Carotenoids in Human Health Johnson Elizabeth J.. Nutrition in Clinical Care.2002;5(2). CrossRef
- Absorption and Metabolism of Xanthophylls Kotake-Nara Eiichi, Nagao Akihiko. Marine Drugs.2011;9(6). CrossRef
- Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products Perry A, Rasmussen H, Johnson EJ. J Food Comp Anal.2009;22:9-15.
- Xanthophyll carotenoids are more bioaccessible from fruits than dark green vegetables O'Connell Orla F., Ryan Lisa, O'Brien Nora M.. Nutrition Research.2007;27(5). CrossRef
- Lutein, zeaxanthin and mammalian development: Metabolism, functions and implications for health Giordano Elena, Quadro Loredana. Archives of Biochemistry and Biophysics.2018;647. CrossRef
- Mammalian Metabolism of β-Carotene: Gaps in Knowledge Shete Varsha, Quadro Loredana. Nutrients.2013;5(12). CrossRef
- Effect of dietary lutein and zeaxanthin on plasma carotenoids and their transport in lipoproteins in age-related macular degeneration Wang Wei, Connor Sonja L, Johnson Elizabeth J, Klein Michael L, Hughes Shannon, Connor William E. The American Journal of Clinical Nutrition.2007;85(3). CrossRef
- The relation between serum lipids and lutein and zeaxanthin in the serum and retina: results from cross-sectional, case-control and case study designs Renzi Lisa M, Hammond Billy R, Dengler Melissa, Roberts Richard. Lipids in Health and Disease.2012;11(1). CrossRef
- Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease Bernstein Paul S., Li Binxing, Vachali Preejith P., Gorusupudi Aruna, Shyam Rajalekshmy, Henriksen Bradley S., Nolan John M.. Progress in Retinal and Eye Research.2016;50. CrossRef
- All three human scavenger receptor class B proteins can bind and transport all three macular xanthophyll carotenoids Shyam Rajalekshmy, Vachali Preejith, Gorusupudi Aruna, Nelson Kelly, Bernstein Paul S.. Archives of Biochemistry and Biophysics.2017;634. CrossRef
- Relationship between Concentrations of Lutein and StARD3 among Pediatric and Geriatric Human Brain Tissue Tanprasertsuk Jirayu, Li Binxing, Bernstein Paul S., Vishwanathan Rohini, Johnson Mary Ann, Poon Leonard, Johnson Elizabeth J. FASEB J.2016;30.
- Identification of StARD3 as a Lutein-Binding Protein in the Macula of the Primate Retina Li Binxing, Vachali Preejith, Frederick Jeanne M., Bernstein Paul S.. Biochemistry.2011;50(13). CrossRef
- Purification and Partial Characterization of a Lutein-Binding Protein from Human Retina Bhosale Prakash, Li Binxing, Sharifzadeh Mohsen, Gellermann Werner, Frederick Jeanne M., Tsuchida Kozo, Bernstein Paul S.. Biochemistry.2009;48(22). CrossRef
- Provitamin A metabolism and functions in mammalian biology von Lintig Johannes. The American Journal of Clinical Nutrition.2012;96(5). CrossRef
- Evidence for compartmentalization of mammalian carotenoid metabolism Palczewski Grzegorz, Amengual Jaume, Hoppel Charles L., Lintig Johannes. The FASEB Journal.2014;28(10). CrossRef
- Substrate Specificity of Purified Recombinant Chicken β-Carotene 9′,10′-Oxygenase (BCO2) dela Seña Carlo, Sun Jian, Narayanasamy Sureshbabu, Riedl Kenneth M., Yuan Yan, Curley Robert W., Schwartz Steven J., Harrison Earl H.. Journal of Biological Chemistry.2016;291(28). CrossRef
- BCDO2 acts as a carotenoid scavenger and gatekeeper for the mitochondrial apoptotic pathway Lobo G. P., Isken A., Hoff S., Babino D., von Lintig J.. Development.2012;139(16). CrossRef
- Safety assessment of lutein and zeaxanthin (Lutemax™ 2020): Subchronic toxicity and mutagenicity studies Ravikrishnan R., Rusia Shraddha, Ilamurugan G., Salunkhe Ulhas, Deshpande Jayant, Shankaranarayanan J., Shankaranarayana M.L., Soni Madhu G.. Food and Chemical Toxicology.2011;49(11). CrossRef
- The Ames Salmonella/microsome mutagenicity assay Mortelmans Kristien, Zeiger Errol. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis.2000;455(1-2). CrossRef
- Methods for detecting carcinogens and mutagens with the salmonella/mammalian-microsome mutagenicity test Ames Bruce N., McCann Joyce, Yamasaki Edith. Mutation Research/Environmental Mutagenesis and Related Subjects.1975;31(6). CrossRef
- Biochemical assays of crude and purified oxycarotenoid extracts isolated from coriander leaves and their effect on oxidative stability of oils by Rancimat assay Sherena PA, Annamala PT, Mukkadan JK, Dinesha Ramadas . Research and reviews: A journal of Biotechnology.2018;8(1):1-10.
- Revised methods for the Salmonella mutagenicity test Maron Dorothy M., Ames Bruce N.. Mutation Research/Environmental Mutagenesis and Related Subjects.1983;113(3-4). CrossRef
- Antioxidant Phytochemicals in Fruits and Vegetables: Diet and Health Implications Prior Ronald L., Cao Guohua. HortScience.2000;35(4). CrossRef
- A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay Wong Chi-Chun, Li Hua-Bin, Cheng Ka-Wing, Chen Feng. Food Chemistry.2006;97(4). CrossRef
- Epidemiologic studies of antioxidants and cancer in humans. Flagg E W, Coates R J, Greenberg R S. Journal of the American College of Nutrition.1995;14(5). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2020
Author Details