Diagnostic utility of Cytokeratin 13 and Cytokeratin 17 in Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma
Download
Abstract
Objective: To determine the immunohistochemical expression of CK 13 and CK 17 in Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma.
Methods: A total of 170 cases were retrieved from record files of Histopathology Department, to conduct a cross sectional study at Armed Forces Institute of Pathology, Rawalpindi, over a period of one year from June 2018 to June 2019 along with their formalin-fixed, paraffin embedded blocks comprising 85 cases of each oral squamous cell carcinoma and oral epithelial dysplasia. Blocks were trimmed and cut into very thin sections of 5 microns using microtome and mounted on slides. Tissue mounted slides were stained with routine haematoxylin and eosin followed by immunohistochemical staining of cytokeratin 13 and cytokeratin 17. New histological diagnosis of each case was made. Mean and standard deviation were calculated for quantitative variables. Frequency and percentages were calculated for qualitative variables. Chi-square test was employed to assess the significance of difference. The P-value <0.05 was considered significant.
Results: In this study, 101 (59.4%) male and 69 (40.6%) female patients with the mean age of 60.63 ± 13.814 (mean ± SD) were collected and buccal mucosa was the most common site of presentation. In a total of 170 cases, 34 (20%) cases showed positive expression of cytokeratin 13 whereas 136 (80%) cases showed negative expression of cytokeratin 13. In comparison, out of 170 cases, 133 (78.2%) cases showed positive expression of cytokeratin 17 whereas 37 (21.8%) cases showed negative expression of cytokeratin 17. The P-value was found to be < 0.001 and 0.001 for expression of cytokeratin 13 and cytokeratin 17 respectively.
Conclusion: Opposite expression of cytokeratin 13 and cytokeratin 17 was seen in this study, in the form of loss of cytokeratin 13 and over expression of cytokeratin 17 with increase in the degree of dysplasia and invasive carcinoma. Correct assessment, diagnosis and management with the help of these markers can lead to early diagnosis and favorable treatment outcome.
Introduction
Head and neck cancer is the sixth most common cancer worldwide, out of which the most common histological type is squamous cell carcinoma [1]. In spite of the extensive advances in the field of research, it is associated with significant mortality and morbidity rates [2]. The risk factors of oral squamous cell carcinoma (OSCC) include tobacco, alcohol, poor nutrition, poor oral hygiene and high risk types of human papillomaviruses [3]. Dysplasia is characterized by disturbed keratinization starting from the basal layer to surface epithelium. It can sometimes be difficult to identify on routine haematoxylin and eosin (H&E) staining and can transform into OSCC if left untreated. Diagnosis at late stages results in high treatment costs along with poor treatment outcomes [4]. This has led to the search of factors with early diagnostic relevance.
Cytokeratins (CK) are a group of the intermediate filament proteins in the epithelium comprising a heterodimer of an acidic and a basic keratin commonly called the keratin pair [5]. In 1982, Moll et al. found that there were 19 subclasses of CK and classified them according to their molecular weights. CK are place specific and may change when the growth rate rises or when the degree of differentiation is altered pathologically [6].
Cytokeratin 13 (CK 13) is an intermediate filament protein which is present in the suprabasal (spinous and upper) cell layers of oral mucosa which due to loss of stratification, is down regulated in squamous dysplasia and squamous carcinoma [7-8]. A total loss of CK 13 in spinous cell layer of carcinoma in situ (CIS) can be considered as an important immunohistochemical (IHC) marker for early diagnosis. The loss of CK 13 depends upon the degree of dysplastic change.
Cytokeratin 17 (CK 17) should also be focused as a diagnostic marker of OSCC, since several studies have reported that the expression of CK 17 could be detected in malignant tissues compared to normal tissues in squamous cell carcinoma of lung [9], cervix, larynx [10] and esophagus.
Most of the cases of OSCC are headed by a pre-invasive stage which sometimes lasts for many years. The epithelium changes from normal, hyperplasia, dysplasia and invasive carcinoma. Sometimes on routine clinical and histopathological examination the initial epithelial alterations may not be easily identifiable. Hence, it is important to differentiate between OED and OSCC as the whole treatment plan of the surgeons and oncologists relies on correct diagnosis.
Materials and Methods
Ethical Approval
Before commencement of this study, an ethical approval was taken from the Institutional Review Board of AFIP (MP-ORP17-16/READ-IRB/18/678).
Sample Collection
In this cross sectional study, a total of 170 cases were retrieved from the record files of Histopathology Department of Armed Forces Institute of Pathology, Rawalpindi, along with their formalin-fixed, paraffin embedded blocks comprising 85 cases of each oral squamous cell carcinoma and oral epithelial dysplasia. Study was carried out over a period of one year from June 2018 to June 2019. Non probability convenience sampling technique was used until the desired sample size was achieved including patients of all ages and both genders while the specimen with poor fixation, scanty or autolyzed tissue were excluded from the study. For each case, demographic and clinical details of the patient were recorded in data collection questionnaire from the histories presented with each case. Consent was taken from all patients. Blocks were trimmed and cut into very thin sections of 5 microns using microtome and mounted on slides. Tissue mounted slides were stained with routine haematoxylin and eosin followed by immunohistochemical staining.
Immunohistochemical Staining
After established diagnosis on H&E, both immunomarkers were applied by using indirect method of IHC. Monoclonal antibody to CK 13 (Clone EP69, Catalogue no: RM0074; Medaysis) and monoclonal antibody to CK 17 (Clone EP98, Catalogue no: RM0077; Medaysis) was used by following the standard protocol of application. Control for both IHC markers was run with each batch. The microscopic and IHC results were also counter checked and verified by the consultant Histopathologist.
Evaluation of Staining
The staining pattern of both immune markers was found to be cytoplasmic. The CK 13 and CK 17 positive cells were defined as distinct yellow to brown staining of the cytoplasm of the cells. Depending upon the intensity of staining of cells, the case was considered as negative or positive.
Statistical Analysis
The information was collected in the form of variables on especially designed data collection questionnaire. Statistical analysis was performed using SPSS software version 24.0. Frequency and percentages were calculated for qualitative variables like gender, CK 13 and CK 17 expression whereas quantitative variables such as age were presented in the form of mean and standard deviation. Chi square test was performed to assess the significance of difference. The P-value of ≤ 0.05 was considered to be significant.
Results
A total of 170 cases were collected for this study. Slides were prepared and stained with H&E and then examined for diagnosis. Of 170 cases, equal number of cases i.e. 85 each of OED and OSCC were included in this study. Pattern of immune markers (i.e. positive or negative) was then analyzed in these cases. In each case, 10 high power fields (HPF) were selected and evaluated for CK 13 and CK 17 expression.
There were 101 (59.4%) male and 69 (40.6%) female patients which corresponds to a male: female ratio of about 1.46:1. In case of OED 46 (54.1%) were male and 39 (45.9%) were female. While in case of OSCC 52 (61.2%) were male and 33 (38.8%) were female. The overall mean age of the patients at which they presented was 60.63 ± 13.814 (mean ± SD) with the youngest of 30 years and the oldest of 95 years. Overall the buccal mucosa was involved in 47 (27.6%) of the cases followed by tongue in 40 (23.5%) of the cases. Buccal mucosa remained the most commonly affected site in case of dysplasia followed by vocal cord. However, in case of OSCC, the most commonly affected site was tongue followed by the buccal mucosa.
In a total of 170 cases, 34 (20%) cases showed positive expression of CK 13 whereas 136 (80%) cases showed negative expression of CK 13. Out of 85 cases of OED, 26 (30.6%) showed positive expression of CK 13 whereas in a total of 85 cases of OSCC, 8 (9.4%) cases showed positive staining of CK 13. The remaining 77 (90.6%) OSCC cases showed near or complete loss of CK 13. Chi Square test was applied to compare the expression of CK 13 with histological diagnosis (Table 1).
| Histological Diagnosis | ||||
| OSCC | OED n (%) | P-Value | ||
| n (%) | ||||
| Expression of CK 13 | Positive | 8 (4.7) | 26 (15.3) | < 0.001* |
| Negative | 77 (45.2) | 59 (34.7) | ||
| Expression of CK 17 | Positive | 74 (43.5) | 59 (34.7) | 0.001* |
| Negative | 11 (6.5) | 26 (15.3) |
*CK, Cytokeratin; OSCC, Oral Squamous Cell Carcinoma; OED, Oral Epithelial Dysplasia; *Significant at 5% level of significance (P<0.05)
P-value of < 0.05 was considered as significant. The statistical estimates and expression of CK 13 with respect to individual grade of OSCC are represented in the Table (Table 2).
| Grades of OSCC | ||||
| WD-OSCC | MD-OSCC n (%) | PD-OSCC n (%) | ||
| n (%) | ||||
| Expression of CK 13 | Positive | 3 (3.5) | 3 (3.5) | 2 (2.6) |
| Negative | 39 (45.9) | 31 (36.5) | 7 (8.0) | |
| Expression of CK 17 | Positive | 38 (44.7) | 30 (35.3) | 6 (7.1) |
| Negative | 4 (4.7) | 4 (4.7) | 3 (3.5) |
*CK, Cytokeratin; OSCC, Oral Squamous Cell Carcinoma; WD, Well Differentiated; MD, Moderately Differentiated; PD, Poorly Differentiated
In a total of 170 cases, 133 (78.2%) cases showed positive expression of CK 17 whereas 37 (21.8%) cases showed negative expression of CK 17. Out of 85 cases of OED, 59 (69.4%) showed positive expression of CK 17 while in total of 85 cases of OSCC, 74 (87.1%) cases showed positive staining of CK 17. The remaining 11 (12.9%) showed near or complete loss of CK 17. The. Chi Square test was applied to compare the expression of CK 17 with histological diagnosis.
P-value of < 0.05 was considered as significant. The statistical estimates and expression of CK 17 with respect to individual grade of OSCC are represented in the Table (Table 2).
The H&E stained slides and the expression of each immuno-marker is also shown in the Figure 1 and 2.
Figure 1: H&E of Oral Squamous Cell Carcinoma (10x).
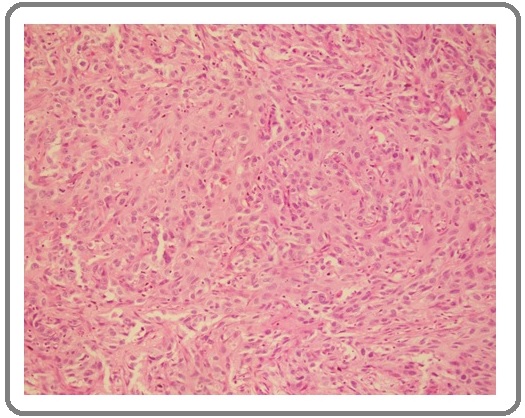
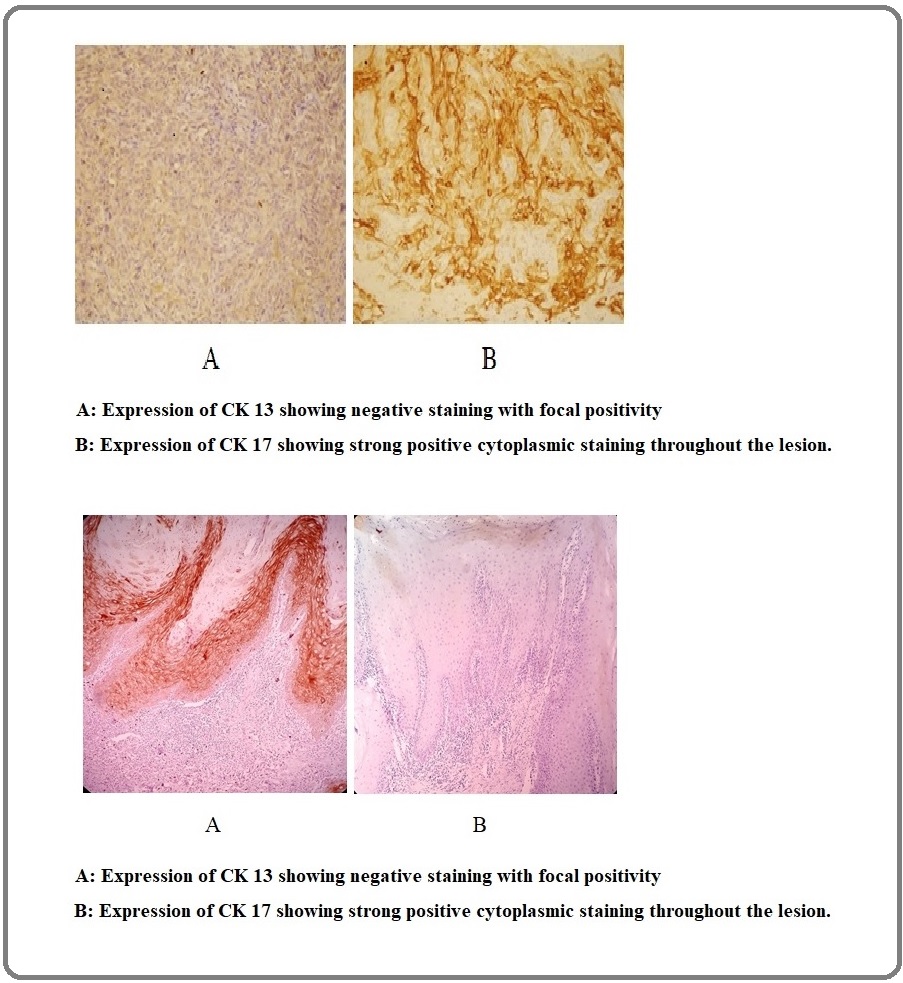
Figure 2: Sections from Oral Mucosal Biopsies Showing Epithelial Dysplasia. A, Expression of CK 13 showing positivity only in the basal epithelial cells (10x); B, Expression of CK 17 showing negative staining (10x).
Discussion
Head and neck cancer is the sixth most common cancer worldwide, out of which the most common histological type is squamous cell carcinoma [1]. It is particularly prevalent in South Asia [11], reaching up to 10% of all cancers in Pakistan and 45% in India [12]. On routine H&E, it is sometimes difficult to differentiate between OSCC and OED and when left untreated OED can transform into OSCC. Oral biopsies are mostly of small size so it becomes extremely difficult to differentiate between both. There are a number IHC markers used to differentiate between OED and OSCC. In our study we assessed the expression of two such IHC markers called CK 13 and CK 17, which can be used in addition to routine H&E staining to assist in differentiating OSCC from OED. CKs are intermediate filament proteins. Loss of CK 13 is reported with loss of stratification in squamous dysplasia and squamous carcinoma while expression of CK17 is reported in malignant tissues compared to normal tissues. Researches have been held out on individual expression of CK 13 and CK 17 in dysplastic and neoplastic oral epithelium and their results are in harmony with our study. In this study, however, we compared the expression of both markers.
In this study, a total number of 170 cases of oral mucosal biopsies were taken in the Department of Histopathology, Armed Forces Institute of Pathology, Rawalpindi. Out of the 170 cases, there were 85 cases each of OSCC and OED. The mean age of the patients at which they presented was 60.63 ± 13.814 (mean ± SD) with the youngest of 30 years and the oldest of 95 years. Many previous studies being conducted in Sweden, Denmark, Norway and Finland showed higher incidence of OSCC in 40 and above ages [13]. Previous studies being conducted in India and Pakistan show the same results.
There were 101 (59.4%) male and 69 (40.6%) female patients which corresponds to a male: female ratio of about 1.46:1, consistent with the previous international and local studies. In current study, the most common site for OSCC was tongue whereas for OED it was buccal mucosa. Previous studies report tongue to be the most commonly affected site, worldwide. However in Asian countries, buccal mucosa is the most commonly affected site followed by tongue [14]. This is attributed to the oral habits of the local population.
In this study, in a total of 170 cases, 34 (20%) cases showed positive expression of CK 13 whereas 136 (80%) cases showed negative expression of CK 13. In contrast to this, out of total 170 cases, 133 (78.2%) cases showed positive expression of CK 17 whereas 37 (21.8%) cases showed negative expression of CK 17. These results are parallel to national and international studies.
The results of the present study are s¡im¡ilar w¡ith the stud¡ies ͻon lͻocal pͻopulat¡iͻon. In a study being conducted in Karachi, in 2015, most of the biopsies labelled as dysplastic showed complete or near complete loss of CK 13 with only few showing focal weak reactivity [15]. Similarly most cases of OSCC showed complete loss of CK13 expression excluding very few cases with minimum focal reactivity in the keratin pearls only. This is in comparison with our study where 80% of all the cases showed near or complete loss of CK 13.
In 2012, a study conducted in Japan by Kitamura and colleagues showed that CK 17 and CK 13 were detected in 101 (96.2 %) and three (2.9 %) of the 105 OSCCs respectively [16]. Furthermore, it stated that there was considerable expression of CK 17 in well differentiated OSCC in comparison to moderately or poorly differentiated OSCC. Similar results are shown in this study where the positive expression of CK 17 and CK 13 is detected in 87.1% and 9.4% of all the OSCC cases. To further validate this, a future study to compare the expression of these markers in individual grades of OSCC keeping the sample size equal within the grades is recommended. The equal number of cases for each grade would help to minimize bias.
In another Japanese study conducted in 2016 induction of keratin 17 was established to be a central feature of OSCC. In 50 biopsy specimens diagnosed as OSCC strong CK 17 expression was noted irrespective of the histological grade [17]. This again, compares with our study.
Sakamoto K et al, conducted a study in 2011 to examine the IHC expression of major keratins including CK 13 in epithelial dysplasia and OSCC [18]. He concluded his study by reporting consistent down-regulation of CK 13 [19]. He stated this was due to changes in the epithelial morphology and dysregulated epithelial differentiated in OED and OSCC.
Yamashina and colleagues conducted a study in 2014 in which they evaluated the morphometry as well as the IHC expression of CK 13 and CK 17. They specifically considered the superficial oral squamous cells in their study. In their study loss of CK 13 was associated with greater cellular atypia, while expression of CK 17 was related to higher-grade cellular atypia [20]. This is similar to current study where greater cellular atypia i.e. transition from increase in degree of dysplasia to invasive carcinoma causes loss of CK 13 and positive expression of CK 17.
Mikami et al, in 2011 conducted a research on “oral borderline malignancies” including OED and OSCC to assess the differential expressions for CK 13 and CK 17. They also included normal oral mucosal biopsies in their study. In normal oral epithelium, none of the biopsies showed CK 17 positivity (0%) in contrast to definite (100%) CK 13 positivity. Same pattern was observed in case of mild to moderate dysplasia. In comparison to this, the cases of severe dysplasia and invasive OSCC, definite (100%) CK 17 positivity was observed. CK 13 positivity was lost with only 7% of the positive cases in OED and 4% in OSCC (7). The results of this study are also parallel to our study which indicate reciprocal relationship of the expressions of CK 13 and CK 17.
The results of these different studies are summarized in the Table (Table 3).
| Study | Area | Year | CK 13 | CK 17 |
| Farrukh et al | Karachi | 2015 | Complete or near loss, few showing focal reactivity | - |
| Kitamura et al | Japan | 2012 | 2.9% positivity in OSCC | 96.2 % positivity in OSCC |
| Mikami et al | Japan | 2011 | 100 % positivity in normal mucosa and mild OED while 4% positivity in OSCC | 100% positivity in severe OED & OSCC while 0% in mild OED and normal mucosa |
| Sakamoto et al | Malaysia | 2011 | Down regulation in OED and OSCC | - |
| Current Study | Pakistan | 2019 | 30.6 % positivity in OED and 9.4 % positivity in OSCC | 69.4 % positivity in OED and 87.1 % positivity in OSCC |
A large sample size can further validate the results of this study. But, because of the time limitation and resources, this study was conducted on an estimated sample size. One future recommendation with respect to this study is to conduct it on a larger sample size and make comparisons within individual grades of OSCC.
In conclusion, this study shows opposite expression of CK 13 and CK 17 in the form of loss of CK 13 and over expression of CK 17 with increase in the degree of dysplasia and invasive carcinoma. With the help of these IHC markers, early diagnosis can be ensured which not only help the clinicians in the assessment of the progression of disease at the time of initial diagnosis but also help in correct treatment planning and early management of the disease.
Acknowledgements
I would express my appreciation for my Institute, Armed Forces Institute of Pathology (AFIP) for providing the excellent research environment and facilities. I am very thankful to the entire staff of Pathology laboratory, AFIP, Rawalpindi who helped me relentlessly from day one.
Source of Funding
No external source of funding was involved in this research. It was partly borne by the institute, National University of Medical Sciences, Rawalpindi and partly by the researcher.
References
- MicroRNAs as new biomarkers for human papilloma virus related head and neck cancers Wang Yuhan, Wang Jie, Huang Yuanshuai. Cancer Biomarkers.2015;15(3). CrossRef
- Analysis of human papilloma virus in oral squamous cell carcinoma using p16: An immunohistochemical study Sanketh DS, Patil S, Rao RS, Amrutha N. Journal of International Society of Preventive and Community Dentistry.2014;4(1). CrossRef
- Global Oral Health Inequalities Challacombe S., Chidzonga M., Glick M., Hodgson T., Magalhães M., Shiboski C., Owotade F., Ranganathan R., Naidoo S.. Advances in Dental Research.2011;23(2). CrossRef
- ‘Oral Cancer and Some Epidemiological Factors : A Hospital Based Study’ Khandekar SP, Bagdey PS, Tiwari RR. Indian Journal of Community Medicine.2006;31(3):157-159.
- Heat shock (stress) proteins and γδ T lymphocytes in oral lichen planus Bramanti Thomas E., Dekker Nusi P., Lozada-Nur Francina, Sauk John J., Regezi Joseph A.. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology.1995;80(6). CrossRef
- The catalog of human cytokeratins: Patterns of expression in normal epithelia, tumors and cultured cells Moll Roland, Franke Werner W., Schiller Dorothea L., Geiger Benjamin, Krepler Reinhard. Cell.1982;31(1). CrossRef
- Emergence of keratin 17 vs. loss of keratin 13: Their reciprocal immunohistochemical profiles in oral carcinoma in situ Mikami Toshihiko, Cheng Jun, Maruyama Satoshi, Kobayashi Takanori, Funayama Akinori, Yamazaki Manabu, Adeola Henry A., Wu Lanyan, Shingaki Susumu, Saito Chikara, Saku Takashi. Oral Oncology.2011;47(6). CrossRef
- ‘Loss of cytokeratin 13 expression in squamous cell carcinoma of the tongue is a possible sign for local recurrence.’ Yanagawa T, et al. . Journal of experimental & clinical cancer research : CR.2007;26(2):215-220.
- Laminin and type VII collagen distribution in different types of human lung carcinoma: correlation with expression of keratins 14, 16, 17 and 18 WETZELS R.H.W., SCHAAFSMA H.E., LEIGH I.M., LANE E.B., TROYANOVSKY S.M., WAGENAAR S.S.C., VOOIJS G.P., RAMAEKERS F.C.S.. Histopathology.1992;20(4). CrossRef
- Cytokeratin-17 as a Potential Marker for Squamous Cell Carcinoma of the Larynx Cohen-Kerem Raanan, Rahat Michal A., Madah Wahid, Greenberg Elhanan, Sabo Edmond, Elmalah Irit. Annals of Otology, Rhinology & Laryngology.2004;113(10). CrossRef
- Current concepts in management of oral cancer – Surgery Shah Jatin P., Gil Ziv. Oral Oncology.2009;45(4-5). CrossRef
- Current Aspects on Oral Squamous Cell Carcinoma Markopoulos Anastasios K. The Open Dentistry Journal.2012;6(1). CrossRef
- Emerging patterns in clinico-pathological spectrum of Oral Cancers Aamir Saadia, Mirza Talat, Mirza M Aamir, Qureshi Masood. Pakistan Journal of Medical Sciences.2013;29(3). CrossRef
- Trends in the epidemiology of oral squamous cell carcinoma in western UP: An institutional study Sharma Preeti, Saxena Susmita, Aggarwal Pooja. Indian Journal of Dental Research.2010;21(3). CrossRef
- Differential Expression of Cytokeratin 13 in Non-Neoplastic, Dysplastic and Neoplastic Oral Mucosa in a High Risk Pakistani Population Farrukh Sanniya, Syed Serajuddaula, Pervez Shahid. Asian Pacific Journal of Cancer Prevention.2015;16(13). CrossRef
- Association of cytokeratin 17 expression with differentiation in oral squamous cell carcinoma Kitamura Ryoji, Toyoshima Takeshi, Tanaka Hideaki, Kawano Shintaro, Kiyosue Takahiro, Matsubara Ryota, Goto Yuichi, Hirano Mitsuhiro, Oobu Kazunari, Nakamura Seiji. Journal of Cancer Research and Clinical Oncology.2012;138(8). CrossRef
- ‘Keratin 17 Is Induced in Oral Cancer and Facilitates Tumor Growth’ Rumana Khanom , Chi Thi Kim Nguyen KK . 2016.
- Down-regulation of keratin 4 and keratin 13 expression in oral squamous cell carcinoma and epithelial dysplasia: a clue for histopathogenesis Sakamoto Kei, Aragaki Tadanobu, Morita Kei-ichi, Kawachi Hiroshi, Kayamori Kou, Nakanishi Shoichi, Omura Ken, Miki Yoshio, Okada Norihiko, Katsube Ken-ichi, Takizawa Toichiro, Yamaguchi Akira. Histopathology.2011;58(4). CrossRef
- Down-regulation of keratin 4 and keratin 13 expression in oral squamous cell carcinoma and epithelial dysplasia: a clue for histopathogenesis Sakamoto Kei, Aragaki Tadanobu, Morita Kei-ichi, Kawachi Hiroshi, Kayamori Kou, Nakanishi Shoichi, Omura Ken, Miki Yoshio, Okada Norihiko, Katsube Ken-ichi, Takizawa Toichiro, Yamaguchi Akira. Histopathology.2011;58(4). CrossRef
- Evaluation of Superficial Oral Squamous Cell Malignancy Based on Morphometry and Immunoexpression of Cytokeratin 13 and Cytokeratin 17 Yamashina Mitsumasa, Sato Kazumichi, Tonogi Morio, Tanaka Yoichi, Yamane Gen-yuki, Katakura Akira. Acta Cytologica.2014;58(1). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2020
Author Details