Diagnostic and Pathologic Value of Programmed Death-Ligand 1 Expression in Colon Carcinoma
Download
Abstract
Objectives: To evaluate the diagnostic value of programmed death-ligand1 expression in colon carcinoma cells and its correlation with different pathological features.
Methods: An immunohistochemical study was conducted on 50 cases diagnosed as colon carcinoma over a one year period from October 2018 to October 2019.The paraffin blocks were received from the Pathology Departments, Faculty of Medicine, Cairo University and Misr University for Science and Technology (MUST). The diagnosis of colon carcinoma for all cases was revised using H&E stain. The immunohistochemical expression of programmed death- ligand1 was assessed using immune reactive score method. The personal data, clinical details and pathological diagnosis were recorded and correlated with programmed death-ligand1 expression.
Results: This study included 50 cases of colon carcinoma. There was a male predominance with female to male ratio 1:1.08.The age of patients was ranged from 30 years to 76 years with mean age 53.3± 11.2 years. The majority of cases (44%) were invasive adenocarcinoma followed by invasive adenocarcinoma with mucoid activity (18%), invasive adenocarcinoma with neuroendocrine differentiation (16%), invasive signet ring carcinoma (14%) and invasive mucinous carcinoma (8%). Immune reactive score for programmed death-ligand1 had a Mean ± Standard Deviation of 8.4 ± 4.2. Strong immune reactive score was represented by 58% of cases, moderate immune reactive score was represented by 24% of cases and only one case (2%) had mild immune reactive score. The remaining 16% of cases were negative. Correlation between immune reactive score of programmed death-ligand1and tumor type, size and lymph-vascular invasion was statistically significant. Cases with marked immune reactive score for programmed death-ligand1 showed highest predilection for invasive adenocarcinoma with neuroendocrine differentiation, large sized tumors and lymph-vascular invasion.
Conclusion: The expression of programmed death-ligand1 using immune reactive score method can estimate the growth of colon carcinoma, invasive adenocarcinoma with neuroendocrine differentiation and lymph-vascular spread. Further large scale studies utilizing programmed death-ligand 1can aid in identifying the prognosis and therapeutic possibilities for patients with colon cancer.
Introduction
Colonowas Colon cancer incidence and mortality rates vary markedly around the world. Globally it is the most frequent malignant disorder of gastrointestinal tract [1]. It is the third most commonly diagnosed malignancy and the fourth leading cause of cancer death [2]. Colon cancer is the 7th commonest cancer in Egypt, representing 3.47% of male cancers and 3% of female cancers [3].
Colon cancer is a disease originating from the epithelial cells lining the colon of the gastrointestinal tract, most frequently as a result of mutations in the wingless-related integration site signaling pathway that increase signaling activity. The mutations can be inherited or acquired and most probably occur in stem cells of the intestinal crypt. The most commonly mutated gene in cancer colon is the adenomatous polyposis coli gene (APC), which produces the APC protein [4]. Programmed death-ligand 1 (PD-L1) is an inhibitory checkpoint molecule that in humans is encoded by the CD274 gene. This trans-membrane protein has been suspected to play a major role in suppressing the immune system during particular events [5]. Programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) is a negative modulatory signaling pathway for activation of T cell. It is recognized that PD- 1/PD-L1 axis plays a crucial role in the progression of tumor by altering status of immune surveillance [6]. The tumor cells will reduce their immunogenicity by expressing PD-L1. Hence, they will not be recognized by the immune system and will evade immune attack [7]. Because immune checkpoints are one of the steps for immune escape and tumor development, inhibition of these molecules with monoclonal antibodies may restore host immune response and, consequently, stop tumor growth and even cause tumor regression [8]. In primary colon cancers, approximately 15% of cases are believed to develop through the serrated neoplasia pathway, showing mucinous and/or poorly differentiated histology, mismatch repair (MMR)-deficiency, mutational B- Raf proto-oncogene (BRAF) activation, and switching off of tumor suppressor gene (CpG) island methylator phenotype. Several studies have identified significant associations between PD-L1 expression and characteristics of serrated neoplasia pathway-driven colon cancers [9]. This study aimed to evaluate the diagnostic value of PD-L1 expression in colon carcinoma cells and its correlation with different pathological features.
Materials and Methods
This study was carried out on 50 cases diagnosed as colon carcinoma in the Pathology Departments, Faculty of Medicine, Cairo University, Kasr El Einy Hospital, Cairo- Egypt and Misr University for Science and Technology (MUST), Giza-Egypt over one year period from October 2018 to October 2019. Available personal data, clinical details and pathological diagnosis of patients were obtained from the histopathology reports. The paraffin blocks of all cases were retrieved and two sections were cut at 5 microns thickness. One slide was stained with Hematoxylin and Eosin for routine histopathological examination. The other was mounted on positively charged slides for immunohistochemical staining. Revision and confirmation of diagnosis for all cases was done and histopathologic types and grades of tumors were reported. Assessment of peri-neural invasion (PNI), lymph- vascular invasion (LVI), and associated adenomatous polyps were revised and recorded. The tumor size (T), lymph node status (N) and metastasis (M) were assessed according to TNM staging system of colon cancer. Modified DUKES staging classification was also applied for all studied cases. The primary antibody used was PD-L1monoclonal rabbit antibody (code no. 901- 3171-081017 ACI 3171 A, C API 3171 A, P DAKO) at dilution 1:1000. PD-L1 expression was assessed at the cell membrane of colon carcinoma cells. The immune reactive score (IRS) was applied for evaluating both the intensity of immunohistochemical staining and proportion of stained cells. The IRS gives a range of 0–12 as a product of multiplication between positive cells proportion score (0–4) and staining intensity score (0–3). Positive cells were quantified as a percentage of the total number of cells, and assigned to one of five categories: 0, no positive cells; 1, <10; 2, 10–50; 3, 51–80; 4, >80%. The staining intensity was sub
classified as 0 (No stain), 1 (mild), 2 (moderate) and 3 (strong). The percentage of positivity of the tumor cells and staining intensity were multiplied to generate the IRS for each tumor specimen. A cut-off of 10% stained tumor cells was chosen to define a positive tumor [10-11].
Statistical analysis: Statistical analysis was conducted using SPSS 23. Descriptive statistics encompassed presentation of categorical data by number and percentage, and continuous data by mean and standard deviation for normally distributed data. Regarding the variables not following normal distribution, the presentation was based on median and range. Normality of continuous variables was tested using Kolmogorov test. Associations between PDL-1 expression and clinico-pathological data of the study cohort were investigated using Mann-Whitney U and Kruskal Wallis tests. Pearson correlation coefficient test was used to estimate the association between age of the patients, size of the tumor and IRS level of PDL-1 expression. The null hypothesis was rejected when P value less than 0.05.
Results
Fifty cases of colonic carcinoma were studied. Male predominance (26 males and 24 female patients) was noticed with a female to male ratio of 1:1.08. The age of patients ranged from 30 year to 76 years with mean age 53.3 ± 11.2 years (Table 1).
| Parameters | Colon cancer patients (n= 50) |
| Age, years (mean ±SD) | 53.3±11.2 |
| Gender, N (%) | |
| Male | 26 (52.0) |
| Female | 24 (48.0) |
The size of tumor ranged from 3 cm to 15 cm in its maximal diameter with median size 7 cm. Concerning the site of the tumors, 26 (52%) cases were located at the ascending colon, 17 (34%) cases were located at the descending colon and 7 (14%) cases were located at sigmoid colon. According to type of tumors, 22 (44%) of cases were invasive adenocarcinoma, 18 (82%) of cases were moderately differentiated grade II and 4 (18%) of cases were poorly differentiated grade III, followed by 9 cases (18%) were invasive adenocarcinoma with mucoid activity, 8 cases (16%) were invasive adenocarcinoma with neuroendocrine differentiation, 7 cases (14%) were invasive signet ring carcinoma, and the remaining 4 (8%) of cases were invasive mucinous carcinoma. Assessment of tumor grade showed grade II in 34 (68%) of cases and 16 (32%) of cases were grade III. PNI was present in 9 (18%) of cases and absent in 41 (82%) of cases. LVI was present in 31 (62%) of cases and absent in 19 (38%) of cases. Associated adenomatous polyps were present in 8 (16%) of cases, while 42 (84%) of cases revealed absent polyps. According to modified Dukes classification, 27 (54%) of cases were class C, 14 (28%) of cases were class B and 9 (18%) of cases were class D (Table 2).
| Parameters | Colon cancer patients (n= 50) |
| Type of tumor: N (%) | |
| Invasive adenocarcinoma | 22 (44.0) |
| Invasive adenocarcinoma with mucoid activity | 9 (18.0) |
| Invasive adenocarcinoma with neuro-endocrine differentiation | 8 (16.0) |
| Invasive signet ring carcinoma | 7 (14.0) |
| Invasive mucinous carcinoma | 4 (8.0) |
| Size tumor, cm, Median (Range) | 7 (3 - 15) |
| Site of tumor: N (%) | |
| Ascending colon | 26 (52.0) |
| Descending colon | 17 (34.0) |
| Sigmoid colon | 7 (14.0) |
| Grade: N (%) | |
| II | 34 (68.0) |
| III | 16 (32.0) |
| Perineural invasion: N (%) | |
| Present | 9 (18.0) |
| Absent | 41 (82.0) |
| Lymph vascular invasion: N (%) | |
| Present | 31 (62.0) |
| Absent | 19 (38.0) |
| Dukes classification: N (%) | |
| B1 | 2 (4.0) |
| B2 | 12 (24.0) |
| C1 | 2 (4.0) |
| C2 | 25 (50.0) |
| D | 9 (18.0) |
| Presence of adenomatous polyp: N (%) | |
| Present | 8 (16.0) |
| Absent | 42 (84%) |
Concerning TNM staging system, 34 (68%) of cases were T3, 12 (24%) of cases were T4 and 4 (8%) of cases were T2. Eighteen (36%) of cases were N0, 17 (34%) of cases were N1 and 15 (30%) of cases were N2. The majority 41(82%) of cases had no metastasis and 9 (18%) of cases had metastasis (Table 3).
| Parameters | Colon cancer patients (n= 50) |
| T stage: N (%) | |
| II | 4 (8.0) |
| III | 34 (68.0) |
| IV a | 7 (14.0) |
| IV b | 5 (10.0) |
| N stage: N (%) | |
| 0 | 18 (36.0) |
| 1A | 4 (8.0) |
| 1B | 9 (18.0) |
| 1C | 4 (8.0) |
| 2A | 5 (10.0) |
| 2B | 10 (20.0) |
| Metastasis: N (%) | |
| Present | 9 (18.0) |
| Absent | 41 (82%) |
As regards to the expression of PD-L1 in tumor cells, the Mean ± SD of IRS was 8.4 ± 4.2. Twenty nine (58%) of cases were strongly positive, 12 (24%) of cases were moderately positive, one (2%) case was mildly positive and the remaining 8 (16%) of cases were negative (Table 4).
| Expression | Colon cancer patients (n= 50) |
| PDL1-intensity: mean (±SD) | 2.3 (±1.0) |
| PDL1-percentage: mean (±SD) | 3.2 (±1.3) |
| IRS: mean (±SD) | 8.4 (±4.2) |
| IRS category: N (%) | |
| Marked | 29 (58.0) |
| Mild | 1 (2.0) |
| Moderate | 12 (24.0) |
| Negative | 8 (16.0) |
As regards to the distribution of IRS of PD-L1 among different tumor types, 29 (58%) of cases were strongly positive IRS (9-12); 12 (24%) of cases were invasive adenocarcinoma, 8 (16%) of cases were adenocarcinoma with neuroendocrine differentiation, 5 (10%) of cases were invasive adenocarcinoma with mucoid activity, 3 (6%) of cases were signet ring carcinoma, and one (2%) case was mucinous carcinoma. Moderate positivity IRS (4-8) was represented by 12 (24%) of cases; 8 (16%) of cases were invasive adenocarcinoma, 2 (4%) of cases were invasive adenocarcinoma with mucoid activity and 2 (4%) of cases were signet ring carcinoma. Mild positivity IRS (2-3) was represented by one case (2%) diagnosed as adenocarcinoma with mucoid activity. 8 (16%) of cases were negative IRS (less than 2); 2 (4%) of cases were invasive adenocarcinoma, 1(2%) case was invasive adenocarcinoma with mucoid activity, 2 (4%) of cases were invasive signet ring carcinoma, and 3 (6%) of cases were invasive mucinous carcinoma (Table 5).
| Type of tumor | IRS " Intensity × Percentage" | |||||||
| Negative (<2) | Mild (2–3) | Moderate (4–8) | Marked (9–12) | |||||
| (n= 8) | (n= 1) | (n= 12) | (n= 29) | |||||
| No. | % | No. | % | No. | % | No. | % | |
| Invasive adenocarcinoma | 2 | 4% | 0 | 0.00% | 8 | 16% | 12 | 24% |
| Invasive adenocarcinoma with mucoid activity | 1 | 2% | 1 | 2% | 2 | 4% | 5 | 10% |
| Invasive adenocarcinoma with neuro-endocrine differentiation | 0 | 0.00% | 0 | 0.00% | 0 | 0.00% | 8 | 16% |
| Invasive signet ring carcinoma | 2 | 4% | 0 | 0.00% | 2 | 4% | 3 | 6% |
| Invasive mucinous carcinoma | 3 | 6.00% | 0 | 0.00% | 0 | 0.00% | 1 | 2% |
The mean value of IRS of PD-L1 in invasive adenocarcinoma (9.6±1.1), invasive adenocarcinoma with mucoid activity (10.0±2), invasive adenocarcinoma with neuro endocrine differentiation (11.6±1.1), invasive signet ring carcinoma (8.0±2), and invasive mucinous carcinoma (3±6). Statistically significant association was found between IRS of PD-L1 and type of tumor, (P value=0.030). Cases of adenocarcinoma with neuroendocrine differentiation showed highest mean value (11.6±1.1). While cases diagnosed as mucinous carcinoma showed lowest mean value (3±6) .Correlation between the size of the tumor and IRS of PD-L1 among the study group showed a statistically significant correlation (P value = 0.025). Cases with strong IRS for PD-L1were associated with large sized tumors. The relation between IRS of PD- L1 and LVI showed a statistically significant correlation, (P value = 0.030). Cases with strong IRS for PD-L1 showed highest predilection for lymph-vascular invasion (Table 7). No statistical significant correlation was found between age, gender, site, grading, PNI, associated adenomatous polyps, modified Dukes staging system or TNM and IRS of PD-L1 in tumor cells (P values = 0.481, 0.523, 0.433, 0.753, 0.440, 0.278, 0.998, 0.162, 0.456 and 0.823 respectively) (Tables 6-7-8).
| Parameters | IRS | P value |
| mean (± SD) | ||
| Age | - | 0.481 |
| Gender | ||
| Male | 8.9 (±3.7) | 0.523 |
| Female | 7.9 (±4.6) |
| Parameters | IRS | P value |
| mean (± SD) | ||
| Size | - | 0.025 |
| Site of tumor | ||
| Ascending colon | 7.7 (±4.4) | 0.433 |
| Descending colon | 9.5 (±3.2) | |
| Sigmoid colon | 8.1 (±5.1) | |
| Grade | ||
| II | 8.3 (±4.0) | 0.753 |
| III | 8.5 (±4.5) | |
| Perineural invasion | 7.4 (±4.7) | 0.44 |
| Lymph vascular invasion | 9.1 (±4.2) | 0.03 |
| Dukes classification | ||
| B1 | 8.0 (±0.0) | 0.456 |
| B2 | 7.2 (±4.1) | |
| C1 | 10.5 (±2.1) | |
| C2 | 9.0 (±4.2) | |
| D | 7.9 (±5.0) | |
| Presence of adenomatous polyp | 9.6 (±4.2) | 0.278 |
| Type of tumor | ||
| Invasive adenocarcinoma | 8.4 (3.2) | 0.03 |
| Invasive adenocarcinoma with mucoid activity | 8.4 (4.4) | |
| Invasive adenocarcinoma with neuro-endocrine differentiation | 11.6 (1.1) | |
| Invasive signet ring carcinoma | 7.6 (5.2) | |
| Invasive mucinous carcinoma | 3.0 (6.0) |
| Parameters | IRS | P value |
| mean (± SD) | ||
| T stage | ||
| II | 9.3 (±1.9) | 0.998 |
| III | 8.4 (±4.2) | |
| IV a | 7.7 (±5.2) | |
| IV b | 8.2 (±4.9) | |
| N stage | ||
| 0 | 6.9 (±3.8) | 0.162 |
| 1A | 7.5 (±5.2) | |
| 1B | 9.4 (±3.8) | |
| 1C | 8.0 (±5.7) | |
| 2A | 9.0 (±4.8) | |
| 2B | 10.1 (±3.8) | |
| M stage | ||
| 0 | 8.5 (±4.0) | 0.823 |
| 1 | 7.9 (±5.0) |
The histopathologic results were summarized in Figures 1- 12.
Figure 1: A case of Invasive Adenocarcinoma Shows Malignant Glands within Desmoplastic Stroma. (H&E x 200).
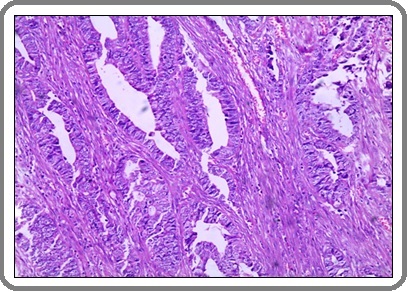
Figure 2: A Case of Invasive Adenocarcinoma Carcinoma with Mucoid Activity Shows Malignant Glands and Mucin Pools within Desmoplastic Stroma. (H&E x 200).
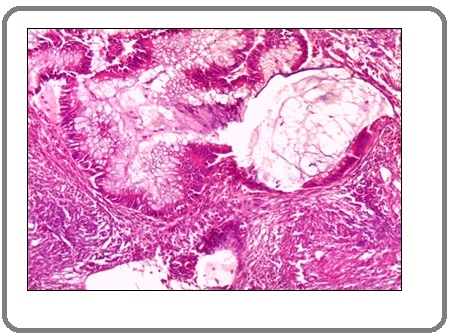
Figure 3:A Case of Invasive Signet Ring Carcinoma Shows Signet Ring Cells within Desmoplastic Stroma. (H&E x 200).
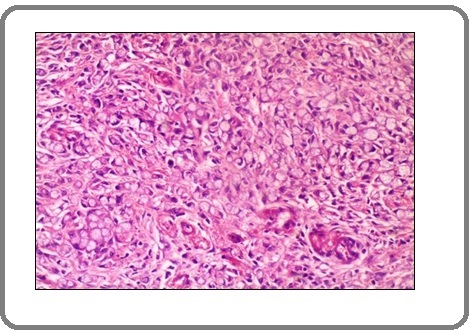
Figure 4:A Case of Invasive Adenocarcinoma Shows mild Positive Membranous Staining for PD-L1 Antibody x100 (IRS=2).
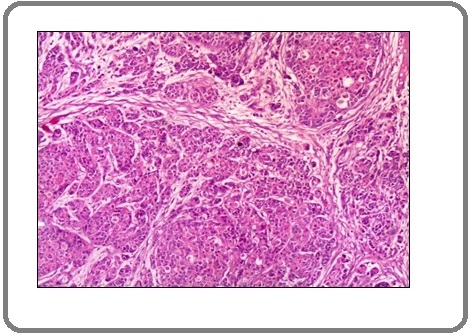
Figure 5: A Case of Invasive Adenocarcinoma Shows Marked Positive Membranous Staining for PD-L1 Antibody x 200 (IRS=12).
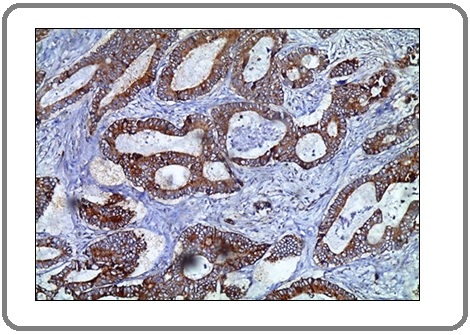
Figure 6:A Case of Invasive Adenocarcinoma Shows Moderate Positive Membranous Staining for PD-L1 Antibody x400 (IRS=8).
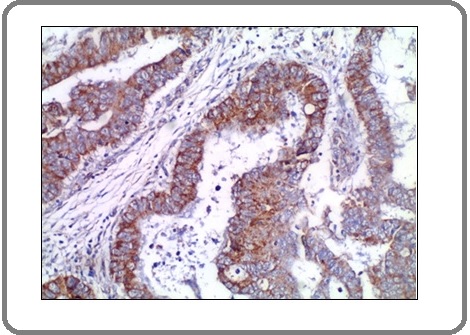
Figure 7: A Case of Invasive Adenocarcinoma with Mucoid Activity Shows Mild Positive Membranous Staining for PD-L1 Antibody x200 (IRS=3).
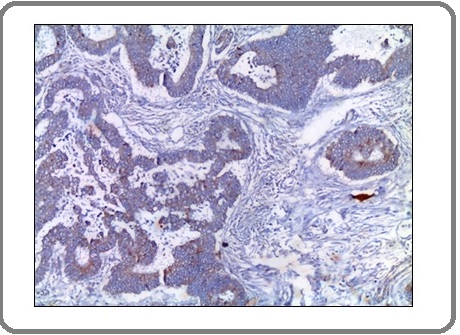
Figure 8: A Case of Invasive Signet Ring Carcinoma Shows Mild Positive Membranous Staining for PD-L1 Antibody x 200 (IRS=2).
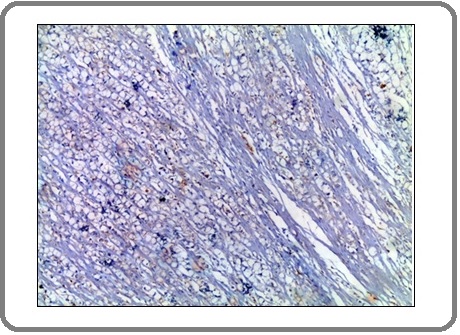
Figure 9: A case of Invasive Signet Ring Carcinoma Shows Moderate Positive Membranous Staining for PD-L1 Antibody x 200 (IRS=6).
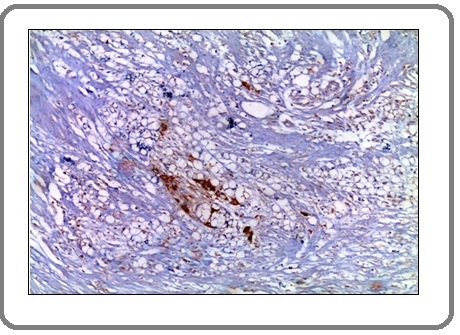
Figure 10: A Case of Invasive Signet Ring Carcinoma Shows Marked Positive Membranous Staining for PD-L1 Antibody x 200 (IRS=12).
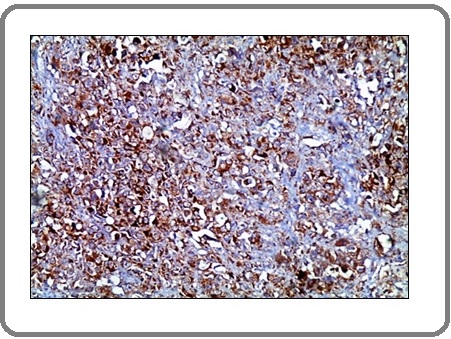
Figure 11: A Case of Invasive Adenocarcinoma with Neuro-endocrine Differentiation Shows Marked Positive Membranous Staining for PD-L1 Antibody x 200 (IRS=12).
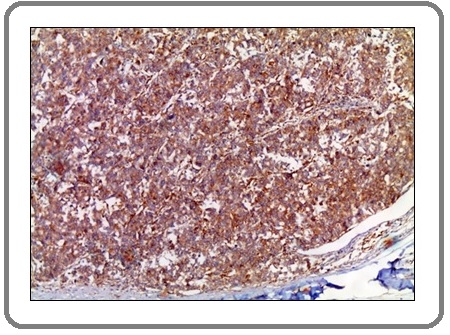
Figure 12: A Case of Invasive Adenocarcinoma with Neuro-endocrine Differentiation Shows Marked Positive Membranous Staining for PD-L1 Aantibody x 200 (IRS=12).
Discussion
In this study, 52% of cases of colon carcinoma were males and 48% were females. The age of studied cases ranged from 30 years to 76 years with mean age 53.3. In parallel study 54% of cases were males and 46% were females and the mean age was 64 [12]. The size of tumor in the current study was ranged from 3 cm to 15 cm in its maximal diameter with median size 7cm. In accordance with our results, a study of Crozier et al., 2007 [13] revealed that the majority of cases have same range of tumor size (4-15 cm). The present study showed that 52% of tumors were located at the ascending colon, 34% of tumors were located at the descending colon and the remaining 14% of tumors were located at sigmoid colon. In agreement with our results, the study of Jung et al., 2017 [14] reported that among the identified patients with colon cancer 33.8% cases had right colon cancer and 66.2% cases had left colon cancer. In this study, 44% of cases had invasive adenocarcinoma, followed by invasive adenocarcinoma with mucoid activity, invasive adenocarcinoma with neuroendocrine differentiation, invasive signet ring carcinoma and invasive mucinous carcinoma represented by ratios of (18%), (16%), (14%) and (8%) respectively. Parallel to our results, a study done by Fleming et al., 2012 [15] revealed that more than 90% of colonic carcinomas were adenocarcinomas. They reported other rare types of colon carcinomas such as neuroendocrine, squamous, adenosquamous, spindle cell and undifferentiated carcinomas. The assessment of PNI and LVI in the current study showed that, the majority of cases (82%) had no PNI, while 62% of cases had LVI. Matching to our results, the study of Esheba 2019 [12] revealed that LVI was absent in 74.1% and while PNI was absent in 84.7%. The TNM staging system in the current study revealed that the majority (68%) of cases was T3, 24% of cases were T4 and only 8% of cases were T2. According to lymph node status, 36% of cases were N0, 34% of cases were N1 and 30% of cases were N2. According to metastasis, the majority of cases (82%) had no metastasis and 18% had metastasis. Similar to our results, the study of Esheba 2019 [12] revealed that T1-T2 were represented by 16.5% of cases, T3-T4 were represented by 83.5% of cases. Lymph node stage was negative in 77.6% of cases and was positive in 22.4% of cases. Metastasis was absent in 87.1% of cases and present in 12.9% of cases. In the present study, 28% of cases were of class B, 54% of cases were of class C and only 18% of cases were of class D of modified Dukes classification. A study of Jung et al., 2017 [14] revealed that the majority (85%) of studied cases was class B and C and the rest 15% of cases were class D. The current study revealed that there was statistically significant association between IRS of PD-L1 and type of the tumor (P value=0.030), where cases of adenocarcinoma with neuroendocrine differentiation showed the highest mean value while cases diagnosed as mucinous carcinoma showed the lowest mean value for IRS. In agreement with our results, a study done by Milione et al., 2017 [16] stated that PD-L1 expression was significantly associated with adenocarcinoma with neuroendocrine differentiation. In the current study, the correlation between the size of the tumor and IRS of PD-L1 in tumor cells was statistically significant (P value = 0.025). Cases with strong IRS for PD-L1were associated with large sized tumors. In accordance to our finding Matthew et al., 2016 [17] detected a correlation between PD-L1 expression and tumor size, where the median tumor size was larger in the PD-L1 positive tumor. On the contrary to our results Li et al., 2019 [18] showed that expression of PD- L1in tumor cells was independent to tumor size. The present study evaluated the correlation between the existence of LVI and IRS of PD-L1. Statistically significant association was detected (P value = 0.030), where cases with strong IRS for PD-L1 showed highest predilection for LVI. Similar finding was reported by Zefeng et al., 2019 [7] who detected that highly expressed PD-L1 was firmly related to lymphatic invasion. On the contrary to our results, Droeser et al., 2013 [19] found that strong PD-L1 expression in tumor cells was associated with early stage, absence of lymph-vascular invasion and absence of lymph node involvement. In this study, there was no statistical significance between IRS of PD-L1 and neither sex, age, site, tumor grade, PIN, associated adenomatous polyps, TNM nor Dukes stage. Keeping with our results, Lianzhou et al., 2019 [20] revealed that the expression of PD-L1 in tumor cells is not related to age, sex, tumor location, tumor differentiation, pT stage or pN stage. In addition, Li et al., 2019 [18] revealed that the expression of PD-L1 was independent of gender, age, tumor stage and metastasis. CONCLUSION: We conclude that the expression of PD-L1 using IRS method can estimate the growth of colon carcinoma, invasive adenocarcinoma with neuroendocrine differentiation and lymph-vascular spread. Further large scale studies utilizing PD-L1can aid in identifying the prognosis and therapeutic possibilities for patients with colon cancer.
References
- Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015 Fitzmaurice Christina, Allen Christine, Barber Ryan M., Barregard Lars, Bhutta Zulfiqar A., Brenner Hermann, et al . JAMA Oncology.2017;3(4). CrossRef
- Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012 Ferlay Jacques, Soerjomataram Isabelle, Dikshit Rajesh, Eser Sultan, Mathers Colin, Rebelo Marise, Parkin Donald Maxwell, Forman David, Bray Freddie. International Journal of Cancer.2014;136(5). CrossRef
- Cancer Incidence in Egypt: Results of the National Population-Based Cancer Registry Program Ibrahim Amal S., Khaled Hussein M., Mikhail Nabiel NH, Baraka Hoda, Kamel Hossam. Journal of Cancer Epidemiology.2014;2014. CrossRef
- WNT Signaling and Colorectal Cancer Schatoff Emma M., Leach Benjamin I., Dow Lukas E.. Current Colorectal Cancer Reports.2017;13(2). CrossRef
- SHP-1 and SHP-2 Associate with Immunoreceptor Tyrosine-Based Switch Motif of Programmed Death 1 upon Primary Human T Cell Stimulation, but Only Receptor Ligation Prevents T Cell Activation Chemnitz Jens M., Parry Richard V., Nichols Kim E., June Carl H., Riley James L.. The Journal of Immunology.2004;173(2). CrossRef
- PD-L1 expression in colorectal cancer defines three subsets of tumor immune microenvironments Valentini Anna Maria, Di Pinto Federica, Cariola Filomena, Guerra Vito, Giannelli Gianluigi, Caruso Maria Lucia, Pirrelli Michele. Oncotarget.2018;9(9). CrossRef
- Clinicopathological and prognostic significance of PD-L1 expression in colorectal cancer: a systematic review and meta-analysis Shen Zefeng, Gu Lihu, Mao Danyi, Chen Manman, Jin Rongjia. World Journal of Surgical Oncology.2019;17(1). CrossRef
- Immunotherapy resistance: the answers lie ahead – not in front – of us Andrews Miles C., Wargo Jennifer A.. Journal for ImmunoTherapy of Cancer.2017;5(1). CrossRef
- Histopathological and genotypic characterization of metastatic colorectal carcinoma with PD-L1 (CD274)-expression: Possible roles of tumour micro environmental factors for CD274 expression Inaguma Shingo, Lasota Jerzy, Felisiak-Golabek Anna, Kowalik Artur, Wang Zengfeng, Zieba Sebastian, Kalisz Joanna, Ikeda Hiroshi, Miettinen Markku. The Journal of Pathology: Clinical Research.2017;3(4). CrossRef
- Recent Findings in the Regulation of Programmed Death Ligand 1 Expression Shen Xiangfeng, Zhang Lihong, Li Jicheng, Li Yulin, Wang Yishu, Xu Zhi-Xiang. Frontiers in Immunology.2019;10. CrossRef
- Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue – a review Fedchenko Nickolay, Reifenrath Janin. Diagnostic Pathology.2014;9(1). CrossRef
- Prognostic Value of Programed Cell Death-1 Ligand Expression in Colorectal Cancer and Its Correlation with Cytotoxic Tumor-Infiltrating Lymphocytes Esheba GE. The Egyptian Journal of Hospital Medicine.2019;76(1):3260-3267.
- Tumor size is associated with the systemic inflammatory response but not survival in patients with primary operable colorectal cancer Crozier Joseph EM, McMillan Donald C, McArdle Colin S, Angerson Wilson J, Anderson John H, Horgan Paul G, McKee Ruth F. Journal of Gastroenterology and Hepatology.2007;22(12). CrossRef
- Is the Location of the Tumor Another Prognostic Factor for Patients With Colon Cancer? Jung Myung-Kyu, Shin Ui Sup, Ki Young-Jun, Kim Yong-Bae, Moon Sun-Mi, Sung Se-Jin. Annals of Coloproctology.2017;33(6). CrossRef
- Colorectal carcinoma: Pathologic aspects Fleming M, Ravula S, Tatishchev S, Wang H. Journal of gastrointestinal oncology.2012;3(3):153-173. CrossRef
- The Clinicopathologic Heterogeneity of Grade 3 Gastroenteropancreatic Neuroendocrine Neoplasms: Morphological Differentiation and Proliferation Identify Different Prognostic Categories Milione Massimo, Maisonneuve Patrick, Spada Francesca, Pellegrinelli Alessio, Spaggiari Paola, Albarello Luca, Pisa Eleonora, Barberis Massimo, Vanoli Alessandro, Buzzoni Roberto, Pusceddu Sara, Concas Laura, Sessa Fausto, Solcia Enrico, Capella Carlo, Fazio Nicola, La Rosa Stefano. Neuroendocrinology.2016;104(1). CrossRef
- PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes Rosenbaum Matthew W, Bledsoe Jacob R, Morales-Oyarvide Vicente, Huynh Tiffany G, Mino-Kenudson Mari. Modern Pathology.2016;29(9). CrossRef
- The Prognostic and Clinicopathological Roles of PD-L1 Expression in Colorectal Cancer: A Systematic Review and Meta-Analysis Li Yan, He Meizhi, Zhou Yaoyao, Yang Chen, Wei Shuyi, Bian Xiaohui, Christopher Odong, Xie Lang. Frontiers in Pharmacology.2019;10. CrossRef
- Clinical impact of programmed cell death ligand 1 expression in colorectal cancer Droeser Raoul A., Hirt Christian, Viehl Carsten T., Frey Daniel M., Nebiker Christian, Huber Xaver, Zlobec Inti, Eppenberger-Castori Serenella, Tzankov Alexander, Rosso Raffaele, Zuber Markus, Muraro Manuele Giuseppe, Amicarella Francesca, Cremonesi Eleonora, Heberer Michael, Iezzi Giandomenica, Lugli Alessandro, Terracciano Luigi, Sconocchia Giuseppe, Oertli Daniel, Spagnoli Giulio C., Tornillo Luigi. European Journal of Cancer.2013;49(9). CrossRef
-
Prognostic and clinicopathological value of PD-L1 in colorectal cancer: a systematic review and meta-analysis
Yang Lianzhou, Xue Rujun, Pan Chunhua. OncoTargets and Therapy.2019;Volume 12. CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2020
Author Details