Characteristics and Survival of 927 Moroccan Adults with Acute Myeloid Leukemia: Monocentric Experience
Download
Abstract
Acute myeloïd leukemia (AML) is the most frequent form of acute leukemia among adults and the most aggressive type of leukemia, which is associated with the lowest survival rate. Patients with AML are treated with intensive chemotherapy and many factors could influence the survival of these patients, such as age, cytogenetic abnormalities; white blood cell (WBC) counts. The aim of this work was to study the epidemiological and response profiles of AML adults patients in Morocco.
Patients and Methods: A prospective, descriptive study conducted in the Hematology and Pediatric Oncology department, 20 August Hospital Casablanca, and concerned adult patients diagnosed with AML through a period of seven years (January 2011 to December 2017). Statistical analysis was performed using SPSS version 20. The overall survival and disease-free survival were evaluated by using the Kaplan–Meier method.
Results: A total of 927 patients diagnosed with AML during the 7 year period. 466 (50.3%) were males and 461 (49.7%) were females. The median age of patients was 46years.The most represented age group was between 18 and 60 years old with a percentage of 83.2%.The FAB subtype M2 occurred most frequently (27%) followed by M1 (24.8%). The cytogenetic study showed that the majority of patients had a normal karyotype. The t (8; 21) was the most detected balanced translocation in our series and the intermediate cytogenetic group was the most represented group (65.4%). A total of 461 patients (53.54%) were treated according to the protocol AML11. The Disease-free survival (DFS) was significantly better for favorable cytogenetic group as compared to other cytogenetic groups (median survival of 41.58 months for the favorable group versus 29.07 months for the adverse group; p-value = 0.02).
Conclusion: The age of AML patients was younger compared to other populations. The majority of patients had a normal karyotype and the commonest balanced translocation was the t (8; 21). Survival was higher in patients with good prognosis.
Introduction
Acute myeloid leukemia (AML) is the most frequent type of acute leukemia among adults and the most aggressive form of leukemia which is associated with the lowest survival rate [1-3]. It is a highly heterogeneous group of hematological disorders that results from the acquisition of chromosomal aberrations and somatic mutations. These abnormalities lead to the accumulation of myeloid precursor cells arrested at early stages of the maturation and differentiation process (myeblasts) [4-5]. In turn, this immature cell accumulation in the bone marrow and blood is responsible for the appearance of insufficiency medullary symptoms such as, anemia,granulocytopenia and thrombocytopenia [6]. The median age of AML at diagnosis (in the late sixties), varied from 63 to 71 years and males are more likely to develop this cancer than females. These results have been reported in developed countries such USA, UK, Canada and Australia [7-12].
AML is a set of diseases with different morphologic, cytochemic, immunophenotypic, cytogenetic, and molecular genetic features. Two classification systems were used to diagnose and classify AML: The first international classification FAB distinguishes eight subtypes of AML based on the morphology of the blast cells [13] and the new classification WHO is based on the immunophenotypic, genetic and cytogenetic characteristics [14]. Cytogenetic abnormalities are described in the most of AML types and implicated in the diagnosis; prognosis and therapeutic response that make karyotype an indispensable examination in evaluating this cancer [15-18]. Immunophenotyping is an essential complement to cytogenetic analysis; it allows to refine the diagnosis of AML or even to modify it [19].
Patients with AML are treated by intensive chemotherapy with high-dose cytarabine, anthracycline or by hypomethylating agents (HMA) [20-22]. Many clinical factors influence the survival of AML patients, such as age, cytogenetic abnormalities, secondary leukemia, complete remission after the first induction and white blood cell (WBC) count [23-24]. For the standard treatment with intensive chemotherapy (anthracycline and cytarabine), complete remission was seen (CR) in 60–80% of younger adults. These frequencies have been found in developing countries such as North America and Western Europe [25-27]. However, in developing countries such as Morocco, there is little published informations about the epidemiology and survival of AML patients and there are big differences among AML patients between countries due to socioeconomic, genetic and environmental factors [28].
The aim of the present work was to study the epidemiological, cytologic, cytogenetic characteristics and response profiles of acute myeloid leukemia patients in Morocco.
Materials and Methods
Study type
This was a prospective, descriptive study conducted at the Hematology and Pediatric Oncology department, 20 August Hospital, University Casablanca, and concerned adultpatients diagnosed with AML through a period of seven years from January 2011 to December 2017. The patients were from different regions of Morocco and classified according to the French American British (FAB) classification. The diagnosis of AML was confirmed by the complete blood count (CBC), bone marrow aspiration, Cytogenetic analysis, immunophenotyping and myeloperoxidase cytochemical analysis (MPO). However; molecular biology analysis was not made in all patients.
Cytogenetics
Cytogenetic analysis was made according to standard techniques with RHG banding and the International System for Human Cytogenetic Nomenclature [29]. The patients were divided into three groups according to cytogenetic features: according to the classification proposed by the Southwest Oncology Group [30].
• Favorable risk group: Consisted of patients with inversion of chromosome 16 (inv 16), with translocation t(8;21)(q22;q22) and patients with translocation t(15;17) (q22; q12-21).
• Unfavorable risk group: Consisted of patients with
chromosomal abnormalities involving the 5, 7, 17 and 3 chromosomes, patients with Complex karyotypes (three or more cytogenetic abnormalities) and patients with 11q23 aberrations other than t (9;11).
• Intermediate Risk group: Patients with normal karyotypes and patients with Chromosomal Abnormalities that do not fall within the other two groups.
AML-MA 2011 protocol
The chemotherapy treated patients were treated according to the AML-MA 2011 protocol with combined induction chemotherapy using cytarabine (cytosine arabinoside) and daunorubicin. According to this treatment protocol, patients received two inductions of daunorubicin and cytarabine for the first one in addition of etoposide for the second one, followed by three consolidation phases with cytarabine plus daunorubicin for the first and the third phases and asparginase for the second phase. Patients with hyperleukocytosis were treated with hydroxyurea for before induction phase
Inclusion and Exclusion Criteria for Chemotherapy Treatment:
•Inclusion Criteria
Patients with age less than or equal to 60 years who
are diagnosed with AML .
Absence of organ dysfunction
• Exclusion criteria
Patients with a confirmed diagnosis aged more than 60 years.
Patients with acute promyelocytic leukemia M3.
Patients with myelodysplastic syndrome (MDS), secondary AML
Remission criteria
After chemotherapy, complete remission (CR) was defined as the absence of circulating blasts, the presence of less than 5% of the blasts in the bone marrow.
Statistical analysis
Statistical analysis was performed with the statistical package for Social Sciences SPSS version 20 (SPSS Inc., Chicago, IL, USA). The values p<0.05 are considered to be significant. The analysis of overall survival (OS) and disease-free survival (DFS) of the patients, survival curves were constructed by the Kaplan–Meier method, using the statistical package SPSS version 16 (SPSS Inc., Chicago, IL, USA). The OS was defined as the interval between the date of diagnosis and the date of death or the date of last follow-up. The DFS was defined as the period between the achievement of complete remission (RC) and relapse or date of death from any cause. The curves of OS and DFS were correlated with cytogenetic. Differences in curves were tested using the log-rank test and p-value < 0.05 being considered statistically significant.
Results
A total of 927 patients were diagnosed with AML between January 2011 and December 2017. 466 (50.3%) were males and 461 (49.7%) were females; with a sex ratio of 1.01. The median age of all patients was 46 years with a minimum age of 18 years and a maximum of 90 years. 771 (83.2%) patients were aged from 18-60 (young-adults) and only 156 patients (16.8%) were aged more than 60 years old (elderly). The white blood cell (WBC) count was less than 50 G/L at diagnosis in 71.8% of patients, ranged between 50 G/L and 100 G/L in 13.1% and greater than 100 G/L in 13.3% of patients. The characteristics of the patients are summarized in Table 1.
| Patients (N=927) | |
| Sex N (%) | |
| Females | 461(49.7) |
| Males | 466 (50.3) |
| M:F ratio | 1.01 |
| Age years (Median) | 46 |
| Range | 18-90 |
| 18-30 | 215 (23.2%) |
| 31-40 | 162 (17.5%) |
| 41-50 | 186 (20.1%) |
| 51-60 | 208 (22.4%) |
| 61-70 | 88 (9.5%) |
| 71-80 | 55 (5.9%) |
| 81-90 | 13 (1.4%) |
| Median WBC (G/L) Median | 17 |
| Range | 0.15-844 |
| <50 | 666(73.6) |
| 50_100 | 119(13.1) |
| >100 | 120 (13.3) |
| FAB classification N (%) | |
| M0 | 32(3.5) |
| M1 | 230(24.8) |
| M2 | 250 (27) |
| M3 | 52 (5.6) |
| M4 | 124 (13.4) |
| M5 | 46(5) |
| M6 | 37 (4) |
| M7 | 3 (0.3) |
| Not classified | 153 (16.5) |
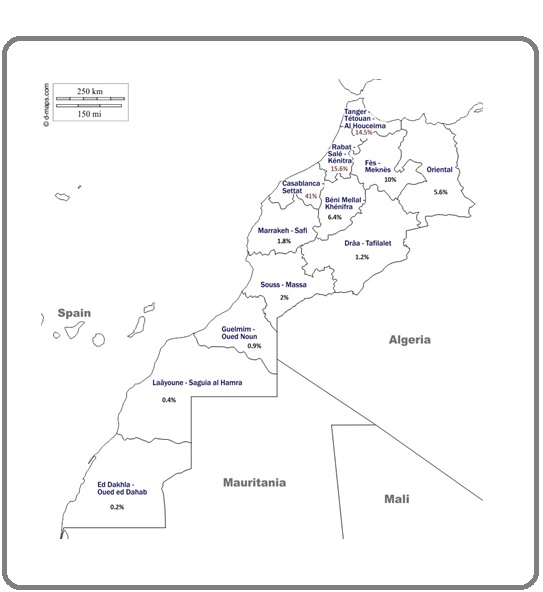
The majority of patients were from the region of Grand Casablanca (41%), 15.6% were from Rabat- sale region and 14.5% were from Tanger-Tetouan region Figure 1.
Figure 1: Map of Morocco Showing the Geographic Distribution of AML Patients.
Regarding the French-American-British (FAB) classification, the M2 subtype was seen in 27% of all patients, followed by M1 (24.8%), and M4 (13.4%) Table1.
85.1% of all patients have benefited from karyotype analysis; the Cytogenetic study revealed that 44% of patients had a normal karyotype, 12% had acomplex karyotype. For the balanced translocations, the t (8; 21) was the most common with a frequency of 8.4%, followed by inv16 (4.7%) and t (15; 17) (3.9%). Trisomy 8 was the most common numerical abnormality in our study(5.3%) Table 2.
| Karyotype | Number of patients | % |
| t (8 ; 21) | 66 | 8,4 |
| t (15 ; 17) | 31 | 3,9 |
| Inv16 | 37 | 4,7 |
| Complex | 95 | 12 |
| Trisomy 8 | 42 | 5,3 |
| Normal | 347 | 44 |
| Trisomy 21 | 10 | 1,3 |
| Trisomy 4 | 4 | 0,5 |
| AnyTrisomy | 26 | 3,3 |
| Monosomy 7 | 21 | 2,7 |
| Monosomy 5 | 7 | 0,9 |
| Monosomy 9 | 3 | 0,4 |
| Any Monosomy | 26 | 3,3 |
| Hyperdiploidy | 15 | 1,9 |
| Monosomy X | 3 | 0,4 |
| Monosomy Y | 7 | 0,9 |
| Del 11 | 19 | 2,4 |
| Inv2 | 1 | 0,1 |
| Inv3 | 1 | 0,1 |
| t (3; 3) | 1 | 0,1 |
| t (3,5) | 1 | 0,1 |
| Der1 | 1 | 0,1 |
| t (11; 3) | 2 | 0,3 |
| t (2,20) | 1 | 0,1 |
| t (3,18) | 1 | 0,1 |
| t (3,21) | 1 | 0,1 |
| t (5,11) | 1 | 0,1 |
| t (5,16) | 1 | 0,1 |
| t (6,11) | 1 | 0,1 |
| t (7,11) | 1 | 0,1 |
| t (8,16) | 1 | 0,1 |
| t (9,11) | 2 | 0,3 |
| t (9,22) | 9 | 1,1 |
| der 11 | 1 | 0,1 |
| t (10,17) | 1 | 0,1 |
| t (11,19) | 1 | 0,1 |
| t (14,15) | 1 | 0,1 |
| Cytogenetic groups | ||
| Favorable | 139 | 17 |
| Intermediaite | 134 | 65,4 |
| Unfavorable | 516 | 17,6 |
| Total | 789 | 100 |
Concerning the cytogenetic groups, the intermediate cytogenetic group was the most represented group (65.4%), followed by unfavorable cytogenetic group (17.6%) and favorable cytogenetic group (17%)Table 2.
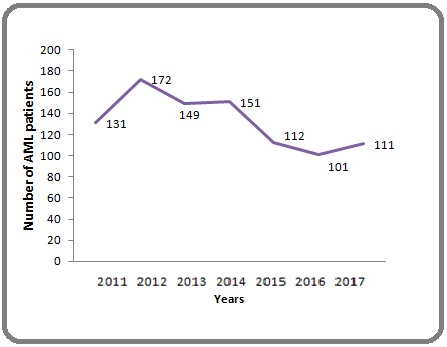
For the annual number of AML patients, the highest annual number was recorded in 2012 (172 cases) and after the 2012 outbreak, the number of AML cases declined Figure 2.
Figure 2: Annual Number of AML Patients.
with cytarabine (cytosine arabinoside) and daunorubicin.
Survival
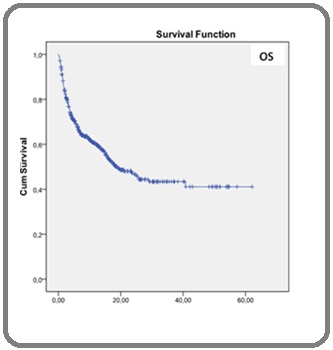
The mean overall survival (OS) was 31 months and the mean free survival (LFS) was 20 months. The 5-year EFS and OS Kaplan-Meier estimate were 19.9% and 41.1%, respectively (Figure 3).
Figure 3: Kaplan-Meier Survival Curves of Overall Survival (OS).
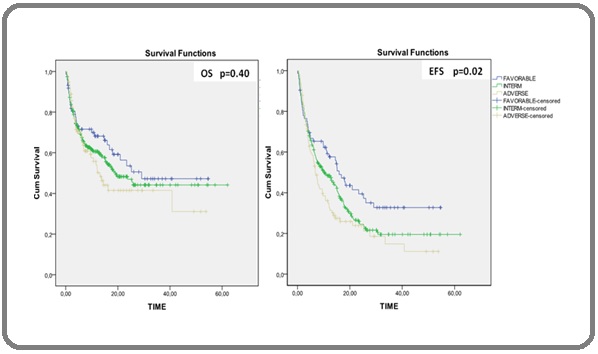
We compared DFS and OS between the different cytogenetic groups, the mean DFS differed significantly between different groups (mean survival of 24.5 months for the favorable group versus 14.83 months for the adverse group; p-value = 0.02). The favorable group had longer EFS and OS than other groups and the adverse cytogenetic group was associated with shorter EFS and OS, but, OS did not differ significantly between these cytogenetic groups (mean OS survival of 31.22 months in the favorable group versus 24.72 months in the adverse group; p-value = 0.40) (Figure 4).
Figure 4: Survival Estimated by Kaplan–Meier Analysis According to Cytogenetic Risk Groups. (A) Overall Survival and (B) Disease-free Survival (DFS).
Discussion
The median age of our patients was 46 years and the extreme range in age was from 18 years to 90 years. This age was similar to that reported by Bekadja et al in the Algerian population [31]. In contrast, this age was lower than that reported in many other studies, which prove the youthful character of the Moroccan AML patients [7-12-23-32-34]. On the other hand study by Sultan et al in the Pakistan population, reported a median age of 37.5 [35] Table 3.
| Study | The current study (Morocco) | Bekadja et al (Algeria) [31] | Sultan et al (Pakistan) [35] | Wahlin et al (Sweden) [32] | Shyshet al (Canada) [33] | Smith et al (UK) [23] | Phekoo et al (England) [34] |
| Number of patients | 927 | 1426 | 125 | 113 | *** | 717 | 507 |
| Sex ratio | 1.01 | 1.16 | 1.5 | *** | 1.25 | 1.25 | 1.53 |
| Age years (Median) | 46 (18-90) | 45 (16-82) | 37.5 (15-85) | 63 (17-91) | 64 (20-64) | 68.7 | 71 (16-98) |
| Most represented age class | 18-60 (83.2%) | *** | <50 years (76%) | *** | *** | ≤55 (52.91%) |
***,Not available
The age between 18 and 60 years was the most represented age bracket in our series (83.2%). In the study of Padilha et al, patients were predominantly younger than 60 years old (81.6%). Similarly, in the study by Sultan et al 76% of all AML patients ( aged between 15-85) were under 50 years old [35-36]. However, according to SEER statistics patients older than age 65 represent approximately 55% of AML cases [37].This disagreement between studies might be explained by the sample size, the recruitment of pediatric patients, differences in demographic characteristics across countries (the pace of population aging, increased life expectancy…..), environmental and genetic factors which could play a crucial role in the appearance of this cancer at a younger age in our AML patients [38-39].The distribution of the population according to sex revealed a slight male predominance; a similar result was reported in most countries [23-31-33-35-40].
The AML Patients come to the Hematology and Pediatric Oncology department, 20 August Hospital (The closest public hospital to Casablanca) from different regions of Morocco and the majority of them were from the most densely populated region of Morocco, Grand Casablanca with a percentage of 39.4%.
In AML, leukocytosis is a major prognostic factor.In our series, 13.3% of patients had WBC counts higher than 100.000/mm3. Our results agreed with previous studies by Xu et al., Viana et al., and Imamura et al. who found that the percentage of patients with WBC counts >100,000/ mm3 is between 12% and 15% [41-43].
In this study, M2 was the most common FAB subtype, which confirmed our previous study results [44]. Many other studies also reported that AML-M2 is the most common subtype among both AML adults and pediatric patients [31-45-46]. However, in the studies of Kakepoto et al and Bennett et al, M4 was the most common FAB subtype [47-48] and M5 was found to be the most predominant subtype in the studies of Mertelsmann et al and van der et al [49-50]. These differences could be caused by variations in the genetic background between populations [47].
The karyotype is an essential examination when evaluating AML; chromosomal abnormalities detected on karyotype are one of the most powerful prognostic factors. 44% of patients had a normal karyotype (NK-AML), this result was in agreement with the reported frequency (40-50%) in the literature [51]. The complex karyotype was seen in 12% of all patients, in the literature, AML patients with complex karyotype account for approximately 10–15% of adult AML, wich is in agreement with our result [52-54]. The t (8; 21) was the most common karyotypic abnormalitieswith a frequency of 8.4%, the frequency of this abnormality varied between 5% and 10% of all AML cases and its incidence decreases with age: it’s the most common among children and young adults not in patients aged more than 60 years old [55-63]. Other authors obtained similar results [58, 59, 64, 65].
The frequency of t (15; 17) was 4%, a similar result was reported by Khoubila et al in Moroccan young AML population [65]. In our series, the inv16 represents 4.7%, this frequency was similar to that reported in a Tunisian population by Gmiddne et al [66]. Trisomy 8 was the most common numerical abnormality in our study with a frequency of 5.3%, a similar result was found by khoubila et al and this frequency was lower than that reported in Tunisian cohort (7%) [65-65].
The frequencies of prognostic groups in our population were 17% for the favorable group, 65.4% for the intermediate group and 17.6% for the adverse group respectively similar results were reported by other authors [48-67, 68]. However, these results were different to the finding of Khoubila et al in Moroccan young AML population (18 to 60 years), who reported that 19.5% had favorable cytogenetics, 68% had intermediate and 12.5% had poor risk,this difference could be explained by the inclusion of patients aged more than 60 years in our study, the decline in the number of the southern Moroccan AML patients, changes in demographic characteristics of our population [65].
In our series, the annual number of AML patients reduced after the 2012 year, due to the construction of health facilities such as the built in 2012 of the new public University Hospital (the Mohammed VI) in Marrakech city in which South Moroccan patients receiving their treatment.
The 5-year overall survival (OS) and event-free survival (EFS) rates were 41.1%, 19.9% respectively. Similarly, other authors reported that, the 5-year survival was less than 50% in adult patients younger than 60 and less than 20% in older patients [4, 69, 70].
In a series of 104 Moroccan AML patients treated according to AML 06/96 protocol, the overall survival at 5 years was 9%, these results were insufficient in comparison with the literature [71], compared to this study, there is a big improvement in our results. However, these improvements would be more satisfactory if we can find positive solutions to the major causes of failure seem to be delayed in diagnosis, early (prior to start of therapy) and induction deaths, induction failures and abandonment of therapy.
Cytogenetics play an important role in the treatment and prognosis of AML. In this work, we observed that patients with poor prognosis and intermediate had worse survival from AML and better survival in patients with good prognosis, which showed that Cytogenetics is an important prognostic factor in AML. Similar to results from other studies [34, 72-76].
In conclusion, the age of AML Moroccan patients was younger compared to other populations; M2 was the commonest FAB subtype of AML among our population followed by M1. The majority of our patients had a normal karyotype. Commonest balanced translocation was the t (8; 21). Survival was better in patients with good prognosis. Further national multicenter studies are needed to confirm these results.
Acknowledgements
Conflicts of Interest
The authors declare no conflicts of interest.
Authors’ contributions:
- Ait Boujmia Oum Kaltoum : Collected and analyzed the data, Wrote the paper.
- Lamchahab Mouna: provided clinical information, evaluation and advice.
- Nezha Hada: performed karyotyping.
- Quessar Asma: supervised the work, discussed the study results and implications and commented on the manuscript at all stages, correted the paper.
References
- Cancer statistics, 2012 Siegel Rebecca, Naishadham Deepa, Jemal Ahmedin. CA: A Cancer Journal for Clinicians.2012;62(1). CrossRef
- Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015 Wang Haidong, Naghavi Mohsen, Allen Christine, Barber Ryan M, Bhutta Zulfiqar A, Carter Austin, Casey Daniel C, et al . The Lancet.2016;388(10053). CrossRef
- Cancer prevalence in the UK: results from the EUROPREVAL study Forman D., Stockton D., Møller H., Quinn M., Babb P., De Angelis R., Micheli A.. Annals of Oncology.2003;14(4). CrossRef
- Acute Myeloid Leukemia Döhner Hartmut, Weisdorf Daniel J., Bloomfield Clara D.. New England Journal of Medicine.2015;373(12). CrossRef
- Acute Myeloid Leukemia Lowenberg Bob, Downing James R., Burnett Alan. New England Journal of Medicine.1999;341(14). CrossRef
- Leukaemia Diagnosis. 3rd ed. Oxford: Blackwell Publishing Bain BJ. Chapter 2, Acute Leukemia: Immunophenotypic, Cytogenetic and molecular genetic analysis in the classification of Acute Leukemia-the EGIL, MIC, MIC-M and WHO Classifications.2003;:p.57-143.
- Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges Shallis Rory M., Wang Rong, Davidoff Amy, Ma Xiaomei, Zeidan Amer M.. Blood Reviews.2019;36. CrossRef
- 8-Acute leukemia incidence and patient survival among children and adults in the United States, 2001-2007 Dores Graça M., Devesa Susan S., Curtis Rochelle E., Linet Martha S., Morton Lindsay M.. Blood.2012;119(1). CrossRef
- Acute myeloid leukaemia in Western Australia 1991-2005: a retrospective population-based study of 898 patients regarding epidemiology, cytogenetics, treatment and outcome Gangatharan S. A., Grove C. S., P'ng S., O'Reilly J., Joske D., Leahy M. F., Threlfall T., Wright M. P.. Internal Medicine Journal.2013;43(8). CrossRef
- Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network Smith A, Howell D, Patmore R, Jack A, Roman E. British Journal of Cancer.2011;105(11). CrossRef
- SEER Cancer statistics review, 1975–2016. Bethesda, MD: National Cancer Institute; 2019https:// seer.cancer.gov/csr/1975_2016/ [based on November 2018 SEER data submission, posted to the SEER web site, April 2019. Accessed 4/18/2019] Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, et al . .
- Data quality in the Danish National Acute Leukemia Registry: a hematological data resource Østgård Lene Sofie Granfeldt, Nørgaard Mette, Nørgaard Jan Maxwell, Severinsen , sengeløv Henrik, Friis Lone Smidstrup, Jensen Morten Krogh, Nielsen Ove Juul. Clinical Epidemiology.2013. CrossRef
- The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes Vardiman James W., Thiele Jüergen, Arber Daniel A., Brunning Richard D., Borowitz Michael J., Porwit Anna, Harris Nancy Lee, et al . Blood.2009;114(5). CrossRef
- The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia Arber Daniel A., Orazi Attilio, Hasserjian Robert, Thiele Jürgen, Borowitz Michael J., Le Beau Michelle M., Bloomfield Clara D., Cazzola Mario, Vardiman James W.. Blood.2016;127(20). CrossRef
- Molecular heterogeneity and prognostic biomarkers in adults with acute myeloid leukemia and normal cytogenetics Marcucci Guido, Mr??zek Krzysztof, Bloomfield Clara D. Current Opinion in Hematology.2005;12(1). CrossRef
- Development of a human acute myeloid leukaemia screening panel and consequent identification of novel gene mutation in FLT3 and CCND3 Smith Matthew L., Arch Rachael, Smith Lan-Lan, Bainton Nigel, Neat Michael, Taylor Claire, Bonnet Dominique, et al . British Journal of Haematology.2005;128(3). CrossRef
- Frontline treatment of acute myeloid leukemia in adults Tamamyan Gevorg, Kadia Tapan, Ravandi Farhad, Borthakur Gautam, Cortes Jorge, Jabbour Elias, Daver Naval, et al . Critical Reviews in Oncology/Hematology.2017;110. CrossRef
- Acute myeloid leukemia in the era of precision medicine: recent advances in diagnostic classification and risk stratification Kansal R. Cancer Biol Med.2016;13(1):41-54. CrossRef
- recent advances in diagnosis of acute myeloid leukaemia:A review Ambayya A. Asian J Multidis Stud.2013;1(5):145-146.
- Acute myeloid leukemia: advancing clinical trials and promising therapeutics Daver Naval, Cortes Jorge, Kantarjian Hagop, Ravandi Farhad. Expert Review of Hematology.2016;9(5). CrossRef
- An update of current treatments for adult acute myeloid leukemia Dombret Hervé, Gardin Claude. Blood.2016;127(1). CrossRef
- New Targeted Agents in Acute Myeloid Leukemia: New Hope on the Rise Bohl Stephan R., Bullinger Lars, Rücker Frank G.. International Journal of Molecular Sciences.2019;20(8). CrossRef
- The changing paradigm of prognostic factors in acute myeloid leukaemia Grimwade David. Best Practice & Research Clinical Haematology.2012;25(4). CrossRef
- Differences in prognostic factors and outcomes in African Americans and whites with acute myeloid leukemia Sekeres M. A.. Blood.2004;103(11). CrossRef
- Anthracycline Dose Intensification in Acute Myeloid Leukemia Fernandez Hugo F., Sun Zhuoxin, Yao Xiaopan, Litzow Mark R., Luger Selina M., Paietta Elisabeth M., Racevskis Janis, et al . New England Journal of Medicine.2009;361(13). CrossRef
- Long-term survival after induction therapy with idarubicin and cytosine arabinoside for de novo acute myeloid leukemia Flasshove M., Meusers P., Schütte J., Noppeney R., Beelen D. W., Sohrab S., Roggenbuck U., et al . Annals of Hematology.2000;79(10). CrossRef
- Addition of cladribine to daunorubicin and cytarabine increases complete remission rate after a single course of induction treatment in acute myeloid leukemia. Multicenter, phase III study Holowiecki J, Grosicki S, Robak T, Kyrcz-Krzemien S, Giebel S, Hellmann A, Skotnicki A, et al . Leukemia.2004;18(5). CrossRef
- Acute myeloid leukemia: Epidemiology and etiology Deschler Barbara, Lübbert Michael. Cancer.2006;107(9). CrossRef
- Use of the International System for Human Cytogenetic Nomenclature (ISCN) Gonzalez Garcia Juan Ramon, Meza-Espinoza Juan Pablo. Blood.2006;108(12). CrossRef
- Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group study Slovak Marilyn L., Kopecky Kenneth J., Cassileth Peter A., Harrington David H., Theil Karl S., Mohamed Anwar, Paietta Elizabeth, et al . Blood.2000;96(13). CrossRef
- A population-based study of the epidemiology and clinical features of adults with acute myeloid leukemia in Algeria: report on behalf of the Algerian Acute Leukemia Study Group Bekadja Mohamed Amine, Hamladji Rose Marie, Belhani Meriem, Ardjoun Fatima Zohra, Abad Mohand Tayeb, Touhami Hadj, Ait-Ali Hocine, Zouaoui Zahia, Sidimansour Noureddine, Hamdi Selma, Grifi Fatiha, Mesli Naima, Saidi Mahdia, Mehalhal Nemra, Bachiri Aissa, Bouhass Rachid, Said Yousuf Mohamed, Midoun Nouri. Hematology/Oncology and Stem Cell Therapy.2011;4(4). CrossRef
- Remission rate and survival in acute myeloid leukemia: Impact of selection and chemotherapy Wahlin Anders, Hörnsten Per, Jonsson Håkan. European Journal of Haematology.2009;46(4). CrossRef
- The incidence of acute myeloid leukemia in Calgary, Alberta, Canada: a retrospective cohort study Shysh Andrea Christine, Nguyen Leonard Tu, Guo Maggie, Vaska Marcus, Naugler Christopher, Rashid-Kolvear Fariborz. BMC Public Health.2017;18(1). CrossRef
- The incidence and outcome of myeloid malignancies in 2,112 adult patients in southeast England Phekoo KJ, Richards MA, Moller H, Schey SA. Haematologica.2006;91(10):1400-1404.
- Demographic and Clinical Characteristics of Adult Acute Myeloid Leukemia - Tertiary Care Experience Sultan Sadia, Zaheer Hasan Abbas, Irfan Syed Mohammed, Ashar Sana. Asian Pacific Journal of Cancer Prevention.2016;17(1). CrossRef
- Acute myeloid leukemia: survival analysis of patients at a university hospital of Paraná Padilha Sergio Lunardon, Souza Emannuely Juliani dos Santos, Matos Marcela Coriolano Cruz, Domino Natália Ramos. Revista Brasileira de Hematologia e Hemoterapia.2015;37(1). CrossRef
- Age and acute myeloid leukemia Appelbaum Frederick R., Gundacker Holly, Head David R., Slovak Marilyn L., Willman Cheryl L., Godwin John E., Anderson Jeanne E., Petersdorf Stephen H.. Blood.2006;107(9). CrossRef
- Recent advances in the study of the Hereditary and environmental basis of childhood leukemia Mizutani S. tnt. J. Hematol.1998;68:131-143. CrossRef
- Dietary and other environmental risk factors in acute leukaemias Kwiatkowski A. European Journal of Cancer Prevention.1993;2(2). CrossRef
- Cancer Incidence in Five Continents Parkin DM WS, Ferlay J, Raymond L, Young J, editors . Lyon, France: IARC Scientific Publications.1997;VII. IARC Scientific Pub(143).
- Long-term outcome of childhood acute myeloid leukemia in a developing country: experience from a children's hospital in China Xu Xiao-Jun, Tang Yong-Min, Song Hua, Yang Shi-Long, Shi Shu-Wen, Wei Jian. Leukemia & Lymphoma.2010;51(12). CrossRef
- Leucemia mielóide aguda na criança: experiência de 15 anos em uma única instituição Viana Marcos B., Cunha Keyla C. C. M. S., Ramos Gilberto, Murao Mitiko. Jornal de Pediatria.2003;79(6). CrossRef
- Outcome in 146 patients with paediatric acute myeloid leukaemia treated according to the AML99 protocol in the period 2003-06 from the Japan Association of Childhood Leukaemia Study Imamura Toshihiko, Iwamoto Shotaro, Kanai Rie, Shimada Akira, Terui Kiminori, Osugi Yuko, Kobayashi Ryoji, Tawa Akio, Kosaka Yoshiyuki, Kato Koji, Hori Hiroki, Horibe Keizo, Oda Megumi, Adachi Souichi. British Journal of Haematology.2012;159(2). CrossRef
- Association of Glutathione S-transferase Genes (M1 and T1) with the Risk of Acute Myeloid Leukemia in a Moroccan Population AitBoujmia OK, Nadifi S, Dehbi H, Kassogue Y, Lamchahab M, Quessar A. Middle East Journal of Cancer.2017;8(1):7-12.
- HAEMATOLOGICAL MANIFESTATIONS AND FREQUENCY OF FAB SUBTYPES IN PATIENTS OF ACUTE MYELOID LEUKAEMIA: A SINGLE CENTER STUDY Muhammad Arif Sadiq , Ghassan Umair Shamshad , Nadir Ali , Eijaz Ghani , Suhaib Ahmed , Ma.ham Arshad . Pakistan Armed Forces Medical Journal.2015;65(5):610-615.
- Acute leukaemia in adults: Morphological profile of 101 patients Asif MJ, Iqbal Z, Iqbal F. Ann King Edward Med Uni.2000;6-4:343-348.
- Long- term outcomes of acute myeloid leukemia in adults in Pakistan Kakepoto GN, Burney IA, Zaki S, Adil SN, Khurshid M. J Pak Med Assoc.2002;52:482-486.
- Eastern Cooperative Oncology Group study of the cytochemistry of adult acute myeloid leukemia by correlation of subtypes with response and survival Bennett JM, Begg CB. Cancer Res.1981;41:4833-4839.
- Morphological classification, response to therapy, and survival in 263 adult patients with acute nonlymphoblastic leukemia Mertelsmann R, Tzvi Thaler H, To L, Gee TS, McKenzie S, Schauer P, Friedman A, Arlin Z, Cirrincione C, Clarkson B. Blood.1980;56(5). CrossRef
- A comparison of surface marker analysis and FAB classification in acute myeloid leukemia van der Reijden HJ, van Rhenen DJ, Lansdorp PM, van't Veer MB, Langenhuijsen MM, Engelfriet CP, von dem Borne AE. Blood.1983;61(3). CrossRef
- A Review of 170 cases of acute leukemias Butt FI, Lodhi Y. Ann King Edward Med Uni .1999;5(1):1-3.
- Clinical relevance of mutations and gene-expression changes in adult acute myeloid leukemia with normal cytogenetics: are we ready for a prognostically prioritized molecular classification? Mrózek Krzysztof, Marcucci Guido, Paschka Peter, Whitman Susan P., Bloomfield Clara D.. Blood.2006;109(2). CrossRef
- TP53 gene mutation is frequent in patients with acute myeloid leukemia and complex karyotype, and is associated with very poor prognosis Bowen D, Groves M J, Burnett A K, Patel Y, Allen C, Green C, Gale R E, Hills R, Linch D C. Leukemia.2008;23(1). CrossRef
- Dismal prognostic value of monosomal karyotype in elderly patients with acute myeloid leukemia: a GOELAMS study of 186 patients with unfavorable cytogenetic abnormalities Perrot Aurore, Luquet Isabelle, Pigneux Arnaud, Mugneret Francine, Delaunay Jacques, Harousseau Jean-Luc, Barin Carole, Cahn Jean-Yves, Guardiola Philippe, Himberlin Chantal, Recher Christian, Vey Norbert, Lioure Bruno, Ojeda-Uribe Mario, Fegueux Nathalie, Berthou Christian, Randriamalala Edouard, Béné Marie C., Ifrah Norbert, Witz Francis. Blood.2011;118(3). CrossRef
- Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials Grimwade David, Hills Robert K., Moorman Anthony V., Walker Helen, Chatters Stephen, Goldstone Anthony H., Wheatley Keith, Harrison Christine J., Burnett Alan K.. Blood.2010;116(3). CrossRef
- Prognostic Impact of Acute Myeloid Leukemia Classification Arber Daniel A., Stein Anthony S., Carter Nora H., Ikle David, Forman Stephen J., Slovak Marilyn L.. American Journal of Clinical Pathology.2003;119(5). CrossRef
- Prognostically Useful Gene-Expression Profiles in Acute Myeloid Leukemia Valk Peter J.M., Verhaak Roel G.W., Beijen M. Antoinette, Erpelinck Claudia A.J., van Doorn-Khosrovani Sahar Barjesteh van Waalwijk, Boer Judith M., Beverloo H. Berna, Moorhouse Michael J., van der Spek Peter J., Löwenberg Bob, Delwel Ruud. New England Journal of Medicine.2004;350(16). CrossRef
- Cytogenetic profile of de novo acute myeloid leukemia: a study based on 1432 patients in a single institution of China Cheng Y, Wang Y, Wang H, Chen Z, Lou J, Xu H, Wang H, Qian W, Meng H, Lin M, Jin J. Leukemia.2009;23(10). CrossRef
- Population-based demographic study of karyotypes in 1709 patients with adult Acute Myeloid Leukemia Sanderson R N, Johnson P R E, Moorman A V, Roman E, Willett E, Taylor P R, Proctor S J, Bown N, Ogston S, Bowen D T. Leukemia.2006;20(3). CrossRef
- Cytogenetic profile in de novo acute myeloid leukemia with FAB subtypes M0, M1, and M2: a study based on 652 cases analyzed with morphology, cytogenetics, and fluorescence in situ hybridization Klaus Mirjam, Haferlach Torsten, Schnittger Susanne, Kern Wolfgang, Hiddemann Wolfgang, Schoch Claudia. Cancer Genetics and Cytogenetics.2004;155(1). CrossRef
- Population-based age-specific incidences of cytogenetic subgroups of acute myeloid leukemia Bacher U, Kern W, Schnittger S, Hiddemann W, Haferlach T, Schoch C. Haematologica.2005;90(11):1502-1510.
- Flt3-mediated signaling in human acute myelogenous leukemia (AML) blasts: a functional characterization of Flt3-ligand effects in AML cell populations with and without genetic Flt3 abnormalities Bruserud Ø1, Hovland R, Wergeland L, Huang TS, Gjertsen BT. Haematologica.2003;88(4):416-428.
- Advances in molecular genetics and treatment of core-binding factor acute myeloid leukemia Mrózek Krzysztof, Marcucci Guido, Paschka Peter, Bloomfield Clara D. Current Opinion in Oncology.2008;20(6). CrossRef
- Comparison of karyotype analysis and RT-PCR for AML1/ETO in 204 unselected patients with AML Mitterbauer M, Kusec R, Schwarzinger I, Haas OA, Lechner K, Jaeger U. Ann Hematol.1998;76:139-143.
- Cytogenetic profile of a representative cohort of young adults with de novo acute myéloblasticleukaemia in Morocco N Khoubila , M Bendari , NHda , M Lamchahab , MQachouh , M Rachid , A Quessar . 2019;238:1-9.
- Cytogenetic profile of a large cohort of Tunisian de novo acute myeloid leukemia Gmidène Abir, Sennana Hlima, Wahchi Ines, Youssef Yosra Ben, Jeddi Ramzi, Elloumi Moez, Saad Ali. Hematology.2012;17(1). CrossRef
- Prognostic Relevance of Integrated Genetic Profiling in Acute Myeloid Leukemia Patel Jay P., Gönen Mithat, Figueroa Maria E., Fernandez Hugo, Sun Zhuoxin, Racevskis Janis, Van Vlierberghe Pieter, Dolgalev Igor, Thomas Sabrena, Aminova Olga, Huberman Kety, Cheng Janice, Viale Agnes, Socci Nicholas D., Heguy Adriana, Cherry Athena, Vance Gail, Higgins Rodney R., Ketterling Rhett P., Gallagher Robert E., Litzow Mark, van den Brink Marcel R.M., Lazarus Hillard M., Rowe Jacob M., Luger Selina, Ferrando Adolfo, Paietta Elisabeth, Tallman Martin S., Melnick Ari, Abdel-Wahab Omar, Levine Ross L.. New England Journal of Medicine.2012;366(12). CrossRef
- Unfavorable-risk cytogenetics in acute myeloid leukemia Hong Wan-Jen, Medeiros Bruno C. Expert Review of Hematology.2011;4(2). CrossRef
- Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet Döhner Hartmut, Estey Elihu H., Amadori Sergio, Appelbaum Frederick R., Büchner Thomas, Burnett Alan K., Dombret Hervé, Fenaux Pierre, Grimwade David, Larson Richard A., Lo-Coco Francesco, Naoe Tomoki, Niederwieser Dietger, Ossenkoppele Gert J., Sanz Miguel A., Sierra Jorge, Tallman Martin S., Löwenberg Bob, Bloomfield Clara D.. Blood.2010;115(3). CrossRef
- Emerging therapeutic drugs for AML Stein Eytan M., Tallman Martin S.. Blood.2016;127(1). CrossRef
- Acute myeloblastic leukemia in adults: evaluation of the AML 06/96 protocol Qachouh M, Quessar A, Harif M, Benchekroun S. Tunis Med.2003;81(7):461-465.
- Risk stratification of intermediate-risk acute myeloid leukemia: integrative analysis of a multitude of gene mutation and gene expression markers Rockova Veronika, Abbas Saman, Wouters Bas J., Erpelinck Claudia A. J., Beverloo H. Berna, Delwel Ruud, van Putten Wim L. J., Löwenberg Bob, Valk Peter J. M.. Blood.2011;118(4). CrossRef
- Impact of Health Care Reform Legislation on Uninsured and Medicaid-Insured Cancer Patients Virgo Katherine S., Burkhardt Elizabeth A., Cokkinides Vilma E., Ward Elizabeth M.. The Cancer Journal.2010;16(6). CrossRef
- Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry Juliusson Gunnar, Antunovic Petar, Derolf Åsa, Lehmann Sören, Möllgård Lars, Stockelberg Dick, Tidefelt Ulf, Wahlin Anders, Höglund Martin. Blood.2009;113(18). CrossRef
- Cytogenetics' impact on the prognosis of acute myeloid leukemia Gupta Monika, Mahapatra Manoranjan, Saxena Renu. Journal of Laboratory Physicians.2019;11(02). CrossRef
- High-Dose Daunorubicin in Older Patients with Acute Myeloid Leukemia Löwenberg Bob, Ossenkoppele Gert J., van Putten Wim, Schouten Harry C., Graux Carlos, Ferrant Augustin, Sonneveld Pieter, Maertens Johan, Jongen-Lavrencic Mojca, von Lilienfeld-Toal Marie, Biemond Bart J., Vellenga Edo, Kooy Marinus van Marwijk, Verdonck Leo F., Beck Joachim, Döhner Hartmut, Gratwohl Alois, Pabst Thomas, Verhoef Gregor. New England Journal of Medicine.2009;361(13). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2021
Author Details