C-MYC Protein Expression and High Ki-67 Proliferative Index are Predictives of Disease Relapse in Diffuse Large B Cell Lymphoma
Download
Abstract
Objective: To evaluate the status of C-MYC protein expression and Ki-67 proliferative index and to clarify their role in predicting relapse of diffuse large B cell lymphoma (DLBL).
Materials and Methods: A retrospective study conducted on 50 cases diagnosed as DLBL in a 3 years’ time period from January 2014 till December 2016, collected from the archive of Pathology Departments of the National Cancer Institute Cairo - Egypt, Misr University for Science and Technology and private labs of authors. The diagnosis of DLBL for all cases, both nodal and extranodal, was confirmed by histopathologic examination and immunophenotyping. Automated immunohistochemical staining using antibodies against C-MYC protein and MIB-1 was used to evaluate the C-MYC expression in tumor cells and to assess their proliferative ability by calculating Ki-67 labelling index. The relation between the percentage of C-MYC protein expression, Ki-67 proliferative index, clinical data and the relapse status during the follow up period were analyzed.
Results: A total of 50 cases of DLBL in both nodal and extra-nodal sites were included. Twenty-three cases (46%) were expressing the C-MYC protein, and 29 cases (58%) showed high Ki-67 proliferative index. Twenty-two cases (44%) relapsed during the follow-up period. Positive C-MYC protein expression was significantly associated with high Ki-67 proliferative index. C-MYC protein expression and high Ki-67 proliferative index were independently associated with disease relapses in 81.8% and 86.4% of cases respectively. Cases with combined C-MYC protein expression and high Ki-67 proliferative index showed statistical prediction of relapse in 81.8% of cases.
Conclusion: C-MYC protein expression and high Ki-67 proliferative index were independently associated with relapse of diffuse large B cell lymphoma. Furthermore, the combined positive C-MYC protein expression and high Ki-67 proliferative index is better than a single positive test in predicting relapses among DLBL patients.
Introduction
C-MYC is one of the most important transcriptional proto-oncogene involved in human carcinogenesis and is the most common mutated gene in lymphomas. This proto-oncogene is regulating a wide range of cellular functions including proliferation, growth and apoptosis [1]. C-MYC oncogene functions as a universal enhancer of already expressed genes in cells rather than directly activating silent genes [2]. Rearrangement of C-MYC gene is crucial in the pathogenesis of B cell lymphomas, but it is rarely reported in T cell lymphomas. The dysregulated gene in B cell lymphoma occurs either as a primary event in Burkitt lymphoma (BL) or secondary event in aggressive lymphomas such as DLBCL [1]. Under normal physiological conditions, C-MYC protein level is low and insufficient to promote cellular proliferation. The tumor transforming activity of C-MYC is also opposed by its ability to induce apoptosis [3]. Loss of gene regulation induces lymphomas by over expression of intact C-MYC protein that transforms cells through three main mechanisms such as insertional mutagenesis, gene amplification and chromosomal translocation [4]. Secondary C-MYC gene rearrangement in low grade B-cell lymphomas usually results in transformation into highly aggressive lymphomas with decreased survival rates. The gene changes in DLBCL most often confer an aggressive clinical behavior and chemotherapy resistance [1-6]. The adverse prognosis associated with C-MYC gene rearrangement is largely derived from concurrent BCL2 or BCL6 gene rearrangement [7]. DLBCL with C-MYC and either BCL2 or BCL6 rearrangements are subsequently termed double-hit lymphomas, while those with all three rearrangements are referred to as triple-hit lymphomas. Double-hit lymphoma is currently classified as high grade B cell lymphoma [8]. The detection of C-MYC gene translocation and C-MYC protein expression has become essential in the clinical diagnosis and prognosis of aggressive B cell lymphoma. Conventional karyotyping and fluorescence in situ hybridization (FISH) are the two techniques clinically available for the detection of C-MYC gene abnormalities [9]. Immunohistochemical (IHC) detection of C-MYC protein expression does not correspond to C-MY C gene rearrangement. However, there is a correlation between C-MYC protein expression immunohistochemically and its gene translocation in aggressive B cell lymphoma, as over expression of C-MYC protein suggests its gene translocation [10-11]. DLBCL with high level of C-MYC protein expression are likely double-hit lymphomas [8]. IHC staining for detection of C-MYC protein expression is important for prognosis in DLBCL. High level C-MYC protein expression is associated with inferior overall survival irrespective of BCL2 expression [6]. Double expressor lymphoma is a DLBCL that exhibits over expression of both C-MYC and BCL2 proteins. It is associated with worse clinical outcome than other DLBCL [12-13]. Unregulated C-MYC expression promotes cell proliferation, but also induces apoptosis in p53-dependent and p53-independent pathways [14]. Ki-67 is a nuclear non-histone protein that is synthesized at the beginning of cell proliferation [15]. Ki-67 expression has been widely used in clinical practice as an index to evaluate the proliferative activity of lymphoma cells. High Ki-67 proliferative index (PI) is highly associated with worse survival for Non Hodgkin lymphoma [16]. In DLBCL, Ki-67 can distinguish patients with a good and bad prognosis when combined with other prognostic factors [17].
This study aimed to evaluate the status of C-MYC protein expression and Ki-67 PI and clarify their role in predicting relapse of DLBCL using immunohistochemical study.
Materials and Methods
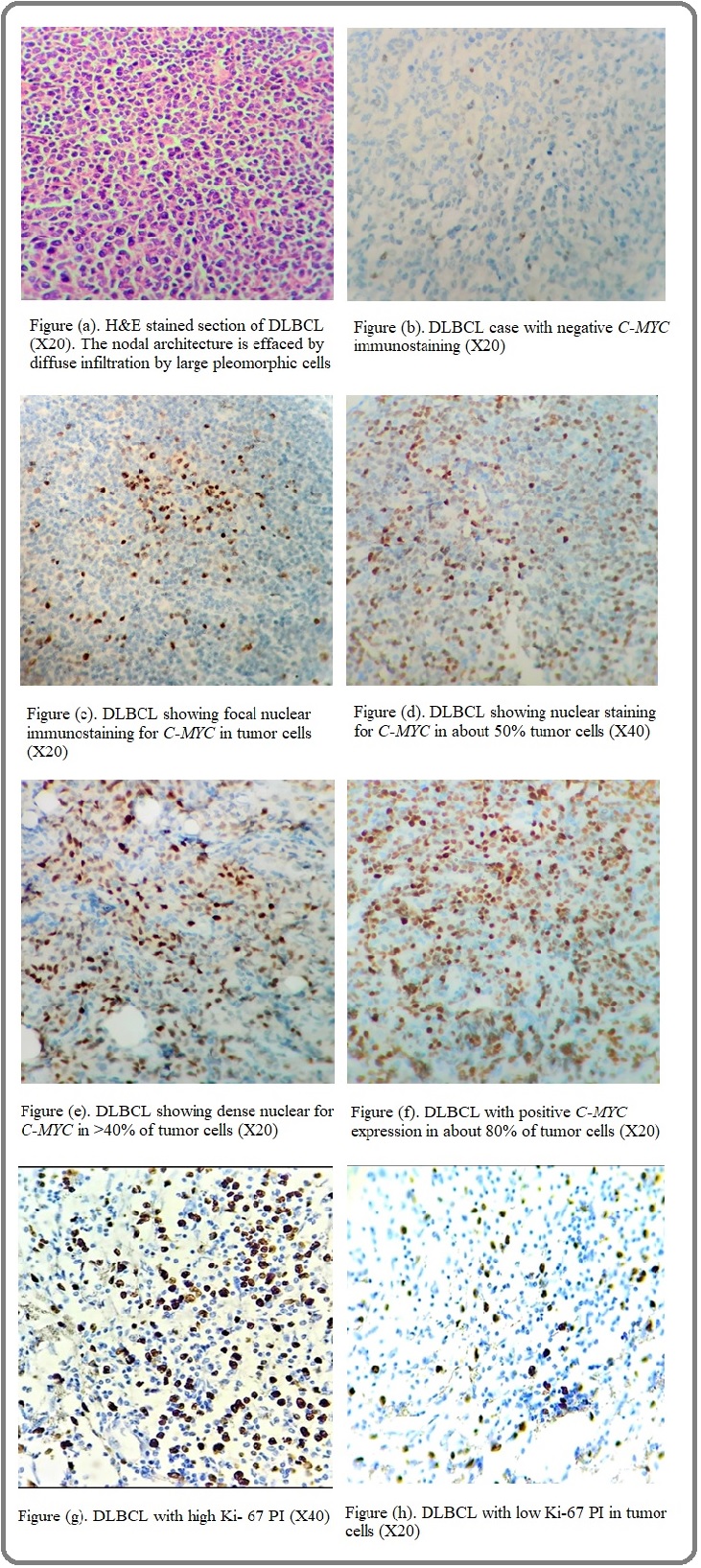
This study was carried out on 50 cases of DLBL diagnosed in the time period from January 2014 till December 2016 in the Pathology Departments of the National Cancer Institute (NCI) Cairo University- Cairo- Egypt, Misr University for Science and Technology (MUST) - Giza- Egypt and private labs of authors, with available follow-up data for the following 3 years from 2015 till the end of year 2017. Approval for this study was obtained from the Medical Ethics Committee of NCI and MUST University. Tissue specimens were preserved in 10% neutral buffered formalin, embedded in paraffin, cut in 4 μm thick tissue sections and stained with H&E stain for conventional histopathologic examination. The diagnosis of DLBL for all cases was confirmed by histologic examination and immunophenotyping using pan B (CD20) and pan T (CD3) markers. All cases revealed diffuse positivity for CD20, while CD3 highlighted only small reactive T lymphocytes. Immunohistochemical analysis was performed using automated Ventana GX immunostainer. The used antibody is C-MYC (EP121) rabbit monoclonal antibody of Cell Marque Tissue Diagnostics, predilute 7 ml vial. C-MYC protein expression was assessed as dense nuclear staining of tumor cells and was judged as positive if the number of stained nuclei is equal to or more than 40% [11]. The used clone for Ki-67 was anti-Ki-67 (30-9) rabbit monoclonal primary antibody from ROCHE Diagnostics. The estimation of Ki-67 PI was done by calculating the percentage of positive tumor nuclei in 1000 tumor cells. The cut-off value of high Ki-67 positivity was set to ≥ 70% [17]. Clinical data as age and gender of the patients were recorded. Excision and core biopsies were obtained from various nodal and extranodal sites, including cervical (n=21), axillary (n=7), inguinal (n=5), spleen (n=4), liver (n=3), colon (n=2), retroperitoneal (n=2), lacrimal gland (n=1), mediastinum (n=1), breast (n=1), supraclavicular (n=1), nasopharyngeal (n=1) and oropharyngeal (n=1) regions. Follow up for all patients included in the study were conducted for the following 3 years and relapsed cases were recorded. The relation between the results of IHC expression of C-MYC protein, Ki-67 PI, clinical data and relapse status during the follow up period were analyzed.
Statistical Analysis
All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS version 25.0; IBM Corporation, Armonk, NY, USA). Demographic and clinical characteristics of the studied patients were presented as frequencies and percentages (%) or mean ± standard deviation (SD). Association between categorical variables were tested for statistical analysis by Chi-square test or Fisher’s exact test (if >20% of expected values were less than 5). Shapiro-Wilk’s test was used to test for data normality. Difference in mean age (a continuous variable) between binary categorical variables was tested for statistical significance by independent-samples t-test. Multivariate analysis with logistic regression was performed to calculate the age- and sex-adjusted odds ratio of combined C-MYC and Ki-67 for predicting relapses. A p-value <0.05 was considered statistically significant.
Results
This study included a total of 50 cases of DLBCL with available follow-up data. Nodal and extra-nodal biopsies were taken from 25 male and 25 female patients. The age of the patients ranged from 4 to 80 years, with a mean age of 56.7± 15 years. The most common biopsy sites were cervical, axillary, and inguinal lymph nodes (42%, 14%, and 10%, respectively). Submitted tissue materials were either core biopsies (56%) or excision biopsies (44%). The Ki-67 PI ranged from 25 to 99 with a mean of 62.40 ± 25.93. Twenty-three cases (46%) had positive C-MYC protein expression, and 29 cases (58%) had high Ki-67 PI (Figure 1).
Figure 1: Histopathological and Immunohistochemical Results.
Twenty-two cases (44%) showed disease relapse during the three years follow-up period (Table 1).
| Age (years) | Mean ± SD (Range) | 56.68 ± 14.99 (4 – 80) |
| Sex, No. (%) | Male | 25 (50.0%) |
| Female | 25 (50.0%) | |
| Site of biopsies, No. (%) | Cervical | 21 (42%) |
| Axillary | 7 (14%) | |
| Inguinal | 5 (10%) | |
| Spleen | 4 (8%) | |
| Liver | 3 (6%) | |
| Colon | 2 (4%) | |
| Retroperitoneal | 2 (4%) | |
| Breast | 1 (2%) | |
| Lacrimal gland | 1 (2%) | |
| Mediastinal | 1 (2%) | |
| Nasopharynx | 1 (2%) | |
| Oropharyngeal | 1 (2%) | |
| Supraclavicular | 1 (2%) | |
| Type of Biopsy, No. (%) | Core | 28 (56.0%) |
| Excision | 22 (44.0%) | |
| C-MYC Protein Expression, No. (%) | Negative | 27 (54.0%) |
| Positive | 23 (46.0%) | |
| Ki-67 Proliferative Index (PI) | Mean ± SD (Range) | 62.40 ± 25.93 (25 – 99) |
| Low | 21 (42.0%) | |
| High | 29 (58.0%) | |
| Relapse on Follow-up | No | 28 (56.0%) |
| Yes | 22 (44.0%) |
Positive C-MYC protein expression was significantly associated with high Ki-67 PI. All 23 cases with positive C-MYC protein expression had high Ki-67 PI, while 22.2% of cases with negative C-MYC protein expression had high Ki-67 PI (Table 2).
| Measured Parameters | C-MYC Expression | Sig | Ki67 PI | Sig | |||
| Negative | Positive | Low | High | ||||
| Age (years) | Mean ± SD (Range) | 54.0 ± 16.5 | 59.9 ± 12.7 | ||||
| (4 – 80) | (33 – 84) | 0.167 | 53.0 ± 17.6 | 59.3 ± 12.5 | 0.147 | ||
| Sex | Female | 11 (40.7%) | 14 (60.9%) | (4 – 80) | (33 – 84) | ||
| Male | 16 (59.3%) | 9 (39.1%) | 0.156 | 9 (42.9%) | 16 (55.2%) | 0.39 | |
| Site of biopsy | Axillary | 3 (11.1%) | 4 (17.4%) | 12 (57.1%) | 13 (44.8%) | ||
| Breast | 1 (3.7%) | 0 | 3 (14.3%) | 4 (13.8%) | |||
| Cervical | 9 (33.3%) | 12 (52.2%) | 1 (4.8%) | 0 | |||
| Colon | 2 (7.4%) | 0 | 6 (28.6%) | 15 (51.7%) | |||
| Inguinal | 1 (3.7%) | 4 (17.4%) | 2 (9.5%) | 0 | |||
| Lacrimal Gland | 1 (3.7%) | 0 | 0 | 5 (17.2%) | |||
| Liver | 2 (7.4%) | 1 (4.3%) | 0 | 1 (3.4%) | |||
| Mediastinal | 1 (3.7%) | 0 | 1 (4.8%) | 2 (6.9%) | |||
| Nasopharynx | 1 (3.7%) | 0 | 1 (4.8%) | 0 | |||
| Oropharyngeal | 1 (3.7%) | 0 | 1 (4.8%) | 0 | |||
| Retroperitoneal | 1 (3.7%) | 1 (4.3%) | 1 (4.8%) | 0 | |||
| Spleen | 4 (14.8%) | 0 | 1 (4.8%) | 1 (3.4%) | |||
| Supraclavicular | 0 | 1 (4.3%) | 4 (19.0%) | 0 | |||
| Type of biopsy | Core | 13 (48.1%) | 15 (65.2%) | 0 | 1 (3.4%) | ||
| Excision | 14 (51.9%) | 8 (34.8%) | 10 (47.6%) | 18 (62.1%) | |||
| Ki67 PI | Low | 21 (77.8%) | 0 | 11 (52.4%) | 11 (37.9%) | ||
| High | 6 (22.2%) | 23 (100.0) | <0.001* |
*. Statistically significant at 0.05 level of significance
Markers high expression and disease relapse have no significant relationship with both patients’ ages and sexes. C-MYC protein expression and high Ki-67 PI were independently associated (significantly associated) with disease relapse in 81.8% and 86.4% respectively. Further, 81.8% of all relapsed patients had combined positive C-MYC protein expression and high Ki-67 PI (Table 3).
| Measured Parameters | Follow-up | Sig. | ||
| Non Relapsing | Relapsing | |||
| (n = 28) | (n = 22) | |||
| Age (years) | Mean ± SD (Range) | 54.5 ± 16.2 | 59.5 ± 13.2 | 0.242 |
| (4 – 80) | (33 – 84) | |||
| Sex | Female | 12 (42.7%) | 13 (59.%) | 0.254 |
| Male | 16 (57.1%) | 9 (40.9%) | ||
| Site of biopsy | Axillary | 6 (21.4%) | 1 (4.5%) | |
| Breast | 1 (3.6%) | 0 | ||
| Cervical | 9 (32.1%) | 12 (54.5%) | ||
| Colon | 2 (7.1%) | 0 | ||
| Inguinal | 1 (3.6%) | 4 (18.2%) | ||
| Lacrimal Gland | 1 (3.6%) | 0 | ||
| Liver | 2 (7.1%) | 1 (4.5%) | ||
| Mediastinal | 1 (3.6%) | 0 | ||
| Nasopharynx | 1 (3.6%) | 0 | ||
| Oropharyngeal | 1 (3.6%) | 0 | ||
| Retroperitoneal | 1 (3.6%) | 1 (4.5%) | ||
| Spleen | 2 (7.1%) | 2 (9.1%) | ||
| Supraclavicular | 0 | 1 (4.5%) | ||
| Type of biopsy | Core | 15 (53.6%) | 13 (59.1%) | |
| Excision | 13 (46.4%) | 9 (40.9%) | ||
| C-MYC Expression | Negative | 23 (82.1%) | 4 (18.2%) | <0.001* |
| Positive | 5 (17.9%) | 18 (81.8%) | ||
| Ki67 PI | Low | 18 (64.3%) | 3 (13.6%) | <0.001* |
| High | 10 (35.7%) | 19 (86.4%) | ||
| Combined C-MYC & Ki67 PI status | Negative & Low | 18 (64.3%) | 3 (13.6%) | <0.001* |
| Positive or High | 5 (17.9%) | 1 (4.5%) | ||
| Positive & High | 5 (17.9%) | 18 (81.8%) |
*. Statistically significant at 0.05 level of significance
Multivariate analysis revealed that patients with combined positive C-MYC protein expression and high Ki-67 PI were 19.6 times more likely to have disease relapse when compared to patients with combined negative C-MYC protein expression and low Ki-67 PI; and 16.2 times when compared to patients with either positive C-MYC protein expression alone or high Ki-67 PI alone. In contrast, patients with either positive C-MYC protein expression or high Ki-67 PI alone were only 1.17 times more likely to have relapses when compared to patients with combined negative C-MYC protein expression and low Ki-67 PI.
Discussion
DLBCL is a mature B-cell neoplasm with diffuse growth pattern and various immune phenotypes and clinical prognosis. DLBCL accounts for approximately 25–30% of non-Hodgkin lymphoma in Western countries [18]. In Egypt, lymphoma is considered the most common hematologic malignancy and accounts for about 8.4% of all new cancer cases diagnosed annually [19]. The role of C-MYC oncogene in the subtypes of B-cell lymphomas was clarified by many previous studies. In Burkitt’s lymphoma, the translocation of C-MYC at 14q32 is the most common, and occurring in approximately 80% of cases [20]. In plasmablastic lymphoma C-MYC rearrangements, most commonly t (8; 14) (q24·1; q32) are found in approximately 49% of cases [21]. On the other hand Kawasaki et al., identified C-MYC gene rearrangement in 5–15% of cases in DLBCL [22]. Previous researches reported that there is a correlation between C-MYC protein expression and its gene translocation in aggressive B cell lymphoma and that over expression of C-MYC protein suggests its gene translocation [10-11].
In this study, C-MYC protein expression and Ki-67 PI were not significantly associated with specific patient’s age and sex. These results were in concordance of those reported by Yun et al., who examined 26 males and 16 females with an average age of 58.9 ± 12.3. Specimens were from various sources such as superficial lymph nodes, subcutaneous soft tissue, gastrointestinal tract, mesentery, spleen, and tonsil. Statistical analysis showed that, there was no significant difference in the expression of C-MYC protein related to age or gender of patients [11].
In this study, the cut-off value of Ki-67 positivity was set to be ≥ 70% .The Ki-67 PI ranged from 25 to 99 with a mean of 62.40 ± 25.93. Broyde et al., reported that the mean Ki-67 PI ranges from 26.6% in indolent lymphomas to 97.6% in very aggressive lymphomas. In patients with DLBCL, a Ki-67 PI of 70% was found to significantly discriminate patients with good or bad prognosis [17].
In this study, 46% of cases had positive C-MYC protein expression, while 58% had high Ki-67 PI. This result was close to the ratio (47.6%) detected by Yun et al., and to the ratio (42%) reported by Julum et al., as regards IHC of C-MYC protein expression [11-23]. In this study, C-MYC protein expression was significantly associated with high Ki-67 PI. All 23 cases with positive C-MYC protein expression had high Ki-67 PI, while 22.2% of cases with negative C-MYC protein expression had high Ki-67 PI. In concordance to our findings, Yun et al. detected that high Ki-67 PI indicated a higher degree of malignancy in patients with C-MYC translocation in DLBCL [11].
In this study 44% of patients relapsed on the three years follow-up period. Relapses were not significantly associated with patient’s age and sex. However, C-MYC protein expression and high Ki-67 PI were independently associated with disease relapse. Multivariate analysis revealed that patients with combined positive C-MYC protein expression and high Ki-67 PI were more likely to have relapses when compared to patients with combined negative C-MYC protein expression and low Ki-67 PI or patients with either positive C-MYC protein expression or high Ki-67 PI alone.
Parallel results were detected by Maria et al., who reported that overall survival and disease-free survival in DLBCL were higher in patients with negative expression for Ki-67 and C-MYC protein [24]. Moreover, higher rates of relapse were observed in the high Ki-67 expression group compared to the low Ki-67 expression group. In multivariate analysis, Ki-67 expression was a significant prognostic factor for event free survival and borderline significance for overall survival. Elevated Ki- 67 expression seems to be associated with higher relapse and inferior free survival in patients with DLBCL [25]. We concluded that C-MYC protein expression and high Ki-67 PI are independently associated with relapses in DLBCL patients. Furthermore, the combined positive C-MYC protein expression and high Ki67 PI test is better than a single positive test in predicting relapses among DLBCL patients.
References
- The Role of c-MYC in B-Cell Lymphomas: Diagnostic and Molecular Aspects Nguyen Lynh, Papenhausen Peter, Shao Haipeng. Genes.2017;8(4). CrossRef
- c-Myc Is a Universal Amplifier of Expressed Genes in Lymphocytes and Embryonic Stem Cells Nie Zuqin, Hu Gangqing, Wei Gang, Cui Kairong, Yamane Arito, Resch Wolfgang, Wang Ruoning, Green Douglas R., Tessarollo Lino, Casellas Rafael, Zhao Keji, Levens David. Cell.2012;151(1). CrossRef
- Omomyc Reveals New Mechanisms To Inhibit the MYC Oncogene Demma Mark J., Mapelli Claudio, Sun Angie, Bodea Smaranda, Ruprecht Benjamin, Javaid Sarah, Wiswell Derek, Muise Eric, Chen Shiying, Zelina John, Orvieto Federica, Santoprete Alessia, Altezza Simona, Tucci Federica, Escandon Enrique, Hall Brian, Ray Kallol, Walji Abbas, O’Neil Jennifer. Molecular and Cellular Biology.2019;39(22). CrossRef
- What retroviruses teach us about the involvement of c-Myc in leukemias and lymphomas Dudley JP, Mertz JA, Rajan L, Lozano M, Broussard DR. Leukemia.2002;16(6). CrossRef
- Elevation of c-MYC Disrupts HLA Class II–Mediated Immune Recognition of Human B Cell Tumors God Jason M., Cameron Christine, Figueroa Janette, Amria Shereen, Hossain Azim, Kempkes Bettina, Bornkamm Georg W., Stuart Robert K., Blum Janice S., Haque Azizul. The Journal of Immunology.2015;194(4). CrossRef
- MYC protein expression and genetic alterations have prognostic impact in patients with diffuse large B-cell lymphoma treated with immunochemotherapy Valera A., Lopez-Guillermo A., Cardesa-Salzmann T., Climent F., Gonzalez-Barca E., Mercadal S., Espinosa I., Novelli S., Briones J., Mate J. L., Salamero O., Sancho J. M., Arenillas L., Serrano S., Erill N., Martinez D., Castillo P., Rovira J., Martinez A., Campo E., Colomo L.. Haematologica.2013;98(10). CrossRef
- Double-hit B-cell Lymphomas With BCL6 and MYC Translocations Are Aggressive, Frequently Extranodal Lymphomas Distinct From BCL2 Double-hit B-cell Lymphomas Pillai Raju K., Sathanoori Malini, Van Oss Stephen Branden, Swerdlow Steven H.. The American Journal of Surgical Pathology.2013;37(3). CrossRef
- The 2016 revision of the World Health Organization classification of lymphoid neoplasms Swerdlow Steven H., Campo Elias, Pileri Stefano A., Harris Nancy Lee, Stein Harald, Siebert Reiner, Advani Ranjana, Ghielmini Michele, Salles Gilles A., Zelenetz Andrew D., Jaffe Elaine S.. Blood.2016;127(20). CrossRef
- ‘Double-Hit’ cytogenetic status may not be predicted by baseline clinicopathological characteristics and is highly associated with overall survival in B cell lymphoma patients Landsburg Daniel J., Nasta Sunita D., Svoboda Jakub, Morrissette Jennifer J. D., Schuster Stephen J.. British Journal of Haematology.2014;166(3). CrossRef
- Expression Profiles of MYC Protein and MYC Gene Rearrangement in Lymphomas Chisholm Karen M., Bangs Charles D., Bacchi Carlos E., Kirsch Hernan Molina-, Cherry Athena, Natkunam Yasodha. The American Journal of Surgical Pathology.2015;39(3). CrossRef
- Correlation Between C-MYC, BCL-2, and BCL-6 Protein Expression and Gene Translocation as Biomarkers in Diagnosis and Prognosis of Diffuse Large B-cell Lymphoma Zhang YunXiang, Wang Hui, Ren Cuiai, Yu Hai, Fang Wenjia, Zhang Na, Gao Sumei, Hou Qian. Frontiers in Pharmacology.2019;9. CrossRef
- MYC Cytogenetic Status Correlates With Expression and Has Prognostic Significance in Patients With MYC/BCL2 Protein Double-positive Diffuse Large B-cell Lymphoma Wang Xuan Julia, Medeiros L. Jeffrey, Lin Pei, Yin C. Cameron, Hu Shimin, Thompson Mary Ann, Li Shaoying. The American Journal of Surgical Pathology.2015;39(9). CrossRef
- C-MYC, BCL2 and BCL6 Translocation in B-cell Non-Hodgkin Lymphoma Cases Salam Dayang Sharyati Datu Abdul, Thit Ei Ei, Teoh Siew Hoon, Tan Soo Yong, Peh Suat Cheng, Cheah Shiau-Chuen. Journal of Cancer.2020;11(1). CrossRef
- Apoptotic signaling by c-MYC Hoffman B, Liebermann D A. Oncogene.2008;27(50). CrossRef
- Ki-67 is a valuable prognostic predictor of lymphoma but its utility varies in lymphoma subtypes: evidence from a systematic meta-analysis He Xin, Chen Zhigang, Fu Tao, Jin Xueli, Yu Teng, Liang Yun, Zhao Xiaoying, Huang Liansheng. BMC Cancer.2014;14(1). CrossRef
- High Ki-67 expression in involved bone marrow predicts worse clinical outcome in diffuse large B cell lymphoma patients treated with R-CHOP therapy Song Moo-Kon, Chung Joo-Seop, Lee Je-Jung, Yang Deok-Hwan, Kim In-Suk, Shin Dong-Hoon, Shin Ho-Jin. International Journal of Hematology.2014;101(2). CrossRef
- Role and prognostic significance of the Ki-67 index in non-Hodgkin's lymphoma Broyde Adi, Boycov Olga, Strenov Yulia, Okon Elimelech, Shpilberg Ofer, Bairey Osnat. American Journal of Hematology.2009;84(6). CrossRef
- Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling Alizadeh Ash A., Eisen Michael B., Davis R. Eric, Ma Chi, Lossos Izidore S., Rosenwald Andreas, Boldrick Jennifer C., Sabet Hajeer, Tran Truc, Yu Xin, Powell John I., Yang Liming, Marti Gerald E., Moore Troy, Hudson James, Lu Lisheng, Lewis David B., Tibshirani Robert, Sherlock Gavin, Chan Wing C., Greiner Timothy C., Weisenburger Dennis D., Armitage James O., Warnke Roger, Levy Ronald, Wilson Wyndham, Grever Michael R., Byrd John C., Botstein David, Brown Patrick O., Staudt Louis M.. Nature.2000;403(6769). CrossRef
- Treatment outcome in Egyptian lymphoma patients, 2-year results, single-center experience Moussa MohamedM, Mostafa NevineN, Helmy MostafaK, ElAzzazi MohamedO, Hegab HanyM, Allah HodaGad, Elafifi AmalM. The Egyptian Journal of Haematology.2014;39(4). CrossRef
- Regulation of Hematopoietic Stem Cell Fate and Malignancy Cho Hee Jun, Lee Jungwoon, Yoon Suk Ran, Lee Hee Gu, Jung Haiyoung. International Journal of Molecular Sciences.2020;21(13). CrossRef
- IG/MYC Rearrangements are the Main Cytogenetic Alteration in Plasmablastic Lymphomas Valera Alexandra, Balagué Olga, Colomo Luis, Martínez Antonio, Delabie Jan, Taddesse-Heath Lekidelu, Jaffe Elaine S., Campo Elías. The American Journal of Surgical Pathology.2010. CrossRef
- Rearrangements of Bcl-1, Bcl-2, Bcl-6, and C-Myc in Diffuse Large B-Cell Lymphomas Kawasaki C., Ohshima K., Suzumtya J., Kanda M., Tsuchiya T., Tamura K., Kikuchi M.. Leukemia & Lymphoma.2001;42(5). CrossRef
- MYC Immunohistochemistry Predicts MYC Rearrangements by FISH Nwanze Julum, Siddiqui Momin T., Stevens Keith A., Saxe Debra, Cohen Cynthia. Frontiers in Oncology.2017;7. CrossRef
- The prognostic role of Bcl-2, Ki67, c-MYC and p53 in diffuse large B-cell lymphoma Maria A, Rotaru I, Olar L, Patrascu S. Romanian journal of morphology and embryology.2017;58(3):837-843.
- Ki-67 expression as a prognostic factor in diffuse large B-cell lymphoma patients treated with rituximab plus CHOP Hyun Yoon Dok, Ro Choi Dae, June Ahn Heui, Kim Shin, Ho Lee Dae, Kim Sang-We, Hee Park Bong, Yoon Sun Ok, Huh Jooryung, Lee Sang-wook, Suh Cheolwon. European Journal of Haematology.2010. CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2021
Author Details