Expression of Simian Virus 40 Large T-Antigen: A Case Control Study of Non-Hodgkin Lymphoma
Download
Abstract
Background: Cancer such as neoplasma lymphoma is related to the immune system. Of all lymphoma cases, about 90% are Non-Hodgkin Lymphoma (NHL). In the United Kingdom, NHL is among the five most frequently diagnosed cancer cases. In Indonesia, based on statistical data from the Dharmais Cancer Center Hospital in 2006, NHL was in the ten most commonly diagnosed cancers. The global incidence of NHL continues to increase annually in western countries, especially in Caucasian populations, men and the elderly. The etiology of NHL is uncertain. Viral infection is thought to be associated with the pathogenesis of NHL. One of them is the Simian 40 virus (SV 40) infection. In some studies, positive SV40 sequencing was found in the majority of NHL cases, but other research found no significant relationship between SV40 infection and NHL.
Objective: This study aimed to identify the frequency of SV40 infection in NHL cases in Yogyakarta and to determine the relationship between SV40 infection in NHL cases and clinical patients.
Methods: This study examined 102 samples of paraffin blocks from subjects diagnosed with NHL in the Anatomical Pathology Laboratory of Dr. Sardjito Hospital from 2014 through 2016. DNA extraction was done and the results were used for PCR. SV40 genome primer sets were used for the PCR process to identify any positive cases.
Results: In this study, all 102 subjects did not indicate genomic L-Tag positive cases of NHL.
Conclusion: It cannot be proved that SV40 has a role in the pathogenesis of NHL in Indonesia.
Introduction
Cancer or malignant tumors are important health problems in Indonesia and the world. Cancer is the leading cause of death in both developing and developed countries, and is increasing especially in the least developed countries where 82% of the world’s population live [1]. Adoption of lifestyle behaviors that are known to be risk factors for cancer, such as smoking, poor diet, lack of physical activity, and changes in reproduction, further increase the incidence of cancer in developed countries [2]. According to data from the World Cancer Research Fund (WCRF) International, one of the most common cancers around the world is lymphoma. In Yogyakarta, lymphoma is included in the top five of the most common malignancies. In contrast to other solid tumors that are often found, such as in the breast, cervix, intestines and nasopharynx, lymphoma has a very heterogeneous subgrouping with an ever-evolving classification [3].
Lymphoma is a tumor of the immune system. Of all types of lymphoma, 10% are subtypes of Hodgkin Lymphoma (HL) while the remaining 90% are Non-Hodgkin Lymphoma (NHL) [2-3]. NHL is one of the five most frequently diagnosed cancer cases in the United Kingdom [4-6]. In Indonesia, based on statistical data from the Dharmais Cancer Center Hospital in 2006, it was reported that NHL was included in the ten most commonly diagnosed cancers [7].
The incidence of NHL continues to increase every year in western countries, especially in Caucasian populations, men and the elderly [8-9]. The incidence of NHL is also increasing in various European countries. In England, Scotland, and Wales, cases increased by 35% at age 30 (1988-2007) [10]. Similar increased rates of incidence were reported from the USA with a percentage increase of 3.7% per year between 1975 and 1991, and 0.3% per year from 1992 to 2002 [2]. The etiology of NHL is not certain. Viral infection is thought to be associated with the pathogenesis of NHL [11].
Simian virus 40 (SV40) is a DNA virus and classified as a double strand polyomavirus. There is speculation that as many as 98 million people in the United States and more worldwide were inoculated with the polio vaccine contaminated with SV40 during the period 1955-1963 [12]. In 2002, Vilchez and Shivapurkar found positive SV40 sequencing in the majority of NHL type lymphoma cases. Although other studies stated that there was no significant relationship between SV40 infection and NHL because there were very few positive results [7].
SV40 is known to induce NHL malignancy through the role of the large T-antigen (L-Tag) [13-14]. L-Tag is a nuclear phosphoprotein which has an important function in viral replication and transformation in rodent cells [12-13]. The L-tag coding genome is found on BK Virus (BKV), JC Virus (JCV), and SV40 so that a specific region of the L-tag is needed to identify SV40. Since there is no cross-reaction between viruses, there will be no false negative results in DNA sequencing. The L-Tag genome in the DNA of the SV40 virus is in the region 2693-4571. The diversity of mutation variants is seen from several regions in the genome [17].
Sequencing of L-Tag in the United States was reported to be 42% in the study conducted by Vilchez [18] and 43% in Shivapurkar’s study [19] in the cases of NHL, while it was 11% in Japan in Nakatsuka’s study [13], 14% in Italy in Martini’s study [16], in Spain Capello’s study reported 3% [20], and in Egypt it was 53% [21]. L-Tag binds and inactivates tumor suppressor genes, such as p53 and pRb, which are involved in regulating abnormal cells and the cell cycles. Their roles are inactivated and disrupted by an infection of L-Tag SV40. Lymphomagenesis happens in the cases of NHL [22]. This study aimed to identify the frequency of SV40 infection in NHL cases in Yogyakarta and to determine the relationship between SV40 infection in NHL cases and clinical patients.
Materials and Methods
The sample used was a paraffin block diagnosed with NHL in the Pathology Anatomy Laboratory of Dr. Sardjito Hospital in the period 2014-2016. Inclusion criteria included paraffin blocks with upright diagnosis of NHL from histopathology diagnosis method.
DNA from paraffin block samples were extracted using a Favorgen kit. The results of DNA extraction were used for polymerase chain reaction (PCR) analysis. The PCR of the SV40 genome used the primer sets of Simian Virus 40 (Table 1).
| 1st Primer | STEV219, 5′-TCCAACCTATGGAACTGATGAATG-3′ |
| STEV189 5′-TAGTTAATTGTAGGCTATCAACCCGC-3′ (820 bp) | |
| 2nd Primer | SVTAGP1 5’-TTAGCAATTCTGAAGGAAAGTCCTTG-3’ |
| SVTAGP2 5’-AGCAGTGGTGGAATGCCTTTCATGAGG-3’ (156 bp) |
DNA detection was conducted using nested PCR techniques. A total of 2 μl of DNA extraction from each sample was amplified in 23 μl of the PCR mixture containing 9.3 μl of nuclease free water, 12.5 μl of PROMEGA green master mix, and 0.6 μl of each primer (forward and reverse) with concentrations of 10 μM.
The PCR program used a Bio-Rad C 1000 PCR machine, and the steps taken were preheating at 95OC for 2 minutes, denaturing at 95OC for 30 seconds, annealing according to Tm annealing for each primer for 30 seconds. (Tm annealing primary STEV 219-STEV189 at 55.6OC and SVTAGP1-SVTAGP2 at 59.1OC) and extension at 72 OC for 30 seconds.
Denaturation, annealing and extension stages were done for 35 cycles, followed by final extension at 72OC for 3 minutes and cooling at 4 ͦ C. This step was followed by the second amplification reaction by adding 2 μl of the first PCR product to 23 μl of the second reaction mixture containing 8.5 μl of nuclease free water, 12.5 μl of Green Master Mix, and 1 μl of each primer with a concentration of 10 μM. Amplification was done under the same conditions as the initial amplification stage, and a total of 35 cycles were used to complete the second amplification.
The amplification results were analyzed on 2% agarose gel and observed under ultraviolet (UV) light to identify any positive or negative results by DNA sequencing.
Results
Total data at Dr. Sardjito Hospital since January 2014 until June 2016 which were diagnosed using biopsy and immunohistochemical staining were 102 samples of paraffin block preparations. The characteristics of the research subjects included data on age, gender, and tumor distribution (Table 2). Detection of SV40 infection in this study was done by the nested-PCR method using two sets of primers. The primers used the region that corresponds to the L-Tag target region of SV40 (Table 1).
This study involved all 102 samples. Of the 102 samples, 51 were male and 44 female, and 7 samples did not have gender data. In accordance with the epidemiology of NHL cases, the ratio of men to women was 1.4: 1 [10] (Table 2).
| Total | Percentage (%) | |||
| Age (year) | Value | 2-87 | ||
| Mean | 51.3656 | |||
| Median | 55.0000 | |||
| <20 | 11 | 10.78 | ||
| 20-60 | 25 | 24.5 | ||
| >60 | 55 | 53.92 | ||
| No Data | 11 | 10.78 | ||
| Gender | Man | 51 | 50 | |
| Woman | 44 | 43.13 | ||
| No Data | 7 | 6.86 | ||
| Extranodal involvement | Yes | 63 | 61.76 | |
| No | 32 | 31.37 | ||
| No Data | 7 | 6.86 |
Most cases were found at the age above 50 years, with 55 samples over 50 years, 25 samples between the age ranges of 20 to 50 years, 11 samples were under 20 years old and the remaining 11 samples had no age data (Table 2).
NHL is a malignancy of the lymphoid tissue that is more commonly found spreading in tissues beyond the node [10]. Cases in Yogyakarta since 2014 until 2016 included 63 extranodal samples, 32 samples in lymphoid tissue and the rest had no data (Table 2).
Final reading of the results of electrophoresis of nested-PCR products using primer SVTAGP1-SVTAGP2 obtained 0 samples with positive results, so all of 102 samples showed negative results (Figure 1).
Figure 1: Electrophoresis of 102 samples.
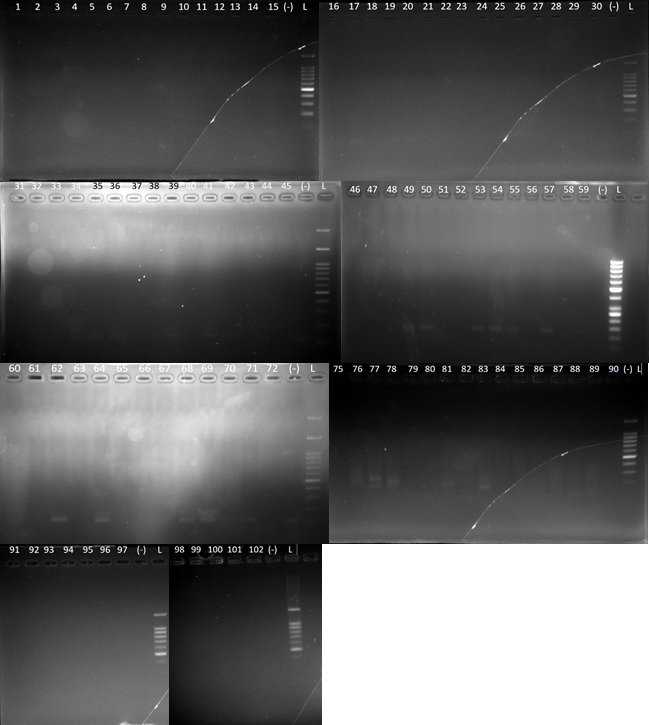
Nested-PCR and electrophoresis were conducted on all 102 samples. The reading of the electrophoresis results of nested-PCR products using STEV219-STEV189 primers showed no positive samples for SV40 infection, so all 102 samples showed negative results (Figure 1).
In this study, all 102 subjects in the study using primer STEV219-STEV189 and SVTAGP1-SVTAGP2 did not indicate genomic L-Tag positive cases of NHL.
Discussion
SV40 was detected in the vaccine from Salk and Sabin which has been widely distributed worldwide and is being administered to millions of children worldwide. The rate of childhood cancer also continued to rise after 1960 [23]. However, several studies looking for a direct link to both the SV40-contaminated polio vaccine and cancer in humans have not yielded consistent conclusions. Some reported an association with cancer risk due to exposure to SV40, including NHL [14]. However, others found that there was no association because the results of the study showed few or no positive SV40 PCR results. This was the same as the results of this study which did not have positive PCR results. Research by Capello and MacKenzie, Brousset, and by Schuler had the same results as this study that 0 samples showed PCR positive results [20], [24], [25]. That was in contrast to studies that were mostly done in the United States where the production of the polio vaccine was contaminated with SV40. Among others, studies conducted by Vilchez, Takashi, Amara, and by Zekri had the results showing some positive samples [15], [26-28]. Research by Zekri in 2007 showed 178 positive samples from a total of 266 samples or 67% of the samples were positive for SV40 [26]. Research on monkey kidney cells used in the manufacture of the oral polio vaccines suspected of being contaminated with SV40 found that it was estimated that only 3% -10% of vaccines contained live SV40. This could be one reason some studies have had so few positive results [29]. All of these studies also faced the same problem that no one knows who actually received the SV40-contaminated vaccines and who did not. Accordingly, further study of the connection between SV40 and existing cancer cases is needed by adding various information regarding the distribution of vaccines and vaccination status in greater detail. Additional research is equally important concerning the pathogenesis of SV40 in NHL cases in a more detailed manner using an adequate number of samples.
The weakness of this study is the sample used was a paraffin block because it would be better to use a fresh tissue sample or a blood sample from a patient to carry out the PCR process in identifying a virus. The study of the relationship between SV40 and NHL cases which had the highest percentage was research by Zekri in 2007 with fresh tissue as a sample with the detection method using nested-PCR [26]. In this study only one pair of primers was used, therefore it is necessary to have a follow-up study of the genome in SV40 associated with the occurrence of NHL so that the associated antigen coding region can be detected. Using a variety of primers to detect the presence of SV40 infection in NHL patients would reduce false-negative results during the study. The study by Zekri in 2007 that had the highest percentage of positive results also used a primer that included the T-antigen (T-ag) genome instead of only the L-Tag. Data on polio vaccines for patients with sample owners could not be obtained, so the study did not get information on polio vaccine administration.
The results of the study were aimed at determining the involvement of the genome L-Tag SV40 in NHL lymphomagenesis. Some research found positive SV40 sequencing in the majority of cases of NHL type lymphoma [22]. However, other studies stated that there was no significant relationship between SV40 infection and NHL because there were very few positive results. Research by Daibata et al. in Japan found 3 positive samples out of 178 samples, while Sui et al. in Australia had no positive results, and Schuler in Germany was also without positive results [30].
The results of this study indicate that of the 102 samples that were subjected to PCR, there were no positive results. If SV40 was involved in the cases of NHL, there would be a sample that will show a positive result from the PCR product in the electrophoresis process. Although this study cannot be interpreted in general, the data of this study are consistent with the results of Daibata’s study [30] and Sui’s study [31] which stated that the L-Tag genome of SV40 infection is not involved in lymphomagenesis.
In conclusions, problem identification including further epidemiological research on the viral spread related to polio vaccine contaminated with SV40 is necessary because several countries have significant positive PCR results against SV40 but still lack the data of the vaccination status of patients. Meanwhile, in this study, none of the samples indicated positive results of NHL.
Acknowledgments
This work was supported in part by a grant from Dana Masyarakat, Medical College of Gadjah Mada University.
References
- Global cancer statistics, 2012 Torre Lindsey A., Bray Freddie, Siegel Rebecca L., Ferlay Jacques, Lortet-Tieulent Joannie, Jemal Ahmedin. CA: A Cancer Journal for Clinicians.2015;65(2). CrossRef
- N. C. Institute, “National Cancer Institute,” http://seer.cancer.gov/statfacts/html/nhl.html/, 2012..
- International analysis of the frequency and outcomes of NK/T-cell lymphomas William Basem M., Armitage James O.. Best Practice & Research Clinical Haematology.2013;26(1). CrossRef
- Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001 Morton Lindsay M., Wang Sophia S., Devesa Susan S., Hartge Patricia, Weisenburger Dennis D., Linet Martha S.. Blood.2006;107(1). CrossRef
- “BRIEF in the United Kingdom,” Mackenzie J, Katherine S, Perry J, Jarrett R. F. 2003;95(13):1001-1003.
- Simian Virus 40 Is Present in Most United States Human Mesotheliomas, but It Is Rarely Present in Non-Hodgkin's Lymphoma Rizzo Paola, Carbone Michele, Fisher Susan G., Matker Christine, Swinnen Lode J., Powers Amy, Di Resta Ilaria, Alkan Serhan, Pass Harvey I., Fisher Richard I.. Chest.1999;116. CrossRef
- “Multicentre Epidemiology and Survival Study of B Cell Non-Hodgkin Lymphoma Patinets in Indonesia,” Reksodiputro A. J. Blood Disord. Transfus.2015;6:2.
- Non-Hodgkin lymphoma Shankland Kate R, Armitage James O, Hancock Barry W. The Lancet.2012;380(9844). CrossRef
- Robbins Basic Pthology, 9th ed Kumar V, Abbas A. K, Aster J. C. 2015.
- “Cancer Statistics Registration: Registration of Cancer Diagnosed in 2007,” 2010 O. for N. Statistics..
- The rise in incidence of lymphomas in Europe 1985–1992 Cartwright R, Brincker H, Carli P.M, Clayden D, Coebergh J.W, Jack A, McNally R, Morgan G, de Sanjose S, Tumino R, Vornanen M. European Journal of Cancer.1999;35(4). CrossRef
- “Simian virus 40, poliovirus vaccines, and human cancer: Research progress versus media and public interests,” Butel J. S. Bull. World Health Organ.2000;78(2):195-198.
- “Simian Virus 40 Sequences in Malignant Lymphomas in Japan,” Nakatsuka S. I, et al . Cancer Res.2003;63(22):7606-7608.
- Reactivation of infectious simian virus 40 from normal human tissues Barbanti-Brodano Giuseppe, Martini Fernanda, Corallini Alfredo, Lazzarin Lorena, Trabanelli Cecilia, Vignocchi Beatrice, Calza Nilla, Iaccheri Laura, Morelli Cristina, Tognon Mauro. Journal of Neurovirology.2004;10(3). CrossRef
- Presence of simian virus 40 DNA sequences in diffuse large B-cell lymphomas in Tunisia correlates with aberrant promoter hypermethylation of multiple tumor suppressor genes Amara Khaled, Trimeche Mounir, Ziadi Sonia, Laatiri Adnene, Hachana Mohamed, Sriha Badreddine, Mokni Moncef, Korbi Sadok. International Journal of Cancer.2007;121(12). CrossRef
- Simian-virus-40 footprints in human lymphoproliferative disorders of HIV− and HIV+ patients Martini Fernanda, Dolcetti Riccardo, Gloghini Annunziata, Iaccheri Laura, Carbone Antonino, Boiocchi Mauro, Tognon Mauro. International Journal of Cancer.1998;78(6). CrossRef
- Specific and quantitative detection of human polyomaviruses BKV, JCV, and SV40 by real time PCR McNees Adrienne L., White Zoe S., Zanwar Preeti, Vilchez Regis A., Butel Janet S.. Journal of Clinical Virology.2005;34(1). CrossRef
- SV40 in human brain cancers and non-Hodgkin's lymphoma Vilchez Regis A, Butel Janet S. Oncogene.2003;22(33). CrossRef
- “Presence of simian virus 40 DNA sequences in human lymphomas Fatal allergic vasculitis associated with celecoxib For personal use. Only reproduce with permission from The Lancet Publishing Group .,” Shivapurkar N, et al . Lancet.2002;359:851-852.
- Simian virus 40 infection in lymphoproliferative disorders Capello Daniela, Rossi Davide, Gaudino Giovanni, Carbone Antonino, Gaidano Gianluca. The Lancet.2003;361(9368). CrossRef
- The Prognostic Role and Relationship between E2F1 and SV40 in Diffuse Large B-Cell Lymphoma of Egyptian Patients Samaka Rehab M., Aiad Hayam A., Kandil Mona A., Asaad Nancy Y., Holah Nanes S.. Analytical Cellular Pathology.2015;2015. CrossRef
- SV40 and human cancer: A review of recent data Shah Keerti V.. International Journal of Cancer.2006;120(2). CrossRef
- History of Sabin attenuated poliovirus oral live vaccine strains Sabin A.B., Boulger L.R.. Journal of Biological Standardization.1973;1(2). CrossRef
- Immunohistochemical investigation of SV40 large T antigen in Hodgkin and non-Hodgkin's lymphoma Brousset Pierre, de Araujo Valquiria, Gascoyne Randy D.. International Journal of Cancer.2004;112(3). CrossRef
- No evidence for simian virus 40 DNA sequences in malignant non-Hodgkin lymphomas Schüler Frank, Dölken Sandra C., Hirt Carsten, Dölken Marc T., Mentel Renate, Gürtler Lutz G., Dölken Gottfried. International Journal of Cancer.2006;118(2). CrossRef
- Detection of simian virus 40 DNA sequences in Egyptian patients with different hematological malignancies Zekri Abdel-Rahman, Mohamed Waleed, Bahnassy Abeer, Refat Lobna, Khaled Mohsen, Shalaby Sameh, Hafez Mohamed. Leukemia & Lymphoma.2007;48(9). CrossRef
- Association between simian virus 40 and non-Hodgkin lymphoma Vilchez Regis A, Madden Charles R, Kozinetz Claudia A, Halvorson Steven J, White Zoe S, Jorgensen Jeffrey L, Finch Chris J, Butel Janet S. The Lancet.2002;359(9309). CrossRef
- Prognostic impact of extranodal involvement in diffuse large B-cell lymphoma in the rituximab era Takahashi Hiroyuki, Tomita Naoto, Yokoyama Masahiro, Tsunoda Saburo, Yano Takahiro, Murayama Kayoko, Hashimoto Chizuko, Tamura Kazuo, Sato Kazuya, Ishigatsubo Yoshiaki. Cancer.2011;118(17). CrossRef
- Variable frequency of polyomavirus SV40 and herpesvirus EBV in lymphomas from two different urban population groups in Houston, TX Toracchio Sonia, Kozinetz Claudia A., Killen Deanna E., Sheehan Andrea M., Banez Eugenio I., Ittmann Michael M., Sroller Vojtech, Butel Janet S.. Journal of Clinical Virology.2009;46(2). CrossRef
- Simian virus 40 in japanese patients with lymphoproliferative disorders Daibata Masanori, Nemoto Yuiko, Kamioka Mikio, Imai Shosuke, Taguchi Hirokuni. British Journal of Haematology.2003;121(1). CrossRef
- Absence of simian virus 40 (SV40) DNA in lymphoma samples from Tasmania, Australia Sui Lee-Fan, Williamson Jan, Lowenthal Ray M., Parker Andrew J.C.. Pathology.2005;37(2). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2021
Author Details