Neuroendocrine Differentiation in Non-Small Cell Lung Cancer and its Relation to Different Pathologic Features: An Immunohistochemical Study
Download
Abstract
Objectives: To identify the relevance of neuroendocrine differentiation in non-small cell lung cancer and its correlation with different pathological features.
Materials and Methods: A total number of 30 cases of per cutaneous CT guided biopsies of primary non-small lung cancer were collected in the pathology department of Misr University for Science and Technology Giza, Egypt and private practice in the time period from January 2018 till December 2020. Immunohistochemical study for neuroendocrine differentiation was performed using mono clonal antibodies against synaptophysin, chromogranin A and CD56. For all selected cases, clinical and pathological data such as age, gender, histologic types, grade and clinical stage were collected, tabulated and statistically analyzed with the results of neuroendocrine markers expression. Cases with incomplete pathological data and cases with histologic picture of neuroendocrine differentiation were excluded.
Results: A total number of 30 cases of primary non-small lung cancer were enrolled in this study. The median age of patients was 61.5 years. There were 21 (70%) males and 9 (30%) females. Regarding neuroendocrine markers, positivity for either marker was identified in 23.3% of non-small cell lung cancer. Chromogranin A was positively expressed in 9 (30%) of cases, synaptophysin was positively expressed in 7 (23.3%) of cases and CD56 was positively expressed in 5 (16.7%) of cases. Only 2 cases (6.7%) showed co-expression of two markers. It was found that there was a high significant relation between chromogranin A expression and clinical stage. Chromogranin A expression was significantly higher in stage III than stage I and II (P<0.001). There was no statistical significant difference between synaptophysin, chromogranin A and CD56 expressions and the rest of the studied pathologic data.
Conclusion: A considerable number of non-small cell lung cancer has neuroendocrine differentiation for at least one neuroendocrine marker despite absence of morphologic features. Much less number of cases showed expression of two markers. A reasonable panel of neuroendocrine markers is recommended to detect this differentiation which may have a clinical impact and optimize an alternative therapeutic option.
Introduction
Lung cancer is the most common cancer worldwide. There are more than 1.3 million new cases every year [1-2]. It’s the first ranked cancer in both sexes and in males [2-3], and the fourth in females. It constitutes 13% of all cancers in both sexes. Fifty five percent of the lung cancer cases occur in the developing countries [2]. In Egypt, lung cancer is one of the most common cancers. It constitutes 5%-7% of all cancers [4]. Lung cancer ranks the fifth in males and both sexes, and ninth among females [3]. Also, lung cancer is a leading cause of death. It represents 25% of all cancer deaths and ranks first in males and second in females [2]. Despite the great efforts and progress in lung cancer research, and the use of multiple aggressive protocols of chemo- and radiotherapy, the overall treatment outcome for lung cancer patients remains poor [5]. Primary carcinomas of the lung are traditionally classified as either small cell lung cancer (SCLC) or non-small cell lung cancer (NSCLC). NSCLC constitutes approximately 85% of all primary lung cancers with adenocarcinoma (ADC), squamous cell carcinoma (SCC) and large cell carcinoma (LCC) constituting the major histological types [6]. The human lung has a resident population of neuroendocrine (NE) cells. These are characterized by the presence of neurosecretory granules and by a number of histochemical and immunocytochemical reactions. NE differentiation is a defining feature of classical and atypical carcinoid tumors and is seen in most SCLC [7]. It has become well recognized that NE differentiation can be demonstrated by immunohistochemistry (IHC) or electron microscopy in10–30% of carcinomas with conventional non-small cell morphology [8]. These tumors show no evidence of NE differentiation histologically and are referred to collectively as non-small cell carcinomas with neuroendocrine differentiation (NSCLC-ND) [9]. NSCLC are often diagnosed at late stages, for patients with advanced stages of NSCLC when surgical excision is not an option, the adjuvant chemotherapy and radiotherapy has been extensively used [10]. However, unlike SCLC that often has NE features, NSCLC is usually chemo- resistant. Of interest, retrospective studies indicated that a subgroup of NSCLC patients with NE features may benefit from chemotherapeutic treatment [11]. On the other hand, SCLC is more chemo- and radio-sensitive, but, despite this, has a worse prognosis. The finding of NE differentiation in some NSCLC has led to the hypothesis that these may form a subgroup with a prognosis and response to treatment somewhere between NSCLC and SCLC [9]. Thus, it is extremely important to be able to clarify this subgroup of NSCLC, and determine the clinical impacts of NE feature in NSCLC patients [11]. This study aimed to identify the relevance of NE differentiation in NSCLC and its correlation with different pathological features.
Materials and Methods
A total number of 30 cases of per cutaneous CT guided biopsies of primary NSCLC were collected in the pathology department of Misr University for Science and Technology (MUST) Giza, Egypt and private practice in the time period from January 2018 till December 2020. Procedures for collection of these tumor tissues were approved by the Medical Ethics Committee of MUST University. The tissue samples were paraffin embedded, cut in 4 μm thick tissue slices and stained with conventional hematoxylin and eosin stain (H&E) for histopathologic examination. All specimens were reviewed according to WHO histological classification of lung cancer [12] and Union for International Cancer Control (UICC) TNM-staging in 2010 [13]. IHC analysis for subclassification of NSCLC was obtained from pathologic reports and slides were revised for diagnostic confirmation. IHC study for NE differentiation was performed using automated Ventana GX Immunostainer. The following neuroendocrine mouse monoclonal antibodies were used: synaptophysin (SYN), chromogranin A (CgA) and neural cell adhesion molecule (CD56). Semi quantitative assessments of cytoplasmic staining intensity (0: none, 1+: weak, 2+: moderate and 3+: strong) and percentage of tumor cells positive (0: none, 1+: 1–33%, 2+: 34–66% and 3+: 67–100%) were made.
For each antibody the score for intensity was multiplied by that for distribution to give an intensity distribution (ID) score. An ID score of > 1 with any marker was used as the criterion for evidence of NE differentiation [9]. For all selected cases clinical and pathological data such as age, gender, histologic types, grade and clinical stage were collected, tabulated and statistically analyzed with the results of NE markers expression. Cases with incomplete pathological data and cases with histologic picture of NE differentiation were excluded.
Technique of per cutaneous CT guided biopsy:
1. Axial 1 mm slice thickness CT was done using 64 MDCT for localizing the site of the lesion.
2. 18 G automatic core biopsy needle was used.
3. Checking bleeding profile of the patient before the procedure.
4. 5-7 cc lignocain was injected subcutaneously opposite the needle entry.
5. The proper section of the lesion was localized. The nearest and largest size to the pleural surface is chosen.
6. The track of the needle was guided via the CT then the biopsy was taken and the core preserved in 10% formalin bottle.
7. After taking the biopsy, post biopsy axial sections were done to detect any possible complications such as pneumothorax or hemothorax and possible lung contusions.
The procedures were done safely in all cases without complications.
Statistical Analysis
The clinical data were recorded on a report form. These data were tabulated and analysed using the computer program SPSS (Statistical package for social science) version 20 to obtain:
Descriptive data
Descriptive statistics were calculated for the data in the form of:
1. Mean and standard deviation for quantitative data.
2. Frequency and distribution for qualitative data.
Analytical statistics
In the statistical comparison between the different groups, the significance of difference was tested using one of the following tests after establishing their non
-normality by K-S test (One-Sample Kolmogorov- Smirnov Test) of normality.
1- Student’s t-test Used to compare mean of two groups of quantitative data of parametric and non-parametric respectively.
2- Inter-group comparison of categorical data was performed by using chi square test (X2-value) and fisher exact test (FET).
A P value <0.05 was considered statistically significant (*) while >0.05 statistically insignificant. P value <0.01 was considered highly significant (**) in all analyses.
Results
A total number of 30 cases of primary NSCLC were enrolled in this study. The age of patients ranged from 51–72 years, mean ± standard deviation of age was 61.7± 6.215 years and the median age was 61.5 years. There were 21 (70%) males and 9 (30%) females. Fourteen (46.7%) patients had squamous cell carcinoma, 11 (36.7%) patients had adenocarcinoma and 5 (16.7%) patients had large cell carcinoma. Twenty two (73.3%) patients were found to have high grade tumors and 8 (26.7%) patients had low grade tumors. Among them, 13 (43.3%) patients had stage I cancer, 6 (20%) patients had stage II and 1 1(36.7%) patients had stage III. Regarding neuroendocrine markers, positivity for either NE marker was identified in 23.3% of NSCLC (Figure 1-14).
Figure 1: Squamous Cell Carcinoma of the Lung (H&E x200).
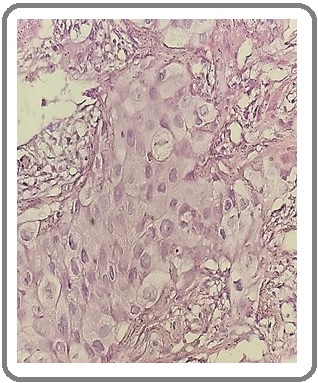
Figure 2: Adenocarcinoma of the Lung (H&E x200).
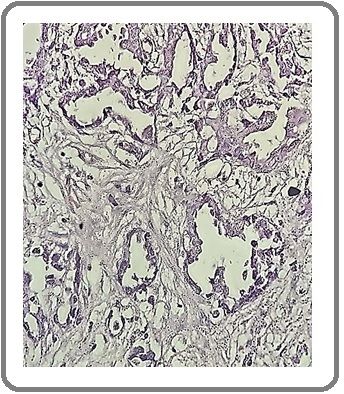
Figure 3: Large Cell Carcinoma of the Lung (H&E x200).
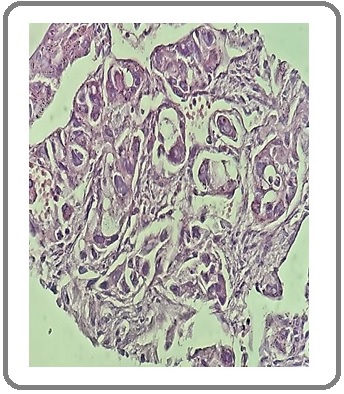
Figure 4: Squamous Cell Carcinoma with NE Differentiation for SYN (IHCx200).
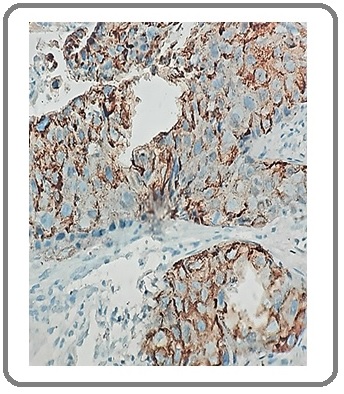
Figure 5: Adenocarcinoma with NE Differentiation for SYN (IHCx200).
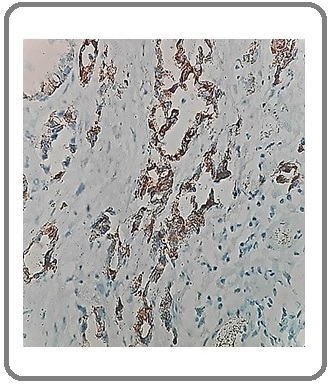
Figure 6: Squamous Cell Carcinoma Negative for NE Differentiation for SYN (IHCx200).
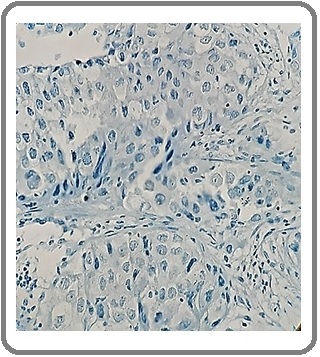
Figure 7: Large Cell Carcinoma with NE Differentiation for SYN (IHCx200).
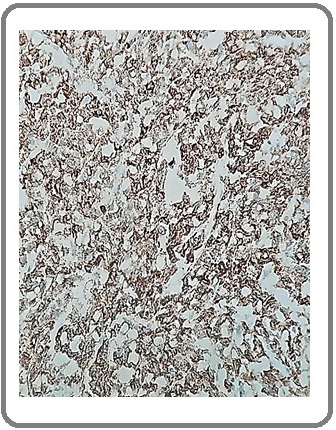
Figure 8: Adenocarcinoma with NE Differentiation for CgA (IHC x200).
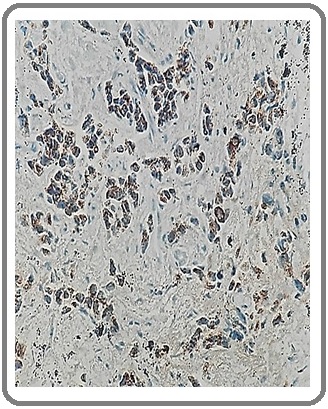
Figure 9: Adenocarcinoma Negative for NE Differentiation for CgA (IHCx200).
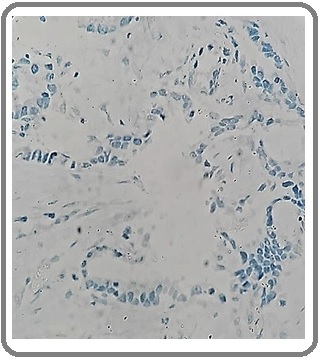
Figure 10: Squamous Cell Carcinoma with NE Differentiation for CgA (IHCx200).
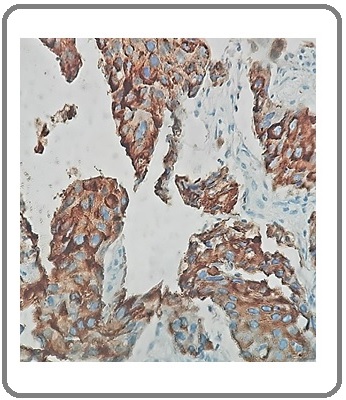
Figure 11: Large Cell Carcinoma with NE Differentiation of CgA (IHCx200).
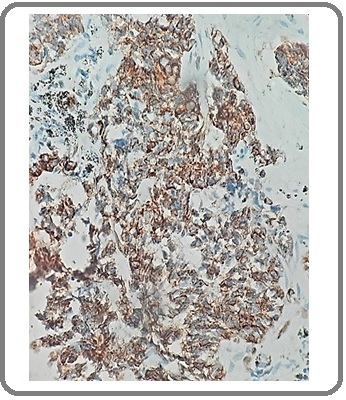
Figure 12: Squamous Cell Carcinoma with NE Differentiation for CD56 (IHCx200).
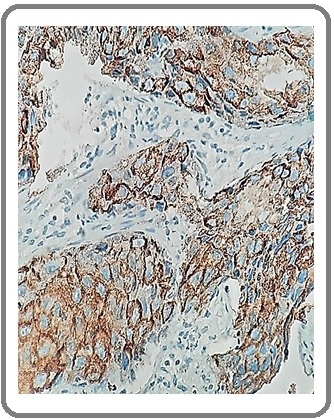
Figure 13: Adenocarcinoma with NE Differentiation for CD56 (IHCx200).
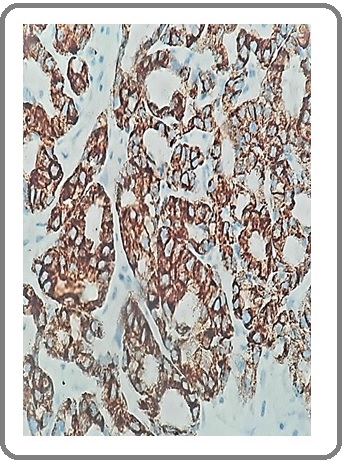
Figure 14: Large Cell Carcinoma with NE Differentiation for CD56 (IHCx200).
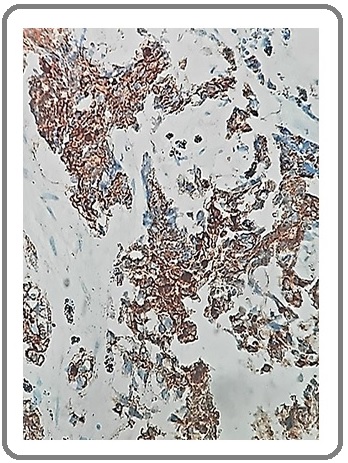
CgA was positively expressed in 9 (30%) of cases, SYN was positively expressed in 7 (23.3%) of cases and CD56 was positively expressed in 5 (16.7%) of cases (Table 1).
| Age | mean± SD | 61.7± 6.215 |
| Range | 51- 72 | |
| Median | 61.5 | |
| Gender | Male, n (%) | 21 (70%) |
| Female, n (%) | 9 (30%) | |
| Histological type | SCC, n (%) | 14 (46.7%) |
| ADC, n (%) | 11 (36.7%) | |
| LCC, n (%) | 5 (16.7%) | |
| Grade | High, n (%) | 22 (73.3%) |
| Low, n (%) | 8 (26.7%) | |
| Clinical stage | I, n (%) | 13 (43.3%) |
| II, n (%) | 6 (20%) | |
| III, n (%) | 11 (36.7%) | |
| SYN | Negative, n (%) | 23 (76.7%) |
| Positive, n (%) | 7 (23.3%) | |
| CgA | Negative, n (%) | 21(70%) |
| Positive, n (%) | 9 (30%) | |
| CD56 | Negative, n (%) | 25 (83.3%) |
| Positive, n (%) | 5 (16.7%) |
Only 2 cases (6.7%) showed co-expression of two markers. The first was SCC and revealed positivity for both SYN and CD56. The other was ADC and revealed positivity for both SYN and CgA.
As shown in (Table 2), there was no significant relation between any of 3 neuroendocrine markers and age (p> 0.05).
| SYN | p- value | CgA | p- value | CD56 | p- value | |||||
| -ve | +ve | -ve | +ve | -ve | +ve | |||||
| Age | Mean± SD | 61.74±5.95 | 61.57±7.55 | 0.95* | 60.67±6.32 | 64.11±5.56 | 0.17* | 61.04±6.17 | 65.00±5.92 | 0.2* |
| Gender | Male, n (%) | 17 | 4 | 0.64¥ | 14 | 7 | 0.68¥ | 20 | 1 | 0.019¥ |
| 73.90% | 57.10% | 66.70% | 77.80% | 80.00% | 20.00% | |||||
| Female, n (%) | 6 | 3 | 7 | 2 | 5 | 4 | ||||
| 26.10% | 42.90% | 33.30% | 22.20% | 20.00% | 80.00% | |||||
| Histological type | SCC, n (%) | 12 | 2 | 0.47ǂ | 11 | 3 | 0.37ǂ | 11 | 3 | 0.54ǂ |
| 52.20% | 28.60% | 52.40% | 33.30% | 44.00% | 60.00% | |||||
| ADC, n (%) | 8 | 3 | 6 | 5 | 9 | 2 | ||||
| 34.80% | 42.90% | 28.60% | 55.60% | 36.00% | 40.00% | |||||
| LCC, n (%) | 3 | 2 | 4 | 1 | 5 | 0 | ||||
| 13.00% | 28.60% | 19.00% | 11.10% | 20.00% | 0.00% | |||||
| Grade | High, n (%) | 15 | 7 | 0.14¥ | 15 | 7 | 1.00¥ | 17 | 5 | 0.29¥ |
| 65.20% | 100.00% | 71.40% | 77.80% | 68.00% | 100.00% | |||||
| Low, n (%) | 8 | 0 | 6 | 2 | 8 | 0 | ||||
| 34.80% | 0.00% | 28.60% | 22.20% | 32.00% | 0.00% | |||||
| Clinical stage | I, n (%) | 12 | 1 | 0.12ǂ | 13 | 0 | <0.001ǂ | 10 | 3 | 0.45ǂ |
| 52.20% | 14.30% | 61.90% | 0.00% | 40.00% | 60.00% | |||||
| II, n (%) | 3 | 3 | 5 | 1 | 6 | 0 | ||||
| 13.00% | 42.90% | 23.80% | 11.10% | 24.00% | 0.00% | |||||
| III, n (%) | 8 | 3 | 3 | 8 | 9 | 2 | ||||
| 34.80% | 42.90% | 14.30% | 88.90% | 36.00% | 40.00% |
*Analysed by Student's t-test; ¥Analysed by Fisher exact test ǂAnalysed by Chi square test; SD, standard deviation; SYN, Synaptophysin; CgA, Chromogranin A; SCC, Squamous cell carcinoma; ADC, Adenocarcinoma; LCC, large cell carcinoma
As regards gender, SYN and CgA markers showed no statistical significant difference between males and females (p> 0.05) while in CD56, males were significantly higher than females in negative expression (p= 0.019). SYN and CgA markers were more positively expressed in adenocarcinoma type, while CD56 was more expressed in squamous cell carcinoma type but this relation was statistically insignificant (p> 0.05). SYN, CgA and CD56 markers were more positively expressed in high grade tumors but without statistical significant difference. It was found that there was a high significant relation between CgA expression and clinical stage. CgA expression was significantly higher in stage III than stage I and II (P<0.001). There was no statistical significant difference between SYN and CD56 expressions and clinical stage.
Discussion
This study included 30 cases of CT guided biopsies of primary NSCLC. We performed IHC analysis for NE markers SYN, CgA and CD56. The median age of patients was 61.5 years. There were 70% males and 30% females. These results were close to Feng et al., 2016 who reported a median age of 60.4 years and 75% of male predominance [5]. In the present study, near half of patients had squamous cell carcinoma followed by adenocarcinoma and large cell carcinoma. In addition, 73.3% of patients were found to have high grade tumors. Among them, 43.3% of patients had stage I cancer, 20% of patients had stage II and 36.7% of patients had stage III. These results were parallel to those detected by Diana et al., 2007 [14]. On the other hand, high grade tumors were detected in 49.9% of cases by Feng et al., 2016. Moreover, 40.6% of their cases were stage I, 24.5% of cases were stage II and 34.9% of cases were stage III [5]. In the present study, positivity for either NE marker was identified in 23.3% of NSCLC. CgA was positively expressed in 30% of cases, SYN was positively expressed in 23.3% of cases and CD56 was positively expressed in 16.7% of cases. Only 6.7% of cases showed co-expression of two markers. In accordance to our results, Feng et al., 2016 reported that NE differentiation in NSCLC was detectable in almost 30% of studied patients. They observed positive immunostaining for CD56, CgA and SYN in 13.3%, 29.7% and 19.1% cases, respectively. Of them, 18.85% of specimens were stained with two or more indicated NE markers [5]. Far from our results, Howe et al., 2005 reported that 36% of cases had positive staining for at least one NE marker. CgA was positive in 5.5% of cases, SYN in 16.5% and CD56 in 28% [9]. In addition, Diana et al., 2007 reported positivity for either marker in 13.6% of NSCLC. CgA was positive in 0.4% of cases, SYN in 7.5% and for CD56 in 8.6%. Only 0.2% of cases showed co-expression of SYN and CgA and none of all 3 markers [14]. In the present study, there was no significant relation between any of 3 neuroendocrine markers and age. As regards gender, SYN and CgA markers showed no statistical significant difference. Males were significantly higher than females in negative expression of CD56. Our results were compared to those of Feng et al., 2016 who found no associations for expressions of NE markers with other pathological factors such as gender and age [5]. In this study, SYN and CgA markers were more positively expressed in adenocarcinoma type, while CD56 was more expressed in squamous cell carcinoma type but this relation was statistically insignificant. Similar to our results Diana et al., 2007 reported that SYN is more likely to be expressed in adenocarcinoma and CD56 in squamous-cell carcinoma. Otherwise there was no correlation between immune-reactivity and tumor morphology [14]. In the present study, SYN, CgA and CD56 markers were more positively expressed in high grade tumors but without statistical significant difference. Unlike our results Feng et al., 2016 reported that tumors in low-middle grade showed higher rates of SYN, CgA and CD56 with statistical significances for expressions of the three NE markers [5]. In the present study, there was a high significant relation between CgA expression and clinical stage. CgA expression was significantly higher in stage III than stage I and II. In contrast to our findings, Feng et al., 2016 found that the expressions of CD56 or SYN, were associated with TNM staging of NSCLC and tumors at later staging had higher percentages of expressions for these molecular markers [5]. Also in disagreement with our results, Howe et al., 2005 reported that there was no association between NE differentiation and either disease stage or nodal status [9].
In conclusion, A considerable number of NSCLC has NE differentiation for at least one NE marker despite absent morphologic features. Much less number of cases showed expression of two markers. A reasonable panel of NE markers should be used to detect this differentiation which may have a clinical impact and optimize an alternative therapeutic option.
References
- Lung cancer around the world and Arab countries Larbi ABID. AMAAC Workshop, Algiers 19.2011.
- Epidemiology of cancer El-Bolkainy M, Nouh M, El-Bolkainy T, Badawy O. Pathology of Cancer.2016;:977-978.
- GLOBOCAN 2008 v1.2, Cancer incidence and mortality worldwide: IARC Ferlay J, Shin H, Bray F, Soerjomataram I, Dikshit R, et al . Cancer Base.10. Lyon, France..
- Cancer Incidence in Egypt: Results of the National Population-Based Cancer Registry Program Ibrahim Amal S., Khaled Hussein M., Mikhail Nabiel NH, Baraka Hoda, Kamel Hossam. Journal of Cancer Epidemiology.2014;2014. CrossRef
- Correlation of neuroendocrine features with prognosis of non-small cell lung cancer Feng Jianguo, Sheng Huaying, Zhu Chihong, Qian Xiaoqian, Wan Danying, Su Dan, Chen Xufeng, Zhu Liming. Oncotarget.2016;7(44). CrossRef
- The pivotal role of pathology in the management of lung cancer Davidson M, Gazdar A, Clarke B. Journal of Thoracic Disease.2013;5:463-479. CrossRef
- NEUROENDOCRINE DIFFERENTIATION IN LUNG CANCER CAREY FRANCIS A., SAVE VICKI E.. The Journal of Pathology.1997;182(1). CrossRef
- The value of immunohistochemical identification of neuroendocrine differentiation in non- small cell lung carcinoma Slodkowska J. Roczniki Akademii Medycznej.1997;42(Suppl. 1):23-27.
- Neuroendocrine differentiation in non-small cell lung cancer and its relation to prognosis and therapy Howe M C, Chapman A, Kerr K, Dougal M, Anderson H, Hasleton P S. Histopathology.2005;46(2). CrossRef
- Effect of Adjuvant Magnetic Fields in Radiotherapy on Non-Small-Cell Lung Cancer CellsIn Vitro Feng Jianguo, Sheng Huaying, Zhu Chihong, Jiang Hao, Ma Shenglin. BioMed Research International.2013;2013. CrossRef
- Neuroendocrine differentiation in non-small cell lung carcinomas Baldi A, Groger A, Esposito V, Di Marino M, Ferrara N, Baldi F. In Vivo.2000;(14):109-114.
- The 2015 World Health Organization Classification of Tumors of the Pleura: Advances since the 2004 Classification Galateau-Salle Francoise, Churg Andrew, Roggli Victor, Travis William D.. Journal of Thoracic Oncology.2016;11(2). CrossRef
- International Union against Cancer Sobin L, Gospodarowicz M, Wittekind C. TNM classification of malignant tumors. Chichester, West Sussex, UK; Hoboken, NJ: Wiley- Blackwell.2010.
- Nonsmall Cell Lung Carcinoma With Neuroendocrine Differentiation—An Entity of No Clinical or Prognostic Significance Ionescu Diana N., Treaba Diana, Gilks Cyril B., Leung Samuel, Renouf Daniel, Laskin Janessa, Wood-Baker Richard, Gown Allen M.. American Journal of Surgical Pathology.2007;31(1). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2021
Author Details