A Study of Molecular Subtypes of Carcinoma Breast by Immunohistochemistry at Tertiary Care Center, Jaipur
Download
Abstract
Introduction: Breast cancer is one of the most common malignant tumors and one of the leading causes of cancer related death amongst females worldwide. The clinical significance of molecular classification of carcinoma breast remains to be established. Molecular subtyping using immunohistochemistry can provide additional prognostic and predictive information. Aim and objectives: To identify morphological variants of carcinoma breast and to determine various proportions of molecular subtypes of carcinoma breast by immunohistochemistry.
Materials and Methods: A descriptive type of observational study was done at SMS Medical College, Jaipur. 65 Modified Radical Mastectomy specimens received in the department were included in the study. The surgical specimens were then evaluated histopathologically and immunohistochemically for ER, PR, HER2/neu, and Ki67 markers.
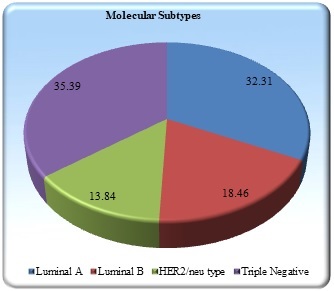
Results: Out of 65 breast carcinoma cases the most common histological type encountered in our study was Invasive ductal carcinoma of no special type (NST) (83.09%) followed by medullary carcinoma (4.63%) and invasive lobular carcinoma (3.09%) and a single case each of tubular, cribriform, metaplastic, lymphoepithelioma like carcinoma, carcinoma with apocrine differentiation and oncocytic carcinoma. The cases were further classified into molecular subtypes using protein expression patterns in IHC. The proportion of tumors found were Luminal A (32.31%), Luminal B (18.46%), HER2/neu overexpressed (13.84%), and Triple-negative (35.39%).
Conclusion: The most common molecular subtype found in our study was Triple negative. The use of IHC markers in the clinical setting could determine the prognosis and benefit the patients with targeted therapies.
Introduction
Breast cancer is one of the most common malignant tumors and one of the leading causes of cancer related deaths amongst females worldwide. Its incidence have been increasing rapidly in both developing and developed countries with approximately 2 million new cases worldwide in 2018, representing 11.6% of all cancers [1]. It is estimated that in 2018 there were approx. 1,62,468 new breast cancer cases in India [1]. However there has been reduction in the morbidity and mortality rates of breast cancer in the developed countries due to increasing early detection by way of mass screening as well as improved targeted therapy.
Breast cancer is heterogenous in terms of histopathological features, metastatic patterns, molecular features, outcome and response to therapy. The heterogeneity affects clinical outcomes accordingly.
Prognosis varies with tumor size, axillary lymph node status, histologic grade, histologic type and biological markers such as estrogen receptor (ER), progesterone receptor (PR), HER2/neu expression profile [2].
The more recent classifications try to categorize the disease at the molecular level and give important predictive information on the potential responsiveness of the tumors to different therapeutic modalities.
Microarray-based gene expression of breast cancer has demonstrated that there are multiple molecular subtypes of breast cancer (luminal A like, luminal B like, HER2 positive and basal-like and these subtypes correlate with prognosis [3].
Presently IHC is accepted as an adequate surrogate marker for molecular subtypes [4]. This surrogate IHC method for the determination of molecular subtypes uses ER, progesterone receptor (PR), HER2 and Ki67 antibodies. It is better than molecular testing as it is more economical and technically simpler.
The luminal molecular subtypes are characterized by estrogen receptor (ER) and progesterone receptor (PR) positivity and further subdivided into Luminal A and Luminal B based on proliferative index. Luminal A subtype are low grade tumors and includes 40-55% of all breast carcinoma. These do not express HER 2/neu and have low proliferative index. Luminal B cancers are approximately 15%–20%, may or may not express HER 2 and have a worse prognosis than luminal A cancers. These often have lower expression levels of HRs, higher Nottingham grade, and higher proliferative rates [5]. A 14% cut-off for Ki-67 was endorsed in St. Gallen 2011 to separate Luminal B from Luminal A tumors [6].
HER2 positive breast cancer lacks expression of ER and PR but is defined by HER2 protein overexpression by IHC and/or HER2/neu gene amplification by in situ hybridization [7]. Breast cancer negative for ER, PR and HER2 protein expression is called triple negative. Triple negative breast cancer represents 10 to 17% of all breast cancers [8].
The clinical significance of molecular classification of carcinoma breast remains to be established. Molecular subtyping using immunohistochemistry can provide additional prognostic and predictive information. In this study the main aim is to discover whether molecular subtyping provides more precise information regarding patient outcome compared to traditional classification system.
Materials and Methods
This descriptive observational study on radical mastectomy specimens received from SMS Medical College and Attached Group of Hospitals at Jaipur, Rajasthan was done after approval of the ethical committee in May 2018 and continued till November 2019. Clinical details such as age, sex, clinical diagnosis and radiological findings were recorded in proforma.
The mastectomy specimens were fixed on 10% neutral buffered formalin in a 10-fold volume immediately after surgery. The specimens were subsequently grossed, paraffin-embedded and stained with hematoxylin and eosin for histopathological study to assess histological type, tumor grade, axillary lymph node status, lympovascular invasion and perineural invasion. Histological typing of breast cancer was performed according to WHO classification. Grading was done according to Modified Bloom-Richardson system and staging according to TNM classification designated by American Joint Committee on Cancer.
Immunohistochemistry was performed on representative tumor paraffin blocks using ER, PR, HER2 and Ki67 antibodies. Allred system was used for ER and PR staining. Allred score is a semi quantitative system that utilizes the proportion of cells which appear positive (scale of 0-5) and intensity of staining (scale of 0-3). The proportion and intensity are added to give total scores of 0 or 2 to 8. HER-2/neu was scored on a scale of 0 to 3 according to the guidelines of American Society of Clinical Oncology (ASCO) and College of American Pathologists (CAP). Breast cancers were classified into four molecular subtypes as mentioned below:
1. Luminal A (ER+ and/or PR+, HER2/neu negative, Ki67< 14%)
2. Luminal B with HER2/neu negative (ER+ and/or PR+, HER-2 negative, Ki-67≥14%) or HER2/neu positive (ER+ and/or PR+, HER-2 positive, any Ki67)
3. HER-2 enriched (ER-, PR-, HER-2 positive)
4. Triple negative(ER-, PR- HER-2 negative)
The statistical analysis was determined using the Pearson Chi-square test. Significance was assumed at P < 0.05.
Results
The age of the patients ranged from second decade to above 8 th decade. In this study most of the cases (29.24%) were 41-50 years of age, followed by 61 to 70 years of age (23.07%). The youngest patient was 24 years old and the oldest patient was 87 years old. The mean age of presentation was 49.30 years. Out of the 65 patients diagnosed with breast carcinoma, 61 cases were females (93.85%) and 4 were males (6.15%).
In our study the tumor size ranged from 1.5 to 9 cms. 4 cases (6.15%) had a tumor size of < 2 cm, 54 cases (83.08%) had tumor size ranging from 2 to 5 cms, 7 cases (10.77%) had tumor size > 5 cms. Lymphovascular invasion was seen in 36 cases (55.39%) out of 65 cases. Perineural invasion (PNI) was seen in 10 cases (15.38%). Lymph node metastasis was seen in 34 cases (52.30%). The Grades were assigned to all the invasive carcinomas according to the Bloom Richardson grading system. Of the 65 cases studied, 10 cases were categorized as GRADE I (15.38), a further 29 cases (44.62%) fulfilled the criteria of GRADE II and 26 cases (40.0%) were GRADE III.
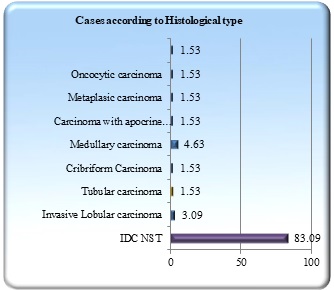
The most common histological type encountered in our study was Invasive carcinoma of no special type (NST) (83.09%) followed by medullary carcinoma (4.63%) and invasive lobular carcinoma (3.09%) and a single case each of tubular, cribriform, metaplastic, carcinoma with apocrine differentiation, lymphoepithelioma like carcinoma and oncocytic carcinoma (Figure 1).
Figure 1. Distribution of Cases According to Histological Type.
ER was positive in 32 cases (49.23%) and PR positivity was noted in 23 cases (35.38%). (Figure 2) (Figure 3,4).
Figure 2. Correlation of ER and PR Receptor Status.
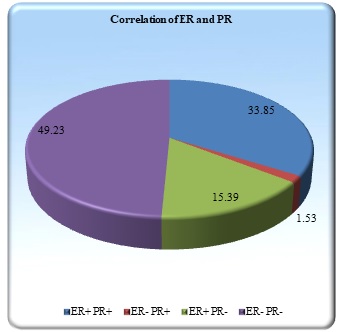
Figure 3. ER Nuclear Positivity, 100x (Allred score 8/8).
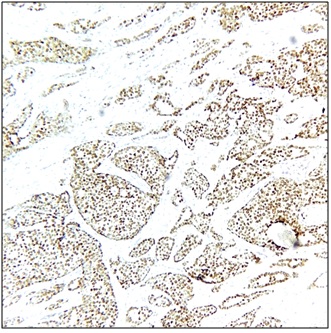
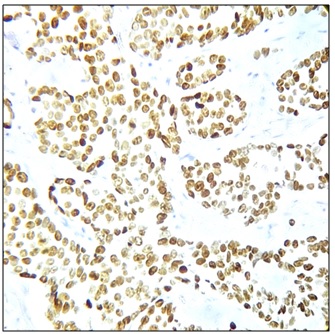
Figure 4. Tumor Cells Positive for PR, 400x (Allred score 8/8).
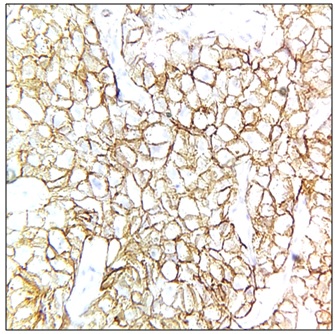
Her2/Neu positivity (Figure 5) was seen in 10 cases (15.38%) and Her2/Neu was negative in 55 cases (84.62%).
Figure 5. HER2/neu Positive, Score 3+, 400x.
The tumors were further classified into molecular subtypes using protein expression pattern in IHC. Proportion of tumors found were Luminal A (32.31%), Luminal B (18.46%), HER2/neu overexpressed (13.84%), and Triple negative (35.39%) (Figure 6).
Figure 6. Distribution of Molecular Subtypes.
Discussion
The age of patients ranged from 24 to 87 years with a mean age group of 49.3 years. Sandhu et al. 2010 [9] found mean age of presentation as 47.8 years.
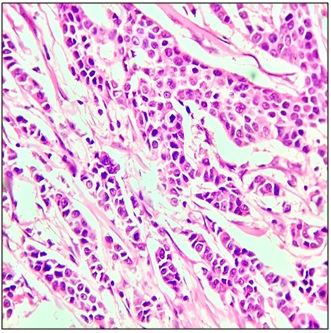
In our study, Invasive ductal carcinoma of no special type (NST) (83.09%) (Figure 7) was the most common histological type followed by medullary carcinoma (4.63%) and invasive lobular carcinoma (3.09%) (Figure 8) and a single case each of tubular, cribriform, metaplastic, lymphoepithelioma like carcinoma, carcinoma with apocrine differentiation and oncocytic carcinoma.
Figure 7. Invasive Ductal Carcinoma, H & E 400x.
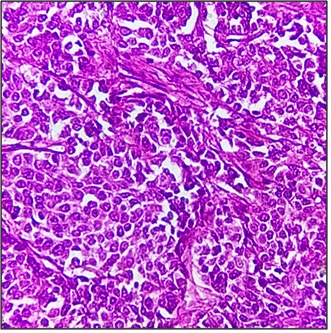
Figure 8. Invasive Lobular Carcinoma, H & E 400x.
This finding is concordant with the study conducted by Gupta et al [10]. in Northern India, showing IDC NST as the predominant histological type in their study. This was also in accordance with a study done by Makki [11], which reported IDC as the commonest histological type. Ductal carcinoma in situ was seen in 27.69% cases in our study (18 cases). In our study, out of 65 cases, lymphovascular invasion was (LVI) seen in 55.39% (36 cases) cases and perineural Invasion was seen in 15.38% cases (10 cases). Lymph node metastasis was seen in 34 cases (52.30%).
ER was positive in 32 cases (49.23%) and PR positivity was noted in 23 cases (35.38%). A prevalence of 32.6% for ER positive and 46.1% for PR positive breast cancers has also been documented in a study carried out in India by Desai et al [12]. In a study done by Col V Dutta et al [13] ER and PR positivity was seen in 30.66% and 42.66% cases respectively. In both these studies percentage of PR positivity was more than ER, compared to our study.
HER2/neu overexpression is associated with poor prognosis and high grade features. In our study Her2/Neu positivity was seen in 10 cases (15.38%) and Her2/Neu was negative in 55 cases (84.62%). The percentage of HER2/Neu postivity in studies conducted by Ghosh et al [14] and Nabi et al [15] was 17.4% and 20% respectively. The tumors were further classified into molecular subtypes using protein expression pattern in IHC. Proportion of tumors found was Luminal A (32.31%), Luminal B (18.46%), HER2/neu overexpressed (13.84%), and Triple negative (35.39%). About 70% triple negative tumors were high grade (Grade III). Out of 65 cases of Triple negative, 3 were reported as Medullary carcinoma, 1 case was reported as Lymphoepithelioma-like carcinoma and rest other cases were IDC NST. All three cases of medullary carcinoma were of triple negative subtype which correlated with studies by Su et al [16], Engstrom et al [2] and Karangadan S et al [17]. Our findings are concordant with the study conducted by Gogoi G. et al [18] in Northeast India showing triple negative as the predominant molecular subtype (38.21%). Similar results were found in the study conducted by Karangadan S et al [17], showing triple negative as the most common molecular subtype (40.00%) followed by luminal B (23.33%). In contrast western studies carried out by Ihemelandu CU et al [19] and Park S et al [20] documented that the most common molecular subtype in their study was Luminal A.
Factors which may account for higher prevalence of Triple-Negative Breast Cancer among Indian breast cancer patients include early age of onset, lifestyle factors such as diet and obesity, reproductive factors such as multiparity, socioeconomic status and screening behaviours [21].
In conclusion, triple negative was the most common molecular subtype found in our study. Molecular techniques have improved our understanding of breast cancer biology, refining molecular classification, and led to the development of novel prognostic and predictive molecular assays.
Immunohistochemistry can be used as a surrogate for molecular testing to determine the molecular subtypes.
The application of these markers in clinical setting determine prognosis and have the potential to benefit patients with targeted therapies.
References
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries Bray F, Ferlay J, Soerjomataram I, Siegel RL , Torre LA , Jemal A. CA: a cancer journal for clinicians.2018;68(6). CrossRef
- Molecular subtypes, histopathological grade and survival in a historic cohort of breast cancer patients Engstrøm M. J., Opdahl S., Hagen A. I., Romundstad P. R., Akslen L. A., Haugen O. A., Vatten L. J., Bofin A. M.. Breast Cancer Research and Treatment.2013;140(3). CrossRef
- Molecular portraits of human breast tumours Perou C. M., Sørlie T., Eisen M. B., Rijn M., Jeffrey S. S., Rees C. A., Pollack J. R., Ross D. T., Johnsen H., Akslen L. A., Fluge O., Pergamenschikov A., Williams C., Zhu S. X., Lønning P. E., Børresen-Dale A. L., Brown P. O., Botstein D.. Nature.2000;406(6797). CrossRef
- Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma Nielsen TO , Hsu FD , Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L, Akslen LA , Ragaz J, Gown AM , Gilks CB , Rijn M, PerouCM . Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2004;10(16). CrossRef
- American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer Hammond MEH , Hayes DF , Wolff AC , Mangu PB , Temin S. Journal of Oncology Practice.2010;6(4). CrossRef
- Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011 Goldhirsch A., Wood W. C., Coates A. S., Gelber R. D., Thürlimann B., Senn H.-J.. Annals of Oncology: Official Journal of the European Society for Medical Oncology.2011;22(8). CrossRef
- Invasive Breast Cancer: Recognition of Molecular Subtypes Strehl JD , Wachter DL , Fasching PA , Beckmann MW , Hartmann A. Breast Care (Basel, Switzerland).2011;6(4). CrossRef
- Triple-negative breast cancer: present challenges and new perspectives Podo F, Buydens LMC , Degani H, Hilhorst R, Klipp E, Gribbestad IS , Van Huffel S, Laarhoven HWM , Luts J, Monleon D, Postma GJ , Schneiderhan-Marra N, Santoro F, Wouters H, Russnes HG , Sørlie T, Tagliabue E, Børresen-Dale A. Molecular Oncology.2010;4(3). CrossRef
- Profile of breast cancer patients at a tertiary care hospital in north India Sandhu D. S., Sandhu S., Karwasra R. K., Marwah S.. Indian Journal of Cancer.2010;47(1). CrossRef
- Comparison of Molecular Subtypes of Carcinoma of the Breast in Two Different Age Groups: A Single Institution Experience Gupta P, Rai NN , Agarwal L, Namdev S. Cureus.2018;10(6). CrossRef
- Diversity of Breast Carcinoma: Histological Subtypes and Clinical Relevance Makki J. Clinical Medicine Insights. Pathology.2015;8. CrossRef
- Hormone receptor status of breast cancer in India: a study of 798 tumours Desai S. B., Moonim M. T., Gill A. K., Punia R. S., Naresh K. N., Chinoy R. F.. Breast (Edinburgh, Scotland).2000;9(5). CrossRef
- Hormone Receptors, Her-2/Neu and Chromosomal Aberrations in Breast Cancer Dutta V., Chopra G. S., Sahai K., Nema S. K.. Medical Journal, Armed Forces India.2008;64(1). CrossRef
- Estrogen, progesterone and HER2 receptor expression in breast tumors of patients, and their usage of HER2-targeted therapy, in a tertiary care centre in India Ghosh J., Gupta S., Desai S., Shet T., Radhakrishnan S., Suryavanshi P., Parmar V., Jalali R., Goyal G., Hawaldar R., Patil A., Nair N., Badwe R. A.. Indian Journal of Cancer.2011;48(4). CrossRef
- Clinicopathological comparison of triple negative breast cancers with non-triple negative breast cancers in a hospital in North India Nabi M. G., Ahangar A., Wahid M. A., Kuchay S.. Nigerian Journal of Clinical Practice.2015;18(3). CrossRef
- Distinct distribution and prognostic significance of molecular subtypes of breast cancer in Chinese women: a population-based cohort study Su Y, Zheng Y, Zheng W, Gu K, Chen Z, Li G, Cai Q, Lu W, Shu XO . BMC cancer.2011;11. CrossRef
- Immunohistochemical characterization of molecular classification of breast carcinoma and its relation with Ki-67 Karangadan S, Patil AG, Andola SK. Clin Cancer Investig J.2016;5(5):430-36.
- Profile of molecular subtypes of breast cancer with special reference to triple negative: A study from Northeast India Gogoi G, Borgohain M, Saikia P, Fazal SA. Clin Cancer Investig J.2016;5(5):374-84.
- Molecular breast cancer subtypes in premenopausal and postmenopausal African-American women: age-specific prevalence and survival Ihemelandu CU , Leffall LD , Dewitty RL , Naab TJ , Mezghebe HM , Makambi KH , Adams-Campbell L, Frederick WA . The Journal of Surgical Research.2007;143(1). CrossRef
- Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry Park S, Koo JS , Kim MS , Park JS , Lee JS , Lee JS , Kim SI , Park B. Breast (Edinburgh, Scotland).2012;21(1). CrossRef
- Triple-negative breast cancer Foulkes WD , Smith IE , Reis-Filho JS . The New England Journal of Medicine.2010;363(20). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2022
Author Details