Evaluation of Posaconazole for Primary Prophylaxis of IFI in Acute Myeloid Leukemia under Intensive Induction Chemotherapy. Comparative Real Life Study with Fluconazole in two Hematological Centers in Algeri
Download
Abstract
Introduction: Acute myeloid leukemia (AML) is a hematological malignancy with a poor prognosis. The early consequences of induction chemotherapy are represented by pancytopenia with severe immunosuppression, which favors the emergence of so-called “opportunistic” infections and in particular invasive fungal infections (IFI) such as invasive aspergillosis (IA). This justifies the implementation of early, empirical, pre-emptive or prophylactic antifungal treatment. The objective of this study is the evaluation of a comparative study before and after the use of primary prevention with posaconazole versus fluconazole, in terms of incidence and mortality of invasive fungal infections (IFIs) during AML inductions, in real life in Algeria.
Patients and methods: This study was performed in two periods in the 2 centers concerning the same AML patients receiving the
same induction chemotherapy (daunorubicin 60 mg/m2 for 3 days and cytarabine 100 mg/m2 for 7 days) but with a different type of hospitalization with common rooms for Tlemcen and protected single rooms for Oran. Period 1: The retrospective study was performed from 2014 to 2016 and involved 188 patients. 70 patients were hospitalized in a common room (Tlemcen) and 118 patients in a protected single room (Oran). All patients received as primary IFI prophylaxis fluconazole 400 mg/day. Period 2: The prospective study was conducted from April 2017 to September 2018 and involved 55 patients. Fourteen patients were hospitalized in Tlemcen and 41 patients in an Oran. These patients received posaconazole prophylaxis at a dose of 200 mgx3/day. The evaluation and comparison of descriptive and incidence data was performed using Chi2 statistical tests using SPSS version 19 software. Overall survival (OS) were calculated using the Kaplan-Meier estimate and were compared using the log-rank test with a significance level α of 0.05.
Results: Period 1: The incidence rate of IFI: 34% at the Oran hospital versus 49% at the Tlemcen hospital (p=0.049). Among the 74 patients who presented with IFI, 39 (53%) died from IFI as the main cause of death, among these deaths, 16 (40%) were recorded at Oran and 23 (68%) at Tlemcen (p=0.011). Period 2: The IFI rate with fluconazole was 39% and that observed with posaconazole 22% (p=0.001) and the cumulative incidence was 39% at 21 days versus 10% at 15 days (p=0.046) with a highly significant decrease in IFI-related deaths: 53% versus 25% (p=0.004).
Conclusion: This work has demonstrated the interest in terms of IFI incidence (17% versus 33%, p=0.04) and IFI-related mortality of posaconazole prophylaxis (25% versus 53%, p=0.004) used during AML in the context of hospitalization in common rooms. Furthermore, this work highlights that the combination of posaconazole prophylaxis and hospitalization in a protected single room allows an optimization of the management of this type of patients.
Introduction
Acute myeloid leukemia (AML) is a hematological malignancy with a poor prognosis. Its incidence in Europe and the USA varies from 3 to 5 per 100 000 inhabitants per year [1-2], while it is lower in Algeria, with an incidence of 0.91 per 100 000 inhabitants per year [3]. AML is common in adults, with an average age at diagnosis of 44 years in Algeria and without predominance of sex [3]. It ranks third among hematological malignancies [4] with an annual number of new cases of around 500 to 600 patients [4]. The early consequences of induction chemotherapy are represented by pancytopenia, and in particular a very deep and long-lasting neutropenia associated secondarily with severe immunosuppression, which favors the emergence of so-called “opportunistic” infections and in particular invasive fungal infections (IFI) such as invasive candidiasis or invasive aspergillosis (IA).
IA, the most serious of the IFIs, is secondary to a filamentous fungus (Aspergillus fumigatus in 80% of cases) which is an environmental mould, called conidia of 2 µm in diameter, suspended in air and water. Under normal physiological conditions, this fungus is harmless when inhaled, and is evacuated through the mucociliary carpet. It becomes pathogenic in the event of deep and lasting neutropenia and/or immunosuppression during germination, leading rapidly to vascular invasion and tissue necrosis [5].
IA is a fungal infection which, despite progress in diagnosis and treatment, remains serious with a poor prognosis and still a high mortality rate often requiring management in intensive care units [6]. Fungal infection remains difficult to diagnose and is sometimes only diagnosed postmortem. This justifies the implementation of early, empirical, pre-emptive or prophylactic antifungal treatment on the basis of (1) clinical, clinical situation and/ or persistent fever under broad-spectrum antibiotic empiric therapy, (2) biological (galactomannan antigenemia, Polymerase chain reaction (PCR) and/or radiological scanners (specific lung lesions and/or “halo” sign). Despite this early take in care, deaths secondary to IFIs remain still high. Therefore, current international recommendations consider the implementation of primary prevention of IFIs and in particular IA by antifungal agents during intensive induction chemotherapy of AML [7]. This primary prevention can be provided by an azole agent and posaconazole has been recommended following the very significant results of a large international randomized multicenter trial comparing posaconazole with fluconazole or itraconazole in AML and myelodysplasias (MDS) under intensive chemotherapy [8].
The objective of this study is the evaluation of a comparative study before and after the use of primary prevention with posaconazole, in terms of incidence and mortality of IFIs during AML inductions, in real life in Algeria.
Materials and Methods
This work concerned a bicentric comparative evaluation (hematological department of the university hospital of Tlemcen and the hematological and cellular therapy department of the university hospital November 1st of Oran) of the IFI incidence during AML induction chemotherapy calculated over two periods using a different prophylaxis. This study was performed in the 2 centers concerning the same AML patients receiving the same induction chemotherapy but with a different kind of hospitalization with common rooms for Tlemcen and protected single rooms for Oran. This study included 2 parts: (1) the first one corresponds to a retrospective study where the primary prevention used during intensive AML chemotherapy for the 2 centers was fluconazole with the only difference being the type of hospitalization (protected single rooms vs. common rooms) (2) the second one corresponds to a prospective study where the primary prevention used in the 2 centers was posaconazole with the same similarities (AML receiving intensive chemotherapy) and the same differences (protected single rooms vs. common rooms).
The inclusion criteria were AML diagnosis patients treated with fluconazole or posaconazole prophylaxis and exclusion criteria were AML patients without prophylaxis. The informed consent was obtained from all individual participants included in the study. The study was approved by the Ethical Committees of both centers.
Retrospective and prospective Studies
The retrospective study was performed from 2014 to 2016 and involved 188 patients with a median age of 54 years (19-65) and a sex ratio of 1.72. All patients presented a de novo AML and received 3+7 induction chemotherapy combining daunorubicin 60 mg/m2 for 3 days and cytarabine 100 mg/m2 for 7 days. In terms of hospitalization, 70 patients were hospitalized in a common room (Tlemcen) and 118 patients in a protected single room (Oran). All patients received as primary IFI prophylaxis fluconazole 400 mg/day from Day 1 of the induction phase until the end of the aplasia phase, characterized by hematopoietic recovery with a absolute neutrophil count (ANC) ≥1.5 G/L.
The prospective study was conducted from April 2017 to September 2018 and involved 55 patients with de novo AML who also received intensive 3+7 chemotherapy, with a median age of 52.5 years (24-65) and a sex ratio of 0.96. Fourteen patients were hospitalized in a common room (Tlemcen) and 41 patients in a protected single room (Oran). Due to the introduction of posaconazole at that period in Algeria, these patients received posaconazole prophylaxis with oral suspension administered on Day 1 of the induction phase, at a dose of 200 mgx3/day at mid-meal to improve absorption. Primary prophylaxis was maintained until recovery from aplasia characterized by a level of ANC≥1.5G/L. Posaconazole tolerance and toxicity were monitored by daily clinical examination and laboratory tests, including a blood count every 3 days and bi-weekly renal and hepatic measures. Infectious surveillance was based on twice-daily temperature measurement, daily clinical examination and chest CT scan in case of cough or respiratory discomfort or unknown origin fever.
Statistical analysis
It included a descriptive analysis of the populations in the retrospective and prospective studies. This was followed by a study of the cumulative incidence of invasive fungal infections with a study of the incidence of global IFIs and the incidence of AI and invasive candidiasis. The evaluation and comparison of descriptive and incidence data was performed using Chi2 statistical tests using SPSS version 19 software. In order to be able to compare IFI incidences in the two periods, we matched the two sets of patients by age and gender. Furthermore, in terms of survival, we calculated overall survival (OS) according to the Kaplan-Meier method for all patients in both retrospective and prospective studies, OS with or without IFI, according to whether or not prophylaxis was implemented, according to the type of azole used for prophylaxis (posaconazole versus fluconazole) and finally according to the kind of hospitalization (protected single room versus common room). The comparison of the survival curves was carried out using the Log Rank test.
Results
Retrospective study
Of the 188 AML patients receiving intensive induction chemotherapy and fluconazole prophylaxis, 74 patients developed an IFI (39%). Among the IFIs, 12 IFIs (16%) were not identified, and of the 62 documented, 50 were classified as invasive aspergillosis (81%): 45 pulmonary aspergillosis and 5 aspergillosis of sinuses and 12 were classified as invasive candidiasis (19%). According to the European Organization for Research and Treatment of Cancer (EORTC) classification [Probable IFI=At least 1 criteria related to the host + one clinical criteria +At least 1 mycological criteria; Possible IFI= At least 1 crite
| TLEMCEN | ORAN | |
| N, patients | 70 | 118 |
| Induction Chemotherapy | «3+7» | «3+7» |
| Daunorubicine/Aracytine | (60 mg/m2/100 mg/m2) | (60 mg/m2/100 mg/m2) |
| Type of hospitalization | Common Rooms | Protected single Rooms |
| IFI Incidence | 34 (49%) | 40 (34%) (p=0.049) |
| IA | 28 | 22 |
| Pulmonary IA | 25 | 20 |
| Sinuses IA | 3 | 2 |
| Invasive Candidosis | - | 12 |
| NP | 6 | 6 |
ria related to the host + one clinical criteria], 56 IFI (76%) were classified as probable IFI and 18 (24%) as possible IFI. We observed a significant difference between the 2 centers in terms of incidence: 34% at the Oran hospital versus 49% at the Tlemcen hospital and this difference was correlated to the difference in terms of hospitalization (protected single rooms vs. common rooms) (p=0.049) (Table 1).
Among the 74 patients who presented with IFI, 39 died from IFI as the main cause of death (53%), among these deaths, 16 (40%) were recorded at Oran and 23 (68%) at Tlemcen (p=0.011).
Prospective study
The prospective study began in April 2017 and the cut-off date for results was September 30, 2018. During this period, 55 AML patients receiving intensive induction 3+7 chemotherapy were included in the study and received primary prophylaxis with posaconazole during the induction phase, 12 patients had an IFI (22%), and among the 9 documented: 8 (89%) had an IA and 1 had an invasive candidiasis (19%). Eight (67%) of the IFIs were reported as probable and 4 (33%) as possible according to the EORTC classification.
Comparative study
When comparing the results in terms of IFI rate, we found a highly significant difference between the rate observed with fluconazole 39% and that observed with posaconazole 22% when considering the whole treated population (p=0.001) and the cumulative incidence was 39% at 21 days versus 10% at 15 days (p=0.046) with a highly significant decrease in IFI-related deaths: 53% versus 25% (p=0.004) (Table 2 and Figure 1).
| Fluconazole | Posaconazole | P-value | |
| IFI+ | 74/188 (39%) | 12/55 (22%) | P=0.001 |
| IFI- | 114/188 (61%) | 43/55 (78%) | P=0.01 |
| Deaths (IFI+) | 39/74 (53%) | 3/12 (25%) | P=0.004 |
Figure 1. Cumulative Incidence of IFI on Fluconazole and Posaconazole Prophylaxies.
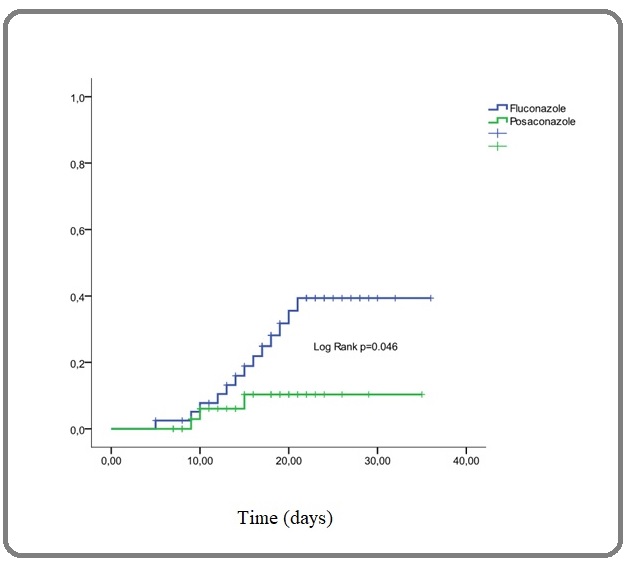
Moreover, our results have shown that when considering the center effect and mainly the common room versus protected single room effect, posaconazole compared to fluconazole improved the incidence of IFI even when patients were in common rooms 49% vs. 43% but not significantly (p=0.8) while the result is highly significant when we considered only patients in protected single rooms with 34% vs. 15% (p=0.017). The number of IFI-related deaths also decreased significantly when comparing fluconazole (33%) and posaconazole (17%). In addition, the number of deaths per center was 16 (40%) and 23 (68%) respectively at Oran hospital (protected single rooms) and Tlemcen hospital (common rooms) with a high significant difference (p=0.011). Table 3 summarized all these last results.
| Type of Hospitalization | Fluconazole Prophylaxis | Posaconazole Prophylaxie | P-value | |
| Tlemcen IFI+ | Common rooms | 34/70 (49%) | 6/14 (43%) | 0.8 |
| Oran IFI+ | Protected single rooms | 40/118 (34%) | 6/14 (15%) | 0.017 |
| Tlemcen (Common rooms) | Oran (Protected single rooms) | P-value | ||
| Posaconazole | 2 (33%) | 1 (17%) | 0.04 | |
| Fluconazole | 23 (68%) | 16 (40%) | 0.011 |
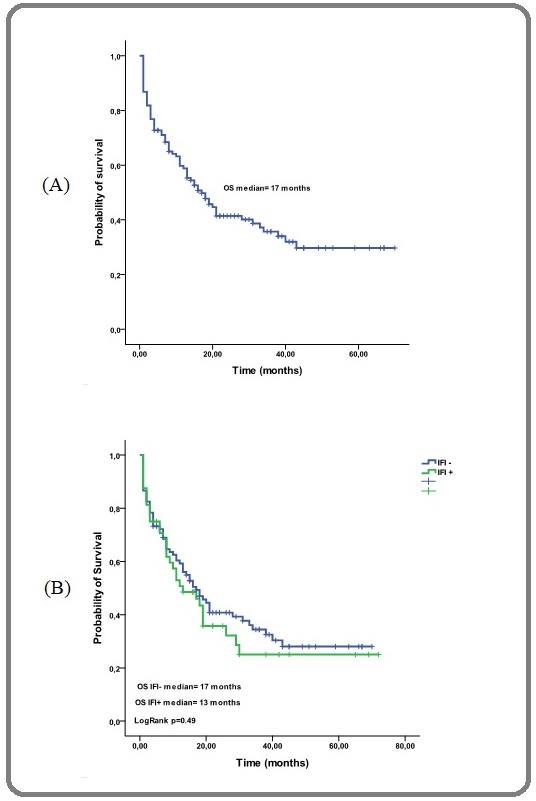
The median overall survival (OS) of the whole cohort was 17 months [Figure 2 (A)], regarding IFI with or without IFI the median OS were 17 months and 13 months respectively (p=0.49) [Figure 2 (B)].
Figure 2. Probability of Overall Survival, (A) for all Patients of Tlemcen and Oran (B) for Patients with or without IFI.
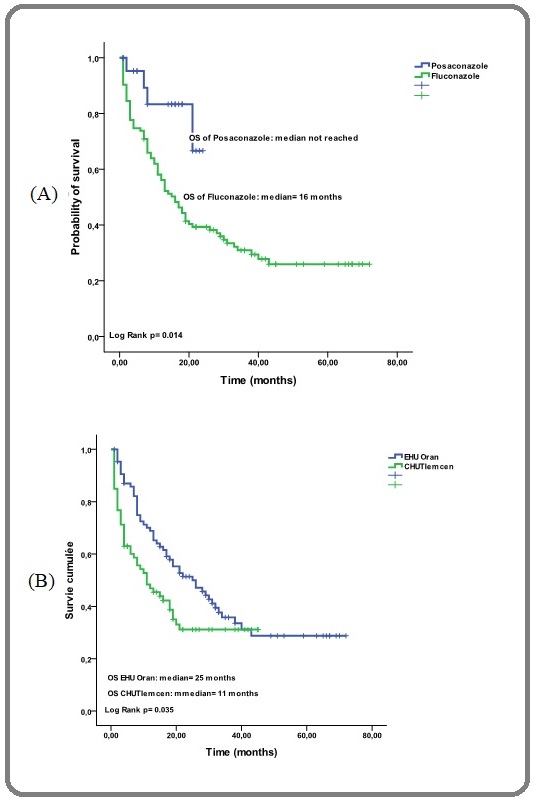
In addition, according to the used azole, we have shown a median OS not reached for posaconazole and at 16 months for fluconazole (p=0.014) [Figure 3 (A)] and finally according to the type of hospitalization the median OS in protected single rooms and common rooms were 25 months and 11 months respectively (p=0.035) [Figure 3 (B)].
Figure 3. Probability of Overall Survival, (A) According to Primary Antifungal Prophylaxis (Posaconazole and Fluconazole)/(B) According to the University Hospital and Kind of Hospitalization (common rooms/ protected single rooms).
Discussion
IFIs remain a serious complication that can occur during the aplasia following intensive induction chemotherapy given to AML patients. Despite the progress concerning diagnostic tools, the diagnosis of IFIs remains still difficult and often late, which often compromises therapeutical results. Among the diagnostic tests used for IFIs, we have galactomannan documentation (aspergillar antigenemia) in serum or bronchoalveolar fluid [9], mannans and antimannans with low sensitivity and low specificity, especially in candidiasis of liver and spleen [10]. PCR can also be used for the IFI diagnosis [11]. Imaging and in particular early CT scan is considered as positive in 90% of cases when showing the sign of halo or peri-lesional frosted glass opacity [12]. The EORTC group has established recommendations for the diagnosis of proven, probable or possible IFI with high sensitivity [13]. Therapeutically, there are several possibilities: prophylaxis, empirical treatment, preemptive treatment and finally curative treatment. Early treatment includes empirical treatment (treatment of persistent fever after 3 to 7 days of broad-spectrum empirical antibiotics or recurrent fever after initial apyrexia) and preemptive treatment (guided by clinical signs, biomarkers, CT data, or a combination of several of these criteria) that reduces the incidence of IFIs and IFI-related death rates [14]. Primary prophylaxis has also been shown to be effective in terms of IFI incidence and IFI-related mortality, including posaconazole prophylaxis. Several publications have demonstrated the superiority of this type of prophylaxis in hematology patients at high risk of IFI with two pivotal studies: during AML and MDS receiving intensive induction chemotherapy and during graft versus host disease (GVHD) on immunosuppression after allogeneic hematopoietic stem cell transplantations [8][15]. Posaconazole is an azole antifungal agent with a mechanism of action focused on membrane destruction at the ergosterol (predominant fungal membrane sterol) level. There are currently 2 galenic forms: the oral suspension and the film-coated tablet form with absorption problems that have been demonstrated during the administration of the oral suspension, which requires for good absorption an oral intake of high-fat meals, which is difficult in the neutropenic period [16]. An alternative in this context has been evaluated with a significant improvement in absorption through the ingestion of coca-cola [17]. The film-coated tablet form allows excellent rapid absorption without significant intra- and inter-individual variation [18], but this form is not currently available in Algeria. It is estimated that an effective rate is reached in terms of prophylaxis using the oral suspension when a plasma level is above > 0.7 µg/mL [19]. However, some studies have shown that despite a lower plasma level, a significant efficacy in terms of IFI incidence has been nevertheless observed. Subsequently, many therapeutic trials have highlighted the value of primary prophylaxis with a drastic reduction in the incidence of IFI and have led to the development of international recommendations [6] [20]. Our study in Algeria has shown a predominance of invasive IA with few reported cases of invasive candidiasis during AML, which is similar to the results in the literature [7] [21]. Regarding the recent introduction of posaconazole in Algeria, it was given as prophylaxis during AML receiving induction chemotherapy in the two hematological departments of Tlemcen and Oran for identical AML patients in terms of disease and chemotherapy treatment but with a difference in terms of hospitalization with common rooms in Tlemcen and protected single rooms in Oran. This difference allows assessing the impact of the environment in the context of antifungal prophylaxis, a missing data in most current studies on prophylaxis. We confirmed in this study in Algeria the results already published in the world [6][20] [8-9] in a prospective randomized and matched study with a highly significant decrease in incidence and mortality related to IFI after using posaconazole prophylaxis during chemotherapy induction in AML patients. In addition, we also demonstrated the value of protected single rooms during prophylaxis with fluconazole. In this context, we have shown that there is a decrease in the incidence of IFI on posaconazole compared to fluconazole whatever is the environment. Moreover, posaconazole significantly decreases the rate of IFI-related deaths especially in the context of protected single rooms, which shows the value of the current optimal management strategy when posaconazole is combined with protected single room.
In conclusion, this work has demonstrated in Algeria the interest in terms of IFI incidence and IFI-related mortality of posaconazole prophylaxis used during AML receiving intensive induction chemotherapy even in the context of hospitalization in common rooms. Furthermore, this work highlights that the combination of posaconazole prophylaxis and hospitalization in a protected single room allows an optimization of the management of this type of patients.
Acknowledgements
I would like to thank the care teams of the Hematology Departments of the Tlemcen University Hospital and the University Hospital 1st November in Oran, as well as the patients and their families.
All the co-authors declare that they have no conflict of interest.
References
- Patterns of leukemia incidence in the United States by subtype and demographic characteristics, 1997-2002 Yamamoto Jennifer F., Goodman Marc T.. Cancer causes & control: CCC.2008;19(4). CrossRef
- Incidence, survival and prevalence of myeloid malignancies in Europe Visser O., Trama A., Maynadié M., Stiller C., Marcos-Gragera R., De Angelis R., Mallone S., Tereanu C., Allemani C., Ricardi U., Schouten H. C.. European Journal of Cancer (Oxford, England: 1990).2012;48(17). CrossRef
- Bekadja MA et al. Hematol Oncol Stem Cell Ther 2011 ; 4 (4) : 161-166 .
- Hamladji RM. État des lieux de la prise en charge des hémopathies malignes en Algérie. Revue Algérienne d’Hématologie, 2013, 8/9 .
- Aspergillus fumigatus and related species Sugui Janyce A., Kwon-Chung Kyung J., Juvvadi Praveen R., Latgé Jean-Paul, Steinbach William J.. Cold Spring Harbor Perspectives in Medicine.2014;5(2). CrossRef
- Invasive aspergillosis: an important risk factor on the short- and long-term survival of acute myeloid leukemia (AML) patients Michallet M., Bénet T., Sobh M., Kraghel S., El Hamri M., Cannas G., Nicolini F. E., Labussière H., Ducastelle S., Barraco F., Thomas X., Chelghoum Y., Nicolle M.-C., Bienvenu A.-L., Persat F., De Monbrison F., Picot S., Vanhems P.. European Journal of Clinical Microbiology & Infectious Diseases: Official Publication of the European Society of Clinical Microbiology.2012;31(6). CrossRef
- ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients Tissot Frederic, Agrawal Samir, Pagano Livio, Petrikkos Georgios, Groll Andreas H., Skiada Anna, Lass-Flörl Cornelia, Calandra Thierry, Viscoli Claudio, Herbrecht Raoul. Haematologica.2017;102(3). CrossRef
- Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia Cornely Oliver A., Maertens Johan, Winston Drew J., Perfect John, Ullmann Andrew J., Walsh Thomas J., Helfgott David, Holowiecki Jerzy, Stockelberg Dick, Goh Yeow-Tee, Petrini Mario, Hardalo Cathy, Suresh Ramachandran, Angulo-Gonzalez David. The New England Journal of Medicine.2007;356(4). CrossRef
- Correlation between galactomannan antigen levels in serum and neutrophil counts in haematological patients with invasive aspergillosis Cordonnier C., Botterel F., Ben Amor R., Pautas C., Maury S., Kuentz M., Hicheri Y., Bastuji-Garin S., Bretagne S.. Clinical Microbiology and Infection: The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases.2009;15(1). CrossRef
- Three-dimensional models of wild-type and mutated forms of cytochrome P450 14alpha-sterol demethylases from Aspergillus fumigatus and Candida albicans provide insights into posaconazole binding Xiao Li, Madison Vincent, Chau Andrew S., Loebenberg David, Palermo Robert E., McNicholas Paul M.. Antimicrobial Agents and Chemotherapy.2004;48(2). CrossRef
- Diagnosis of invasive fungal infections by a real-time panfungal PCR assay in immunocompromised pediatric patients Landlinger C., Preuner S., Bašková L., Grotel M., Hartwig N. G., Dworzak M., Mann G., Attarbaschi A., Kager L., Peters C., Matthes-Martin S., Lawitschka A., Heuvel-Eibrink M. M., Lion T.. Leukemia.2010;24(12). CrossRef
- Thoracic manifestations of aspergillosis Klein D. L., Gamsu G.. AJR. American journal of roentgenology.1980;134(3). CrossRef
- Primary prophylaxis of invasive fungal infections in patients with haematological malignancies: 2017 update of the recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society for Haematology and Medical Oncology (DGHO) Mellinghoff Sibylle C., Panse Jens, Alakel Nael, Behre Gerhard, Buchheidt Dieter, Christopeit Maximilian, Hasenkamp Justin, Kiehl Michael, Koldehoff Michael, Krause Stefan W., Lehners Nicola, Lilienfeld-Toal Marie, Löhnert Annika Y., Maschmeyer Georg, Teschner Daniel, Ullmann Andrew J., Penack Olaf, Ruhnke Markus, Mayer Karin, Ostermann Helmut, Wolf Hans-H., Cornely Oliver A.. Annals of Hematology.2018;97(2). CrossRef
- Empirical versus preemptive antifungal therapy for high-risk, febrile, neutropenic patients: a randomized, controlled trial Cordonnier Catherine, Pautas Cécile, Maury Sébastien, Vekhoff Anne, Farhat Hassan, Suarez Felipe, Dhédin Nathalie, Isnard Francoise, Ades Lionel, Kuhnowski Frédérique, Foulet Françoise, Kuentz Mathieu, Maison Patrick, Bretagne Stéphane, Schwarzinger Michaël. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America.2009;48(8). CrossRef
- Comparative clinical effectiveness of prophylactic voriconazole/posaconazole to fluconazole/itraconazole in patients with acute myeloid leukemia/myelodysplastic syndrome undergoing cytotoxic chemotherapy over a 12-year period Ananda-Rajah Michelle R., Grigg Andrew, Downey Maria T., Bajel Ashish, Spelman Tim, Cheng Allen, Thursky Karin T., Vincent Janette, Slavin Monica A.. Haematologica.2012;97(3). CrossRef
- Oral bioavailability of posaconazole in fasted healthy subjects: comparison between three regimens and basis for clinical dosage recommendations Ezzet Farkad, Wexler David, Courtney Rachel, Krishna Gopal, Lim Josephine, Laughlin Mark. Clinical Pharmacokinetics.2005;44(2). CrossRef
- Effect of pH and Comedication on Gastrointestinal Absorption of Posaconazole Walravens Jeroen, Brouwers Joachim, Spriet Isabel, Tack Jan, Annaert Pieter, Augustijns Patrick. Clinical pharmacokinetics.2011;50. CrossRef
- Antifungal Prophylaxis with Posaconazole Delayed-Release Tablet and Oral Suspension in a Real-Life Setting: Plasma Levels, Efficacy, and Tolerability Lenczuk David, Zinke-Cerwenka Wilma, Greinix Hildegard, Wölfler Albert, Prattes Jürgen, Zollner-Schwetz Ines, Valentin Thomas, Lin Timothy C., Meinitzer Andreas, Hoenigl Martin, Krause Robert. Antimicrobial Agents and Chemotherapy.2018;62(6). CrossRef
- Risk factors for invasive aspergillosis in acute myeloid leukemia patients prophylactically treated with posaconazole Michallet Mauricette, Sobh Mohamad, Morisset Stéphane, Kraghel Samira, Nicolini Franck-Emmanuel, Thomas Xavier, Bienvenu Anne-Lise, Picot Stéphane, Nicolle Marie-Christine, Vanhems Philippe. Medical Mycology.2011;49(7). CrossRef
- European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3--2009 Update Maertens J., Marchetti O., Herbrecht R., Cornely O. A., Flückiger U., Frêre P., Gachot B., Heinz W. J., Lass-flörl C., Ribaud P., Thiebaut A., Cordonnier C.. 2011. CrossRef
- Population-based analysis of invasive fungal infections, France, 2001-2010 Bitar Dounia, Lortholary Olivier, Le Strat Yann, Nicolau Javier, Coignard Bruno, Tattevin Pierre, Che Didier, Dromer Françoise. Emerging Infectious Diseases.2014;20(7). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2021
Author Details