Hematologic Presentations of COVID-19 Can be Misinterpreted as Acute Myeloid Leukemia
Download
Abstract
Introduction: COVID-19 infection prompts inflammatory responses and acute lung injury in human beings. Complete blood count with differential is essential investigative tool in its managing. However, very few studies revealed the variations of blood cell morphology in this disease.
Case report: We reported a 39-years- old female patient complained of respiratory distress one week prior to hospitalization. The patient suffered from cough, fever, and molecular test was reported positive for COVID-19 infection. Laboratory data revealed severe permanent leukopenia and peripheral blood smear examination showed blastoid cells after remission of respiratory signs. Patient underwent bone marrow biopsy for rule out acute myeloid leukemia. But, on bone marrow sample, only viral cytopathic effects were seen. COVID-19 virus stimulates inflammatory cells to produces various inflammatory cytokines and as a result, viral cytopathic effects on white blood cells is seen.
Conclusion: We have described how the characteristic peripheral blood findings of COVID‐19 infection can be misinterpret as acute myeloid leukemia.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) caused an epidemic of coronavirus disease 2019 (COVID‐19). First known in December 2019, it was confirmed a pandemic by the World Health Organization (WHO) on 11 March 2020 as it has rapidly extent over the world [1]. The standard test for COVID‐19 presents the reverse transcriptase polymerase chain reaction (RT‐PCR) to recognize viral RNA from clinical specimens [2]. COVID-19 is a rapid spreading pandemic with high mortality rate. Assessable hematologic deviations have been distinct in the first COVID-19 patient studies [3]. The hematopoietic system actions a thoughtful character in the notable hyperinflammation, mainly in severely ill patients [4]. Complete blood count (CBC) is a routine test throughout preliminary biological assessment of patients. CBC analyzers such as SYSMEX® (Sysmex Corporation, Kobe, Japan), make available a white blood cell (WBC) with differential count which exhibiting a sorting of WBCs founded on their morphology and their intracellular components. But, the abnormal leukocyte result is rechecked by peripheral blood smear (PBS) examination for reassessment. Herein, we report a COVID-19 positive patient with severe permanent leukopenia on CBC and blastoid cells on PBS who underwent bone marrow biopsy for rule out acute leukemia as first diagnosis.
Case report
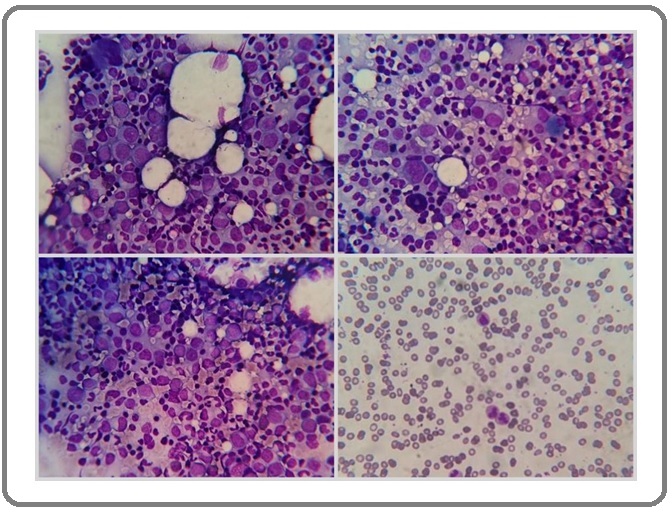
A 39-year-old woman with no known medical history presented to the emergency department in Sina hospital affiliated with Tehran University of Medical Sciences with a 1-week history of fevers, chills, worsening shortness of breath, headache, and malaise. Upon entrance to the hospital, her vital signs included a temperature of 38° C, heart rate of 100 beats/min, respiratory rate of 28 breaths/min, and oxygen saturation of 90% on room air. She needed oxygen at 6 L/min via a nasal cannula when she was in the emergency department. The first chest x-ray revealed perihilar opacification. She had positive RT-PCR test for COVID-19 and was consequently admitted to the COVID-19 unit for controlling of acute hypoxemic respiratory failure and COVID-19. Laboratory data documented before this encounter included hemoglobin of 10.2 g/dL, red blood cells of 3.8x106/µL, platelet of 163x10³/µL, C-reactive protein of 36.8 mg/L, prothrombin time of 12.2, and a partial prothrombin time of 33.8. The patient’s leukocyte (1.5x10³/µL with differential count: 84% neutrophils and 16% lymphocytes) persistent decreased with immature and blastoid cells on PBS after remission of respiratory symptoms that was requested hematology consultation for her. Because, the patient had persistent leukopenia with suspicious immature and blastoid cells on PBS, she underwent bone marrow biopsy (BMB) and aspiration. Bone marrow aspiration showed shift to left in myeloid series with presentation of extensive immature myeloid series and had about 30% atypical cells which were suspicious to blast (Figure 1).
Figure 1. Bone Marrow Aspiration and Peripheral Blood Smear (right lower) Examination Showed Immature Myeloid Cells and Blastoid Cells (Wright&Giemsa stain, x400).
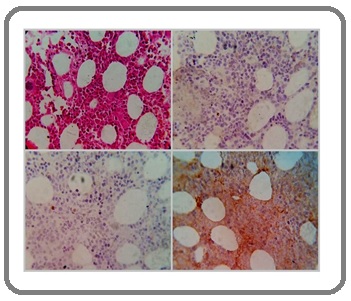
First, we considered atypical cells were blast and acute myeloid leukemia was main diagnosis. So, immunohistochemistry staining on bone marrow biopsy was done. But, CD34, C-Kit, and terminal deoxynucleotidyl transferase (TDT) were unremarkable (Figure 2).
Figure 2. Bone Marrow Biopsy Showed Immature Myeloid Series and Immunohistochemistry Staining Revealed CD34 Positive in 2-3% Blasts (left upper), TDT Positive in 1% Blasts (right lower) and C-Kit had Nonspecific Staining (left lower).
Slides sent to expert hematologic center (Shariati hospital) for rule out leukemia. So, according to patient’s history, IHC staining, and result of consultation, viral hematologic effect was established. Base on the diagnosis, the patient received no chemotherapy. After 6 months of follow-up examinations, leukocyte count increased gradually and was 8.1x10³/µL.
Discussion
COVID-19 is a disease initiated by SARS-CoV-2, which mostly causes pulmonary inflammatory lesions, and can also make damage and related symptoms of intestinal tract, liver and the nervous system. The virus spread through droplets and contact. The main clinical presentations are fever, fatigue, dry cough, dyspnea, etc. In their recent publication, Mina and colleagues introduced some of the usual hematological exhibitions of the COVID-19 viral pandemic [5]. Laboratory tests discovered that white blood cells were normal or decreased and lymphocytes decreased [6]. Like most viruses that influence hematopoiesis and the immune system through progressive stages, COVID-19 enhances release of immature blood cells from bone marrow by inflammatory mechanisms and impress the myelopoiesis system [7]. Patients with COVID-19 have abnormal peripheral blood routine examination results [8]. This is similar to our patient who had immature and blastoid cells on PBS. Leukocyte and neutrophil counts were lower in young patients with COVID‐19. Lymphopenia and reactive lymphocytosis, dysplastic changes of granulocytes, and platelets, even though not specific and diagnostic, could be prominent [9] which in our reported case, firstly, leukopenia was seen in laboratory data. Schapkaitz E, et al. study showed on PBS examination, atypical lymphocytes, which are well demarcated in the contextual of viral infections, was discovered in 57.8% patients. Other common morphological discoveries contained; dysplastic neutrophils and a myeloid left shift [10] which in our case, blastoid cells were dysplastic immature neutrophils series and established by BMB and IHC study. By noticing blood smears colored by Wright-Giemsa stain, we distinguished the characteristics of a neutrophil granulocyte with dysmorphic morphology marked by hypogranular cytoplasm and hyposegmented nucleus due to the upregulation of proinflammatory cytokines in patients with COVID-19 infection were realized [4]. Inhibitory effects of cytokines from virus-involved cells on hematopoiesis might also be responsible for dysplastic changes in myeloid series [11]. Awareness of these patterns of morphologic changes in peripheral blood, if confirmed with greater studies, may aid physicians in diagnose COVID-19 in the lack of a negative RT-PCR test.
In addition, some authors have formerly recommended an association among morphological changes in CBC and disease development or outcome [12]. Considering these morphologic variations and hematologic parameters by sequential PBS and CBC can help in the diagnosis of COVID-19 and accurate management of COVID-19 patients.
In conclusion, observation of blood cells is able to be a simple alternative tool for the first triage and initial identification of the infection. CBC with PBS can identify the impression of the virus on the blood, reflecting early inflammatory signs. Dysplastic cells due to viral effect should be considered.
References
- Frontline Science: COVID‐19 infection induces readily detectable morphologic and inflammation‐related phenotypic changes in peripheral blood monocytes Zhang Dan, Guo Rui, Lei Lei, Liu Hongjuan, Wang Yawen, Wang Yili, Qian Hongbo, Dai Tongxin, Zhang Tianxiao, Lai Yanjun, Wang Jingya, Liu Zhiqiang, Chen Tianyan, He Aili, O'Dwyer Michael, Hu Jinsong. Journal of Leukocyte Biology.2020;109(1). CrossRef
- Rapid screening of COVID‐19 patients using white blood cell scattergrams, a study on 381 patients Osman Jennifer, Lambert Jérome, Templé Marie, Devaux Floriane, Favre Rémy, Flaujac Claire, Bridoux Delphine, Marque‐Juillet Stéphanie, Bruneel Fabrice, Mignon François, Diaz‐Flores Ernesto, Hentgen Véronique, Greder‐Belan Alix, Azarian Reza, Koukabi Mehrsa, Rousselot Philippe, Raggueneau Victoria, Manéglier Benjamin. British Journal of Haematology.2020;190(5). CrossRef
- Morphological anomalies of circulating blood cells in COVID ‐19 Zini Gina, Bellesi Silvia, Ramundo Francesco, d'Onofrio Giuseppe. American Journal of Hematology.2020;95(7). CrossRef
- Coronavirus disease 2019 induces multi‐lineage, morphologic changes in peripheral blood cells Lüke Florian, Orsó Evelyn, Kirsten Jana, Poeck Hendrik, Grube Matthias, Wolff Daniel, Burkhardt Ralph, Lunz Dirk, Lubnow Matthias, Schmidt Barbara, Hitzenbichler Florian, Hanses Frank, Salzberger Bernd, Evert Matthias, Herr Wolfgang, Brochhausen Christoph, Pukrop Tobias, Reichle Albrecht, Heudobler Daniel. eJHaem.2020;1(1). CrossRef
- Anemia and red blood cell abnormalities in COVID-19 Murphy Philip, Glavey Siobhan, Quinn John. Leukemia & Lymphoma.2021;62(6). CrossRef
- A mild type of childhood Covid-19 - A case report Yin Xiaoping, Dong Li, Zhang Yu, Bian Weilin, Li Hongjun. Radiology of Infectious Diseases.2020;7(2). CrossRef
- Peripheral Blood Smear Findings in COVID-19 Ahnach Maryame, Ousti Fadwa, Nejjari Sara, Houssaini Mohammed Sqalli, Dini Nouzha. Turkish Journal of Hematology.2020;37(4). CrossRef
- Abnormalities of peripheral blood system in patients with COVID-19 in Wenzhou, China Sun Suyu, Cai Xuejiao, Wang Huaguo, He Guiqing, Lin Yin, Lu Bibi, Chen Chaoyue, Pan Yong, Hu Xingzhong. Clinica Chimica Acta.2020;507. CrossRef
- Hematological parameters and peripheral blood morphologic abnormalities in children with COVID‐19 Yarali Neşe, Akcabelen Yunus Murat, Unal Yasemin, Parlakay Aslı Nur. Pediatric Blood & Cancer.2020;68(2). CrossRef
- The characteristic peripheral blood morphological features of hospitalized patients infected with COVID‐19 Schapkaitz Elise, De Jager Tanya, Levy Brian. International Journal of Laboratory Hematology.2020;43(3). CrossRef
- Dysplastic Changes of Peripheral Blood Cells in COVID-19 Infection Akçabelen Yunus Murat, Gürlek Gökçebay Dilek, Yaralı Neşe. Turkish Journal of Hematology.2021;38(1). CrossRef
- Morphologic Changes in Circulating Blood Cells of COVID-19 Patients Kaur Gagandeep, Sandeep FNU, Olayinka Oluwaseyi, Gupta Gunjan. Cureus.2021. CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2021
Author Details