A Prospective Study of I.V. Vinflunine in the Treatment of Patients with Advanced or Metastatic Urothelial Carcinoma after Failure of a Platinum-containing Regimen and Biomarker Correlates
Download
Abstract
Background: Vinflunine is the only cytotoxic agent that had been tested as a second line therapy in platinum refractory urothelial carcinoma patients in a phase III clinical trial. The aim of our study was to evaluate the efficacy and safety of vinflunine as a second line after failure of platinum containing regimen.
Patients and methods: We prospectively included 27 patients of locally advanced or metastatic urothelial cancer who presented to the National Cancer Institute (NCI) of Egypt. The primary objective was to assess the disease control rate. However, the secondary objectives were to assess the progression free survival (PFS) and overall survival (OS).
Results: A total of 27 patients were treated at the NCI of Egypt. Median age was 64.1 years (42.3-76.8). Male to female ratio was 26:1. Eastern Cooperative Oncology Group performance status was zero in 2 patients, one in 23 patients while the ECOG PS 2 was in only 2 patients. The vast majority of the patients received 2 cycles (12 patients), one patient received 3 cycles, 5 patients received 4 cycles, and 8 patients received 6 cycles while one patient received 8 cycles. A complete response was observed in one patient, partial response in 9 patients and stable disease in 12 patients and progressive disease in 5 patients with a disease control rate of 81.4%. Median progression free survival (PFS) and overall survival for the entire population were 3.45 months and 3.22 months respectively. Median OS for the responders was 7.24 months. Toxicity was mild, and grade 3-4 adverse events were anemia (11.1%), neutropenic fever (4%), fatigue (14.8%) and constipation (7.4%).
Conclusion: Vinflunine is an efficient and tolerable second line treatment in advanced urothelial carcinoma.
Introduction
Urothelial tract carcinoma represents a major health problem worldwide. In fact, it is the sixth most common type of cancer in western countries [1]. Traditionally, advanced urothelial carcinomas have been considered chemo sensitive tumors based on high radiological response rates of 40-70% with cisplatin-based schemes such as gemcitabine-cisplatin (GC), methotrexate, vinblastine, doxorubicin, and cisplatin (M-VAC) or paclitaxel, cisplatin, and gemcitabine (PCG) [2]. Unfortunately, responses are not maintained over time and median progression free and overall survivals rarely exceed 8 and 15 months, respectively, when metastatic urothelial carcinoma patients are treated in first-line [3]. Patients who fail the initial systemic approach for advanced disease represent a challenge in daily clinical practice.
In the last decade, wide ranges of single agents or combination schemes have been tested for activity in patients who are resistant to previous platinum approaches. The drugs explored in this setting included paclitaxel, nab- paclitaxel, irinotecan, ixabepilone, bortezomib, pemetrexed, oxaliplatin, ifosfamide, lapatinib, docetaxel, gemcitabine, topotecan, gefitinib, sorafenib, sunitinib, and pazopanib. The most promising combined chemotherapy schemes among those studied were paclitaxel plus gemcitabine [4], ifosfamide plus gemcitabine [5] or carboplatin plus paclitaxel [6]. Despite the great efforts and resources devoted to all these trials, together with the number of patients involved, in most cases the clinical outcomes were disappointing with objective response rates ranging between 10 and 20%, median progression free survivals of 2–3 months, and median overall survivals of 6–9 months [7]. However other agents have been introduced and are showing promising results [8].
Vinflunine is the newest member of the vinca alkaloids family available to clinical practice [9]. As with other tubulin inhibitors, vinflunine prevents microtubule assembly during mitosis and induces apoptosis [10]. The main differentiating feature that distinguishes vinflunine from others vinca alkaloids is the affinity profile of vinflunine which has a greater effect on mitotic rather than axonal tubulin. Therefore, the result is a significantly reduced rate of neurotoxicity which allows for greater plasma concentrations of the drug [11]. The clinical activity of vinflunine in patients with metastatic transitional cell carcinoma of the urothelial cancer (TCCU) was initially assessed in two non-randomized phase II trials [12]. The earlier phase II trials showed that the activity of vinflunine in 51 and 175 platinum-resistant urothelial carcinoma patients achieved response rates of 18% and 15%, respectively, and median duration of responses were 9.1 and 6 months. Median progression free survival and overall survival were 3.0 and 6.6 months in the first trial, and 2.8 and 8.2 months in the second one. These consistent results led to a pivotal, multinational, and randomized study that compared vinflunine and best supportive care in the second-line treatment of advanced urothelial carcinoma patients who had previously progressed after a platinum-containing regimen [13]. A total of 370 patients were recruited and vinflunine had shown to be superior to the control arm in terms of the considered primary endpoint of the study which was overall survival in the intention to treat population (6.9 months vs. 4.6 months).
However, these results were not found to be statistically significant (HR 0.88; 95% CI, 0.69-1.12: P = 0.287).
All other efficacy parameters favored vinflunine clinically and were statistically significant, such as overall survival in the analysis per protocol population (6.9 vs. 4.3 months: P = 0.04), overall response rate (16% vs 0%: P = 0.0063), disease control rate (41.1% vs. 24.8%: P = 0.0024), and median progression free survival (3.0 months vs. 1.5 months: P = 0.0012). The duration of objective responses was 7.4 months (95% CI 4.5 to 17.0 months) in those patients treated with vinflunine.
Long-term overall survival data from this registration trial after a follow-up of more than 45 months confirmed the increase in total median overall survival with vinflunine compared to best supportive care in the intention to treat population (6.9 months vs. 4.6 months) and the statistically significant increase in the eligible population (6.9 vs. 4.3 months; HR 0.78; 95% CI 0.61- 0.96: P = 0.00227) [14].
As a result of this study, vinflunine was the first drug to receive approval from the European Medicine Agency (EMA) for use in platinum resistant metastatic urothelial carcinoma patients. Astudy was conducted retrospectively, observational, and a non-interventional study (according to the classification of the Spanish Health Authorities) to assess the impact of treatment with vinflunine in the daily practice in terms of toxicity, response rate, duration of response, progression free survival, and overall survival in an unselected subgroup of patients with metastatic urothelial carcinoma who had progressed after only one previous line of platinum-containing regimen for advanced disease, and furthermore assessed the reproducibility of the clinical trial results in routine clinical practice.
Materials and Methods
Patients
This is a prospective single center open label phase II study conducted at the medical oncology department of the National Cancer Institute of Cairo University. Patients were randomly assigned to receive second line single agent vinflunine chemotherapy drug after documented progression on the first line platinum containing regimen of locally advanced or metastatic carcinoma of the urothelial tract.
Twenty-seven patients were enrolled from August 2013 till October 2017. The study duration was planned to continue until the last patient withdrawn from the treatment. After withdrawal from the study treatment each patient was be followed until death.
Our primary objective was to evaluate the disease control rate as defined by RECIST (version 1.1) assessment criteria [Complete Response (CR) + Partial Response (PR) + Stable Disease (SD) rates] in patients with advanced or metastatic urothelial carcinoma previously treated with a platinum-containing chemotherapy as 1st line treatment. However, the second objective was to evaluate other efficacy parameters as objective response rate (ORR) = CR + PR Rate, time to response, duration of response, progression free survival (PFS) as well as to assess the safety profile of vinflunine (Javlor®) in this category of patients.
We calculated the number of risk factors exhibited by each patient including:
- The time from previous chemotherapy (less than 3 months),
- ECOG PS (more than zero),
- Liver metastasis,
-Hemoglobin level (less than 10gm/dl) and
- Albumin level (< LLN)
We calculated the overall survival for risk factors (0-1), (2) and (3+).
Furthermore, we have applied the Bajorin risk stratification by using the performance status and the visceral metastasis (liver, lung, bone) [15]. We calculated the risk factors based on (zero), (1 risk factor) and (2 risk factors).
Results
We prospectively included 27 patients of locally advanced or metastatic urothelial cancer who presented to the National Cancer Institute (NCI) of Egypt. The baseline characteristics of the study population are listed in Table 1.
| Characteristics | Total, N=27 (%) |
| Gender: | |
| · Male | 26 (96.3) |
| · Female | 1 (3.7) |
| Age: | |
| ·Median | 64.1 years |
| ·Range | 42.3-76.8 |
| Age group: | |
| ·≤50 | 2 (7.4) |
| · ˃50---≤60 | 8 (29.6) |
| · ˃60---≤70 | 13 (48.2) |
| · ˃70 | 4 (14.8) |
| ECOG PS when starting vinflunine | |
| · PS 0 | 2 (7.4) |
| · PS 1 | 23 (85.2%) |
| · PS 2 | 2 (7.4) |
| Hemoglobin level (gm/dl): | |
| · Median | 10.8g/dL |
| · Range | 8.1-15 |
| Creatinine level (mg/dl) | |
| · Median | 1.4 |
| · Range | 0.7-3.7 |
| Total Bilirubin (mg/dl) | |
| · Median | 0.9 |
| · Range | 0.1-6.6 |
| Bilharzial history: | |
| · Yes | 11 (40.7) |
| · No | 16 (59.3%) |
| Prior surgery for cancer: | |
| · None | 13 (48.1) |
| · Radical cystectomy | 7 (25.9) |
| · Partial cystectomy | 1 (3.7) |
| · TURBT | 5 (18.5) |
| · Radical nephrectomy | 1 (3.7) |
| Prior radiotherapy: | |
| · Yes (bladder and pelvis) | 3 (11.2) |
| · No | 24 (88.8) |
The male to female ratio was 26:1. The median age group was 64.1 years ranging from 42.3 to 76.8 years. Out of the 27 patients, 11 patients had bilharzial history. Two patients had an Eastern Cooperative Oncology Group performance status (ECOG PS) of zero while the vast majority (23 patients) had ECOG PS 1, and two patients had ECOG PS of 2.
The median hemoglobin level was 10.8gm/dl (ranging from 8.1 to 15gm/dl). The median creatinine level was 1.4 mg/dl (ranging from 0.7 to 3.7mg/dl), and the median bilirubin level was 0.9 (ranging from 0.1 to 6.6mg/dl).
Thirteen patients had no prior surgery for their tumors. Seven patients were subjected to radical cystectomy and one patient had partial cystectomy. Five patients had transurethral resection of their superficial tumor (TURBT).Only one patient had radical nephrectomy. While 3 patients had previously received radiotherapy to the bladder and pelvis, 24 patients didn’t receive radiation.
The tumor characteristics at the time of first diagnosis are shown in Table 2.
| Location of the disease | |
| · Locally advanced | 4 (14.9%) |
| · Metastatic | 23 (85.1%) |
| Visceral metastasis: | |
| · Liver | 8 |
| · Lung | 6 |
| · Pleural effusion | 1 |
| · Pancreas | 1 |
| · Peritoneum | 1 |
| Non visceral metastasis | |
| · Lymph nodes | 6 |
Four patients presented with locally advanced disease and 23 patients had metastases. Liver metastases were present in 8 patients and 6 patients had lung metastases. Distant lymph nodal metastases were present in 6 patients. Finally, pleural effusion was present in one patient.
Tumor characteristics at the time of first diagnosis are shown in Table 3.
| Tumor description | Number (%) |
| Primary tumor type: | |
| · Squamous cell carcinoma of the urothelial tract (Sq cell Ca) | 5 (18.5) |
| · Transitional cell carcinoma of urothelial tract (TCC) | 22 (81.5) |
| Primary tumor location | |
| · Upper urinary tract/urethra | 4 (14.8) |
| · Bladder | 23 (85.2) |
| T-stage | |
| · T2 | 4 (14.8) |
| · T3 | 21 (77.8) |
| · T4 | 2 (7.4) |
| N-stage | |
| · N0 | 6 (22.2) |
| · N1 | 12 (44.4) |
| · N2 | 3 (11.1) |
| · N3 | 6 (22.2) |
| M-stage | |
| · M0 | 4 (22.2) |
| · M1 | 23 (70.4) |
Twenty-two patients (81.5%) presented with transitional cell carcinoma of the urothelial tract while five patients (18.5%) had squamous cell carcinoma of the urothelial tract.
The primary tumor was located in the bladder in 23 (85.2%) patients. However, in 4 (14.8%) patients the primary tumor was located in the upper urinary tract/ urethra.
Based on the American Joint Committee on Cancer (AJCC, 8th edition) TNM staging system for bladder cancer, 4 patients (14.8%) presented with T2 disease and 21 patients (77.8%) presented with T3 disease, while only 2 patients (7.4%) presented with T4 disease. While 6 patients (22.2%) had N0 disease, 12 patients (44.4%) presented with N1 disease. Three patients (11.1%) had N2 disease and 6 patients had N3 disease. Finally, 4 patients (14.8%) had M0 and 23 patients (85.2%) had M1 disease. Twelve patients (44.5%) received 2 cycles. one patient received 3 cycles (3.7%), and 5 patients (18.5%) reached to 4 cycles. Furthermore, 8 patients (29.6%) continued to 6 cycles, and only one patient (3.7%) succeeded to receive a total of 8 cycles.
Patients were then classified as: low risk if had 0-1 risk factors, intermediate risk with 2 risk factors and high risk with 3+ risk factors.
Fourteen patients out of the 27 patients had the time progression from the prior chemotherapy less than 3 months. Twenty-five patients had PS more than zero. Liver metastases were present in 8 patients, 4 patients had haemoglobin level less than 10gm/dl, and 13 patients had an albumin level less than the normal level. This is shown in Table 4.
| Prognostic factors | Yes | No |
| Time from prior chemotherapy (<3 months) | 14 | 13 |
| Performance status (> 0) | 25 | 2 |
| Liver metastases | 8 | 19 |
| Haemoglobin level (<10gm/dl) | 4 | 23 |
Albumin level (|
13 |
14 |
|
So, only 5 patients were at low risk. Nine patients were classified with an intermediate risk, and 13 patients were classified with high risk factors (Table 5).
| Risk factors groups | Number of patients | Percentage (%) |
| Low risk (0-1) | 5 | -18.5 |
| Intermediate risk (2) | 9 | -33.3 |
| High risk (3) | 13 | -48.2 |
Furthermore, we have applied the Bajorin risk stratification by using the performance status and the visceral metastasis (liver, lung, bone). As shown in Table 6, 25 patients had ECOG PS (0-1) and visceral metastases were present in 13 patients.
| Prognostic factors | Yes | No |
| Performance status (2 or more) | 2 | 25 |
| Visceral metastasis (liver, lung, bone) | 13 | 14 |
Accordingly, 12 patients had zero risk factors and 15 patients had one risk factor while none of the patients had 2 risk factors (Table 7).
| Bajorin risk factors group | Number of patients | Percentage (%) |
| Zero risk factors | 12 | (44.40) |
| (1) Risk factor | 15 | (55.60) |
| (2) Risk factors | 0 | (0) |
Toxicity
The most common adverse event as shown in Table 8 and Figure 1 presented to us during the treatment course was the constipation. Seventeen patients (68%) developed constipation ranging from grade 1 to grade 3 toxicity. The second evident adverse event was the fatigue in 15 patients (60%). That was followed by anemia where 8 patients suffered from grade 1 to grade 3 toxicity.
Figure 1. Treatment Related Adverse Events.
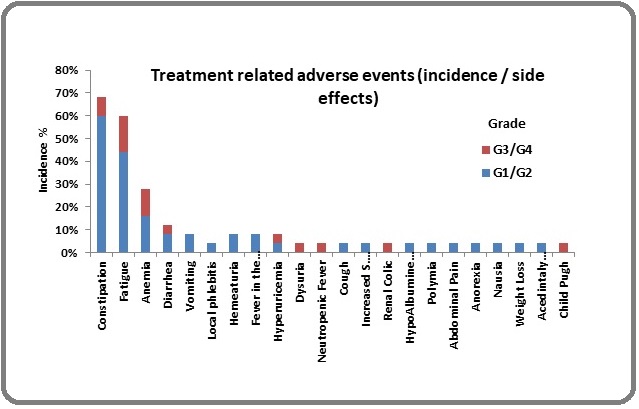
| AE | N (%) | G1 | G2 | G3 |
| None | 1 (4) | |||
| Constipation | 17 (68) | 1 | 14 | 2 |
| Fatigue | 15 (60) | 3 | 8 | 4 |
| Anemia | 8 (32) | 1 | 3 | 3 |
| Diarrhea | 3 (12) | 2 | 1 | |
| Vomiting | 2 (8) | 1 | 1 | |
| Local phlebitis | 2 (8) | 2 | ||
| Hematuria | 2 (8) | 2 | ||
| Fever in absence of neutropenia | 2 (8) | 1 | ||
| Hyperuricemia | 1 (4) | |||
| Dysuria | 1 (4) | 1 | ||
| Neutropenic fever | 1 (4) | 1 | ||
| Cough | 1 (4) | |||
| Increased creatinine | 1 (4) | 1 | ||
| Renal colic | 1 (4) | 1 | ||
| Hypoalbuminemia | 1 (4) | 1 |
Fatigue, anemia and finally constipation were the most common side effects that developed grade 3 toxicity without any grade 4 toxicity. Four patients (16%) developed grade 3 fatigue while 3 patients (12%) developed grade 3 anemia.
However, 2 patients only (8%) developed grade 3 anemia.
Three patients (12%) developed diarrhea where 2 of them had grade 2 toxicity and only one patient developed grade 3 diarrhea. Grade 2 vomiting was associated with 1 patient only and the other one was of grade 3. Local phlebitis developed in 2 patients only with grade 2 toxicity. There were no statistically significant differences in the previously reported adverse event except in constipation with a p value of 0.012.
Disease control rate included patients who achieved a partial response, complete response and stable disease and who maintained this response for duration of at least one month. Evaluation was done every 2 cycles.
The median observation time of the study was 14 weeks (range: 5-92 weeks). The overall response rate was 37% (10/27 patients). Twenty-two out of 27 (81.4%) of enrolled subjects achieved positive disease control (partial response, complete response and stable disease) and maintained this response for a duration of at least one month. Five patients had disease progression (Table 9, and Figure 2).
| Response after 30 days | N | % | |
| Complete Response | 1 | 3.7 | |
| Partial Response | 9 | 33.3 | |
| Positive Disease Control | |||
| Stable Disease | 12 | 44.4 | |
| Disease control | 22 | 81.4 | |
| Negative Disease Control | Progressive Disease | 5 | 18.6 |
| Total | 27 | 100 |
Figure 2. Change in Tumour Size by Waterfall Plot.
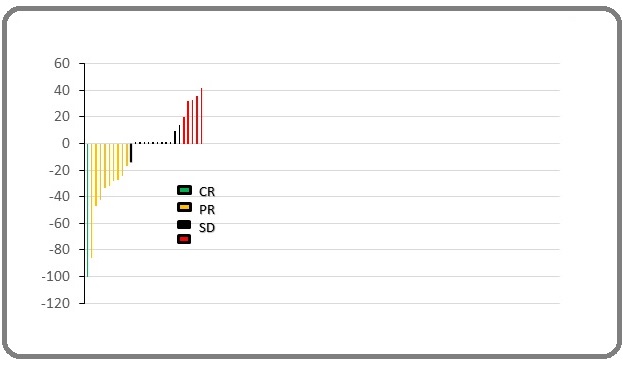
Response duration was calculated from the time that measurement criteria are met for complete or partial response until the documentation of progression or death or start of new anticancer therapy. As shown in Figure 3, out of the 10 responding patients, response duration was assessed for 8 patients. The other 2 patients were lost to follow up after responding.
Figure 3. Change in Tumour Size from Baseline by Spider Plot.
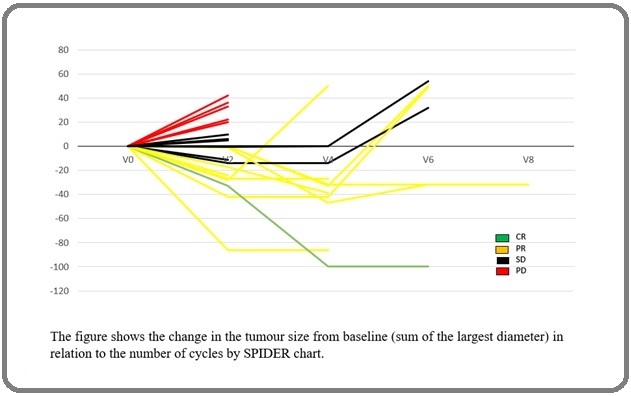
The median estimate response duration for patients who received vinflunine was 17.5 weeks with a mean value of 29 weeks (range: 4-86 weeks) [95% Confidence Interval (CI) 10.72 – 47.28 weeks] before disease progression or death.
The Progression free survival (PFS) was calculated from the date of study entry until the date of first progression or the date of death (whatever the reason of death) if no progression was recorded before.
For the 10 patients who have achieved PR/CR on treatment, 22 weeks (ranging from 12 to 90 weeks) was the median duration time before disease progression or death with a mean value of 35.6 [95% Confidence Interval (CI) 17.03 – 54.17 weeks]. Three patients had disease progression, and one of them died and the other 2 were lost to follow up. Two other patients died, 3 patients were alive at the end of the study, and the remaining 2 cases were lost to follow up after final evaluation (Figure 4).
Figure 4.Progression Free Survival Rates of the 10 Patients who have Achieved PR/CR.
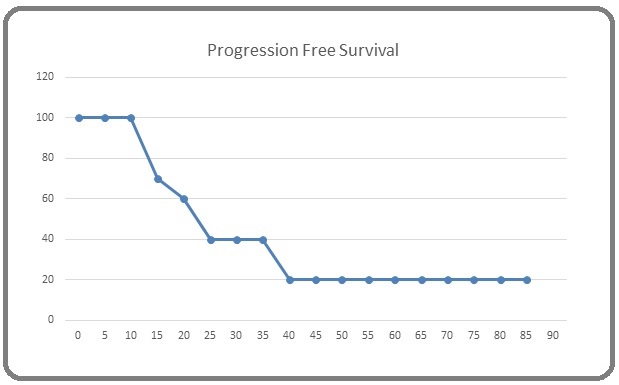
On the other hand, the median PFS for the whole group of patients (22 cases) who have achieved disease control (SD, PR, and CR) was 15 weeks (3.45 months) (range: 7-90 weeks) with a mean value of 23.59 [95% Confidence Interval (CI) 15.52 – 31.66 weeks].
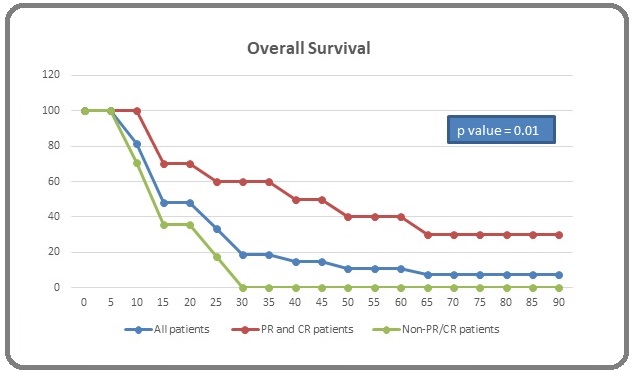
Overall survival was calculated from the date of study entry up to the date of death or last follow up. Mean overall survival time for the total population was 25.1 weeks [95% confidence interval (CI) ranging from 16.24 to 33.86 weeks] with a median value of 14 weeks (3.22 months). For the 10 responding patients the median survival time was 31.5 weeks (7.24 months) with a mean value of 40.1 weeks (9.22 months) [95% confidence interval (CI) ranging from 22.37 to 57.73 weeks]. On the other hand, the median survival time for non-PR/CR patients was 13 weeks with a mean value of 15.3 weeks [95% confidence interval (CI) ranging from 11.5 to 19.1weeks] with a p value of 0.01 between both groups (Figure 5).
Figure 5. Overall Survival Rates of the 27 Patients Included in the Study.
Discussion
This study aimed at assessing the impact of the drug Vinflunine prospectively in terms of efficacy and safety in the treatment of Egyptian patients with advanced or metastatic urothelial carcinoma progressing after a platinum-containing regimen.
Our current study included 27 patients. Most of them were males with a median age group of 64.1 years ranging from 42.3 to 76.8 years which is concordant to [16] who reported a range from 50 to 75 years. The study showed that vinflunine is an active and safe drug for patients who had previously failed to one prior platinum containing regimen. The safety and efficacy of vinflunine that we obtained were comparable to the results achieved in most of the other published trial.
To support the concept of good tolerability and comparing several adverse events of grade 3 and grade 4 observed in different trials, constipation in our study was 7.4% while the percentage of constipation was 5.9% in Castellano study and 5% in Passalacqua trial and finally 8% in Hussein et al trial. Four per cent of our patients had vomiting grade 3 and 4 while it was 2% in Castellano, 3% in Karin trial and 6% in Pistamaltzian trial. In our study, neutropenia and neutropenic fever occurred in 4% while neutropenia was present in 12.8% in Castellano study and 10.7% in Schinzari trial and 50% in Bellmunt trial. Shah et al, reported neutropenic fever in 27% while Karin et al reported 31% febrile neutropenia. Finally anemia was present in 11% of our patients while Passalacqua trial reported 6% and Bellmunt trial 19%.
As per the efficacy that our data were similar to the results achieved in most of previous trials. In our 27 patients, the CR and PR rates were 3.7%, and 33.3% respectively with a DCR of 81.4% and ORR of 37%. The median PFS for the whole group was 3.45 months. These data are concordant with the above mentioned different responses of the controlled trials. Our median OS for the whole group was 3.22 months. However, in the subgroup analysis the OS for the 10 responder patients (CR/PR) was 7.24 months while the OS for the non CR/ PR patients was 2.99 months with a p value 0.01. So this data was expected because of small sample size and substantial heterogeneity.
Recently, the IMvigor 210 study using the immune checkpoint inhibitor, atezolizumab, the median progression free survival was 2.1 months and an overall survival of 9 months [17, 18]. Also, in Key Note 045 study using the drug pembrolizumab, a progression free survival of 2.1 months and an OS of 10.3 months were reported [19]. Furthermore, in Check Mate 275 which used the nivolumab a progression free survival of 2 months and an OS of 7.74 months were reported [20]. Finally, Durvalumab was used showing a PFS of 1.5 months and an OS of 18.2 months [21].
So, in countries with limited resources like Egypt, and in view of the very high cost of the immune checkpoint inhibitors, it is possible to use Vinflunine as second line therapy for patients with advanced/metastatic bladder cancer patients.
In conclusion, our study showed the benefit of vinflunine and its impact on treatment in the daily practice in terms of toxicity, disease control rate, duration of response, progression free survival and overall survival in an unselected subgroup of patients with metastatic urothelial carcinoma who had progressed after only one previous line of platinum-containing regimen for advanced disease.
So, this consolidated the data that JAVLOR has demonstrated to be effective after failure of a platinum- based regimen and the consistency of results with significant and meaningful benefits through the different efficacy parameters.
The value of Ki-67 for molecular staging of urinary bladder cancer needs to be further confirmed in adequately designed prospective trials involving larger number of patients before any definitive conclusions can be made.
Nevertheless, there is an overwhelming need to incorporate new objective translational biomarkers that might help us better select the right treatment for our patients.
Multivariate analysis is the best way to evaluate independent factors that may affect treatment outcome. However due to the relatively small number of patients included in the current study, application of multivariate analysis may not be optimum.
References
- Cancer statistics, 2013 Siegel Rebecca, Naishadham Deepa, Jemal Ahmedin. CA: A Cancer Journal for Clinicians.2013;63(1). CrossRef
- Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma Bellmunt Joaquim, de Wit Ronald, Vaughn David J., Fradet Yves, Lee Jae-Lyun, Fong Lawrence, Vogelzang Nicholas J., Climent Miguel A., Petrylak Daniel P., Choueiri Toni K., Necchi Andrea, Gerritsen Winald, Gurney Howard, Quinn David I., Culine Stéphane, Sternberg Cora N., Mai Yabing, Poehlein Christian H., Perini Rodolfo F., Bajorin Dean F.. New England Journal of Medicine.2017;376(11). CrossRef
- Bladder Cancer Clark Peter E., Agarwal Neeraj, Biagioli Matthew C., Eisenberger Mario A., Greenberg Richard E., Herr Harry W., Inman Brant A., Kuban Deborah A., Kuzel Timothy M., Lele Subodh M., Michalski Jeff, Pagliaro Lance C., Pal Sumanta K., Patterson Anthony, Plimack Elizabeth R., Pohar Kamal S., Porter Michael P., Richie Jerome P., Sexton Wade J., Shipley William U., Small Eric J., Spiess Philippe E., Trump Donald L., Wile Geoffrey, Wilson Timothy G., Dwyer Mary, Ho Maria. Journal of the National Comprehensive Cancer Network.2013;11(4). CrossRef
- Gemcitabine and paclitaxel combination therapy in transitional cell carcinoma of the urothelium Meluch A.A, Burris H.S, Greco F.A, Hainsworth J.D. European Journal of Cancer.2000;36. CrossRef
- Final Results of Sequential Doxorubicin Plus Gemcitabine and Ifosfamide, Paclitaxel, and Cisplatin Chemotherapy in Patients With Metastatic or Locally Advanced Transitional Cell Carcinoma of the Urothelium Milowsky Matthew I., Nanus David M., Maluf Fernando C., Mironov Svetlana, Shi Weiji, Iasonos Alexia, Riches Jamie, Regazzi Ashley, Bajorin Dean F.. Journal of Clinical Oncology.2009;27(25). CrossRef
- Weekly Paclitaxel and Carboplatin against Advanced Transitional Cell Cancer after Failure of a Platinum-Based Regimen Kouno Tsutomu, Ando Masashi, Yonemori Kan, Matsumoto Koji, Shimizu Chikako, Katsumata Noriyuki, Komiyama Motokiyo, Okajima Eijiro, Matsuoka Naoki, Fujimoto Hiroyuki, Fujiwara Yasuhiro. European Urology.2007;52(4). CrossRef
- Second-line systemic therapy and emerging drugs for metastatic transitional-cell carcinoma of the urothelium Sonpavde Guru, Sternberg Cora N, Rosenberg Jonathan E, Hahn Noah M, Galsky Matthew D, Vogelzang Nicholas J. The Lancet Oncology.2010;11(9). CrossRef
- “Immunotherapyin Urothelial Cancer: Recent Results and Future Perspectives,”Drugs M. S. Farina , K. T. Lundgren , J. Bellmunt . 2017;77(10):1077-1089.
- Vinflunine Anton Aparicio Luis Miguel, Pulido Enrique Grande, Gallego Guadalupe Aparicio. Anti-Cancer Drugs.2012;23(1). CrossRef
- Antitumor Activity of Vinflunine: Effector Pathways and Potential for Synergies Braguer Diane, Barret Jean-Marc, McDaid Hayley, Kruczynski Anna. Seminars in Oncology.2008;35. CrossRef
- Tubulin-based Structure-affinity Relationships for Antimitotic Vinca Alkaloids Coderch Claire, Morreale Antonio, Gago Federico. Anti-Cancer Agents in Medicinal Chemistry.2012;12(3). CrossRef
- Vinflunine in platinum-pretreated patients with locally advanced or metastatic urothelial carcinoma Vaughn David J., Srinivas Sandy, Stadler Walter M., Pili Roberto, Petrylak Daniel, Sternberg Cora N., Smith David C., Ringuette Sarah, de Wit Edwin, Pautret Virginie, George Claude. Cancer.2009;115(18). CrossRef
- Phase III Trial of Vinflunine Plus Best Supportive Care Compared With Best Supportive Care Alone After a Platinum-Containing Regimen in Patients With Advanced Transitional Cell Carcinoma of the Urothelial Tract Bellmunt Joaquim, Théodore Christine, Demkov Tomasz, Komyakov Boris, Sengelov Lisa, Daugaard Gedske, Caty Armelle, Carles Joan, Jagiello-Gruszfeld Agnieszka, Karyakin Oleg, Delgado François-Michel, Hurteloup Patrick, Winquist Eric, Morsli Nassim, Salhi Yacine, Culine Stéphane, von der Maase Hans. Journal of Clinical Oncology.2009;27(27). CrossRef
- Long-term survival results of a randomized phase III trial of vinflunine plus best supportive care versus best supportive care alone in advanced urothelial carcinoma patients after failure of platinum-based chemotherapy Bellmunt J., Fougeray R., Rosenberg J.E., von der Maase H., Schutz F.A., Salhi Y., Culine S., Choueiri T.K.. Annals of Oncology.2013;24(6). CrossRef
- Long-Term Survival in Metastatic Transitional-Cell Carcinoma and Prognostic Factors Predicting Outcome of Therapy Bajorin Dean F., Dodd Paul M., Mazumdar Madhu, Fazzari Melissa, McCaffrey John A., Scher Howard I., Herr Harry, Higgins Geralyn, Boyle Mary G.. Journal of Clinical Oncology.1999;17(10). CrossRef
- Familial aggregation of urothelial cell carcinoma Aben Katja K.H., Witjes J. Alfred, Schoenberg Mark P., Hulsbergen-van de Kaa Christina, Verbeek Andr� L.M., Kiemeney Lambertus A.L.M.. International Journal of Cancer.2002;98(2). CrossRef
- Atezolizumab in locally advanced or metastatic urothelial cancer: a pooled analysis from the Spanish patients of the IMvigor 210 cohort 2 and 211 studies Sotelo M., Alonso-Gordoa T., Gajate P., Gallardo E., Morales-Barrera R., Pérez-Gracia J. L., Puente J., Sánchez P., Castellano D., Durán I.. Clinical and Translational Oncology.2020;23(4). CrossRef
- Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial Balar Arjun V, Galsky Matthew D, Rosenberg Jonathan E, Powles Thomas, Petrylak Daniel P, Bellmunt Joaquim, Loriot Yohann, Necchi Andrea, Hoffman-Censits Jean, Perez-Gracia Jose Luis, Dawson Nancy A, van der Heijden Michiel S, Dreicer Robert, Srinivas Sandy, Retz Margitta M, Joseph Richard W, Drakaki Alexandra, Vaishampayan Ulka N, Sridhar Srikala S, Quinn David I, Durán Ignacio, Shaffer David R, Eigl Bernhard J, Grivas Petros D, Yu Evan Y, Li Shi, Kadel Edward E, Boyd Zachary, Bourgon Richard, Hegde Priti S, Mariathasan Sanjeev, Thåström AnnChristine, Abidoye Oyewale O, Fine Gregg D, Bajorin Dean F. The Lancet.2017;389(10064). CrossRef
- Randomized Phase III Study Comparing Paclitaxel/Cisplatin/ Gemcitabine and Gemcitabine/Cisplatin in Patients With Locally Advanced or Metastatic Urothelial Cancer Without Prior Systemic Therapy: EORTC Intergroup Study 30987 Bellmunt Joaquim, von der Maase Hans, Mead Graham M., Skoneczna Iwona, De Santis Maria, Daugaard Gedske, Boehle Andreas, Chevreau Christine, Paz-Ares Luis, Laufman Leslie R., Winquist Eric, Raghavan Derek, Marreaud Sandrine, Collette Sandra, Sylvester Richard, de Wit Ronald. Journal of Clinical Oncology.2012;30(10). CrossRef
- Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial Sharma Padmanee, Retz Margitta, Siefker-Radtke Arlene, Baron Ari, Necchi Andrea, Bedke Jens, Plimack Elizabeth R, Vaena Daniel, Grimm Marc-Oliver, Bracarda Sergio, Arranz José Ángel, Pal Sumanta, Ohyama Chikara, Saci Abdel, Qu Xiaotao, Lambert Alexandre, Krishnan Suba, Azrilevich Alex, Galsky Matthew D. The Lancet Oncology.2017;18(3). CrossRef
- Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma Powles Thomas, O'Donnell Peter H., Massard Christophe, Arkenau Hendrik-Tobias, Friedlander Terence W., Hoimes Christopher J., Lee Jae Lyun, Ong Michael, Sridhar Srikala S., Vogelzang Nicholas J., Fishman Mayer N., Zhang Jingsong, Srinivas Sandy, Parikh Jigar, Antal Joyce, Jin Xiaoping, Gupta Ashok K., Ben Yong, Hahn Noah M.. JAMA Oncology.2017;3(9). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2022
Author Details