Anemia in Lymphoma Patients in Indonesia: The Prevalence and Predictive Factors
Download
Abstract
Background: The burden of lymphoma is intensified with the presence of anemia. The type of anemia in lymphoma is predominantly anemia of chronic disease. Severe anemia is also often associated with advanced stages leading to poor prognosis and survival as well as a worse quality of life.
Objective: In this study, we aimed to observe the incidence of anemia in lymphoma and to identify any associated clinical and laboratory factors.
Methods: Data from lymphoma patients admitted between 2012 to 2018 with complete hemoglobin (Hb) levels were collected from the medical records in Dr. Sardjito Hospital, Yogyakarta, Indonesia. Clinical and laboratory parameters included were age, sex, nutritional status, Ann Arbor staging, extranodal involvement, number of extranodal sites, Lactate Dehydrogenase (LDH) level, Eastern Cooperative Oncology Group (ECOG) performance status, platelet count, absolute lymphocyte count (ALC), white blood cell count (WBC), and lymphoma prognostic score (Non-Hodgkin Lymphoma/NHL using Index Prognostic International (IPI), Hodgkin’s Lymphoma/HL using International Prognostic Score (IPS)). Statistical analysis was done to observe the difference in any parameters between patients with anemia and non-anemia. Logistic regression was employed to model the relationship between associated or predictive factors and anemia incidence.
Results: Six hundred eleven (611) lymphoma patients were involved in this study, 296 (48.5%) had anemia and 314 (51.5%) did not. Anemia was more prevalent in HL (17/ 33 cases or 51.5%) than in NHL (272/ 564 cases or 48.1%). Patients with anemia frequently presented with mild anemia in 142 (48%), followed by moderate anemia in 139 (46.9%). The incidence of anemia were significantly associated with male sex, advanced Ann Arbor stage (III-IV), underweight, elevated LDH level, abnormal platelet, absolute lymphocyte counts less than 600/mm3, elevated WBC count more than 15,000/mm3, and high total prognostic score (>3). Multivariate analysis demonstrated low or elevated platelet (P=0.044; 95% CI=1.03-8.09) as an independent predictor, meanwhile lymphocytopenia as protective factor (OR=0.05; 95% CI=0.00-0.54; P=0.013).
Conclusion: Anemia commonly occurs in Indonesian lymphoma patients. There is an association and increased risk to develop anemia in male, Ann Arbor stage III-IV, underweight, elevated LDH, abnormal platelet, leukocytosis, and high total prognostic score. Abnormal platelet was an independent predictive factor, and lymphocytopenia is one of the protective factor.
Introduction
Non-Hodgkin Lymphoma (NHL) is the 7th most frequent cancer in Indonesia with death rate of 4.25% [1]. On the other side, with 1,047 new cases in 2018, Hodgkin’s lymphoma (HL) represents 0.3% of all new cancer cases and is placed in rank of 27th most frequent cancer in Indonesia [1]. Apparently, more than half of lymphoma patients had anemia before diagnosis [2]. Anemia has been considered as an important adverse prognostic factor of treatment outcome especially with bone marrow involvement which becomes another factor associated with poor prognosis. Patient’s quality of life is also affected with anemia. Anemia may cause shortness of breath, cardiovascular complication, poor performance status, impairment of cognitive, and fatigue in lymphoma patients [3]. The occurrence of anemia may be associated with some underlying conditions such as bleeding due to lymphoma, anemia of chronic disease, infiltration of tumor cells to bone marrow, autoimmune hemolytic anemia, or chemotherapy-induced anemia [4].
The most common type of anemia in patients with lymphoma is anemia of chronic disease, followed with secondary anemia due to marrow involvement, iron deficiency anemia (IDA), vitamin B-12 deficiency, and hemolytic anemia [3]. Different from prognostic score in Non Hodgkin Lymphoma (NHL), anemia is included in the prognostic score component of HL and is almost found in all HL patients. Anemia of HL is usually mild and normocytic- normochromic type, although it rarely presents as microcytic-hypochromic type [5].
Generally, anemia is more frequent in patients with higher stage of lymphoma due to the possible bone marrow involvement. The correlation of anemia with higher stage of lymphoma has been reported [6]. In developing countries, majority of the lymphoma patients had high prevalence of anemia with bone marrow involvement as one of the causative factors and most of them were diagnosed with advanced stages of disease [3].
Anemia in NHL patients without bone marrow involvement may be caused by the production of tumor necrosis factor (TNF) alpha [7]. Inflammatory mediators, such as IL-6, IL-10, IL-1, thymus and activation-regulated chemokine (TARC), gamma interferon, and tumor necrosis factor, will increase the level of hepcidin. In addition, the presence of IL-6 was significantly associated with older age, stage IV disease, B symptoms, and a high-risk International Prognostic Score (IPS) in HL, however, elevated IL-6 inversely correlated with hemoglobin levels [8].
Materials and Methods
Patients
Data was collected from 689 lymphoma patients in Dr. Sardjito Hospital, Yogyakarta, Indonesia during 2012-2018, prior to treatment. The baseline informations including demographic characteristics, clinical features, and laboratory parameters including age, sex, nutritional status, Ann Arbor staging, extranodal involvement, number of extranodal sites, Lactate Dehydrogenase (LDH) level, Eastern Cooperative Oncology Group (ECOG) performance status, platelet count, absolute lymphocyte count (ALC), white blood cell count (WBC), and prognostic score.
The diagnosis of lymphoma was based on histopathology from anatomical pathology laboratory according to WHO classification of Tumors of Hematopoietic and Lymphoid Tissue (2016) [9]. Seventy nine patients were excluded due to missing hemoglobin levels and presumptive diagnosis of lymphoma, hence 610 lymphoma patients were eventually included for further analysis. The prognostic score were defined in accordance to the type of lymphoma: NHL using Index Prognostic International (IPI), meanwhile HL using International Prognostic Score (IPS). Since IPS components also include haemoglobin level of <10.5 g/dl as one of their variables, this component was excluded to avoid overlapping data and total IPS score was subsequently categorized to produce a cut-off point.
Statistical analysis
According to World Health Organization (WHO), anemia is defined as mild (Hb level 11.0-12.9 g/dL for men and 11.0-11.9 g/dL for women), moderate (Hb level 8-10.9 g/dL) and severe (Hb level lower than 8 g/dL). The collected data from Dr. Sardjito Hospital within 2012 to 2018 were checked, coded, cleaned, and entered for analysis. The association between anemia incidence with associated factors was analyzed using Pearson Chi-square or Fisher-Exact test at a significance level ≤0.05. Odds ratio (OR) were used to explore the association of anemia with each component of prognostic score and to determine degree of association. Furthermore, the significant variables in the univariate analysis (p value ≤0.05) were selected for multivariate analysis using logistic regression to model the relationship of the associated or predictive factors and anemia incidence.
Results
Prevalence of anemia in patients with lymphoma
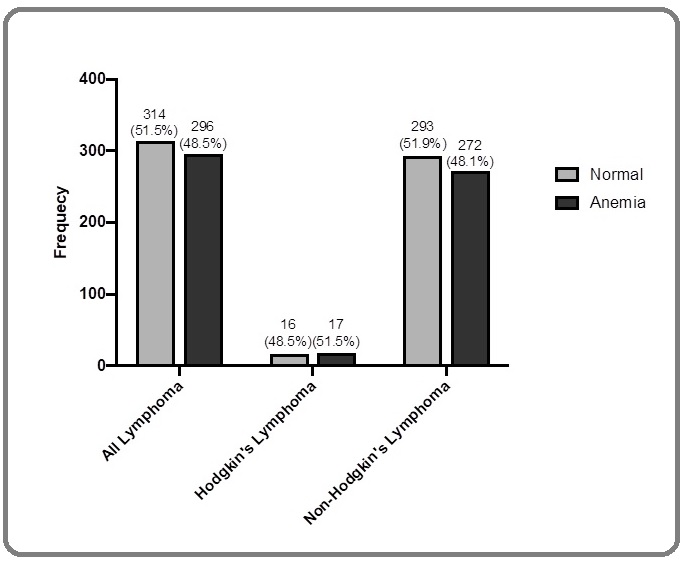
A total number of 611 lymphoma patients with complete hemoglobin level were categorized according to the severity of anemia. Based on the occurrence of anemia, 296 lymphoma patients were reported to have anemia (48.5%) while 314 patients did not (51.5%). In HL patients particularly, more than half of the patients had anemia (n=17/33, 51.5%), whereas NHL were dominated by non-anemic population (n=293/564, 51.9%) (Figure 1).
Figure 1. Prevalence of Anemia among Lymphoma Patients at Dr. Sardjito Hospital in the Period of 2012- 2018.
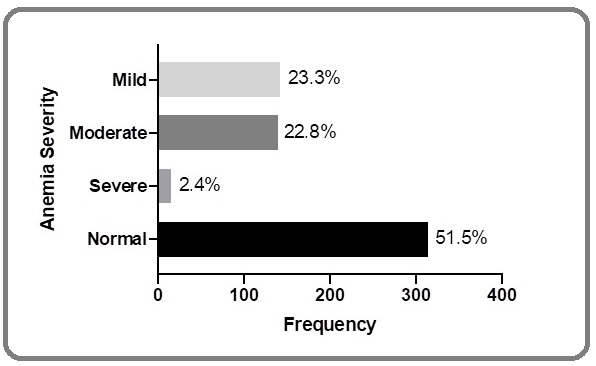
Hemoglobin level for the all lymphoma patients ranged from 5.7 g/dl to 16.7 g/dl with a mean of 12.27 ± 1.93 (mean ± SD). One hundred forty two (23.3%) were mild anemia, 139 (22.8%) moderate anemia, and 15 (2.4%) severe anemia (Figure 2).
Figure 2. Severity of Anemia among all Lymphoma Patients Submitted at Dr. Sardjito Hospital in the Period of 2012-2018.
In addition, the anemic population was higher in male (n = 186, 62.8%) compared to female (n = 110, 37.2%). Moreover, our result demonstrated that anemia occurred more frequently in younger population (age ≤60 years) than in older population (age >60 years) (193 (65.2%) vs 103 (34.8%)).
Predictive factors of the incidence of anemia in lymphoma patients
The bivariate Chi-square test result shown in Table 1. revealed that there were some predictive factors that were protective in nature and some variables that increased the risk of anemia.
| Anemia Status | p-value | ||
| Anemia n (%) | Normal n (%) | ||
| Age (610) | |||
| >60 | 103 (54.2) | 87 (45.8) | 0.059 |
| ≤60 | 193 (54) | 227 (46) | |
| Sex (610) | |||
| Male | 186 (53.9) | 159 (46.1) | 0.002 |
| Female | 110 (41.5) | 155 (58.5) | |
| Ann Arbor Stage (609) | |||
| III-IV | 97 (62.2) | 59 (37.8) | <0.001 |
| I-II | 198 (43.7) | 255 (56.3) | |
| Nutritional Status (595) | |||
| Underweight | 94 (75.9) | 65 (83.1) | 0.001 |
| Normal, overweight, obese | 190 (43.6) | 246 (56.4) | |
| Extranodal Involvement (610) | |||
| Present | 162 (51.1) | 155 (48.9) | 0.185 |
| Absent | 134 (45.7) | 159 (54.3) | |
| Number of Extranodal Sites (317) | |||
| Multiple | 44 (60.3) | 29 (39.7) | 0.074 |
| Single | 118 (48.4) | 126 (51.6) | |
| LDH level (209) | |||
| Elevated (>250 U/L) | 83 (55.3) | 67 (44.7) | 0.019 |
| Normal | 22 (37.3) | 37 (62.7) | |
| ECOG PS (484) | |||
| >3 | 4 (80) | 1 (20) | 0.197 |
| ≤2 | 227 (47.4) | 252 (52.6) | |
| Platelet (610) | |||
| Low or elevated (<150×109/L or >450×109/L) | 73 (65.8) | 38 (34.2) | <0.001 |
| Normal | 223 (44.7) | 276 (55.2) | |
| Absolute lymphocyte count (579) | |||
| <600/mm3 and/or <8% | 262 (47.4) | 291 (52.6) | 0.01 |
| >600/mm3 and/or >8% | 19 (73.1) | 7 (26.9) | |
| White Blood Cell Count (609) | |||
| >15,000/mm3 | 36 (70.6) | 15 (29.4) | 0.001 |
| ≤15,000/mm3 | 260 (46.6) | 298 (53.4) | |
| Prognostic Score (610) | |||
| >3 | 38 (73.1) | 14 (26.9) | <0.001 |
| 0-2 | 258 (46.2) | 300 (53.8) |
Abbreviations, ECOG, Eastern Cooperative Oncology Group; PS, performance status; LDH, lactate dehydrogenase.
Anemic lymphoma patients were found to have statistically significant association with male sex (P=0.002), advanced Ann Arbor stage (III-IV) (P≤0.001), underweight (P=0.001), elevated LDH level (P=0.019), abnormal platelet (P≤0.001), absolute lymphocyte count less than 600/mm3 (P=0.010), elevated WBC count more than 15,000/mm3 (P=0.001), and high total prognostic score (>3) (P=<0.001). There were no significant association of lymphoma patients to develop anemia in person who was more than 60 years old (P=0.059), had extranodal involvement (P=0.185), had multiple extranodal site of the disease (P=0.074), and had ECOG performance status more than 1 (P=0.197) (Table 1).
The result of univariate and multivariate analyses of several factors influencing the incidence of anemia were conveyed in Table 2.
| Clinical and Prognostic Factors | Univariate Analysis | Multivariate Analysis | ||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age >60 | 1.39 | 0.99-1.96 | 0.059 | |||
| Male sex | 1.65 | 1.19-2.28 | 0.002 | 1.38 | 0.73-2.59 | 0.321 |
| Ann Arbor Stage (III-IV) | 2.12 | 1.46-3.08 | <0.001 | 1.36 | 0.62-2.98 | 0.449 |
| Nutritional Status (underweight) | 1.87 | 1.23-2.71 | 0.001 | 2.09 | 0.98-4.44 | 0.056 |
| Extranodal Involvement | 1.24 | 0.90-1.71 | 0.185 | |||
| Number of extranodal site >1 | 1.62 | 0.95-2.76 | 0.074 | |||
| LDH elevated (>250 U/L) | 2.08 | 1.12-3.87 | 0.02 | 1.63 | 0.80-3.29 | 0.175 |
| ECOG PS >1 | 4.44 | 0.49-40.02 | 0.184 | |||
| Abnormal platelet (<150×109/L or >450×109/L) | 2.38 | 1.55-3.65 | <0.001 | 2.88 | 1.03-8.09 | 0.044 |
| Low lymphocyte (<600/mm3) | 0.33 | 0.14-0.80 | 0.014 | 0.05 | 0.00-0.54 | 0.013 |
| Elevated WBC (>15,000/mm3) | 2.75 | 1.47-5.14 | 0.002 | 1.54 | 0.42-5.57 | 0.512 |
| Prognostic Score >3 | 3.16 | 1.67-5.95 | <0.001 | 1.54 | 0.57-4.15 | 0.394 |
Abbreviations, ECOG, Eastern Cooperative Oncology Group; PS, performance status; LDH, lactate dehydrogenase; WBC, White Blood Cells
Univariate regression analysis showed that the following predictors significantly increased the risk of patient in developing anemia: male (OR=1.65, 95% CI=1.19-2.28, P=0.002),
advanced Ann Arbor stage (III-IV) (OR=2.12, 95% CI=1.46-3.08, P≤0.001), underweight (OR=1.87, 95% CI=1.23-2.71, P=0.001), elevated LDH level (OR=2.08, 95% CI=1.12-3.87, P=0.020), abnormal platelet (OR=2.38, 95% CI=1.55-3.65, P=<0.001),
elevated WBC count more than 15,000/mm3 (OR=2.75, 95% CI=1.47-5.14, P=0.002), and high total prognostic score (>3) (OR=3.16, 95% CI=1.67-5.95, P=<0.001)
(Table 2).
Age more than 60 years old (P=0.059), extranodal involvement (P=0.185), number of extranodal sites >1 (P=0.074), and ECOG performance status (P=0.184) had a risk of developing anemia approximately 1.39, 1.24, 1.62, and 4.44 times higher, respectively; however, they were not found to have significant association. Patient who had lower absolute lymphocyte count (less than <600/ mm3 and/or <8%) was associated with a 67% reduction to develop anemia (P=0.010; 95% CI=0.14-0.80) (Table 2).
According to the significant predictors from univariate analysis, multivariate analysis was subsequently performed, and it was shown that a person with abnormal platelet was 2.88 times more likely to develop anemia compared to person who had normal platelet (P=0.044; 95% CI=1.03-8.09), and person with low lymphocyte was protected against manifesting anemic condition (OR=0.05; 95% CI=0.00-0.54; P=0.013). Both of these
results were considered as an independent predictors for the occurrence of anemia in lymphoma in this study (Table 2).
Discussion
Almost half of the lymphoma patients in our study presented with anemia (n=296, 48.5%). Anemic condition were also reported in other studies, which accounts for 45.1% and 40% for NHL and HL respectively [3,10]. In addition, most of our lymphoma patients had mild (n=142, 48%) and moderate anemia (n=139, 46.9%). Similar findings were also reported by Hohaus et al. (2010) and Yasmeen et al. (2019) [3,10].
Anemic condition in our study population was observed naïve to treatments, hence the most possible pathogenesis was due to inflammation [3]. Inflammation has been recognized to play massive role in the development of lymphoma [11]. The pathogenesis of anemia in lymphoma is suggested to be related with inflammation in B cell NHL patients indicated by increased level of inflammatory mediators such as IL-6, TNF-α, IL-1, and gamma interferon [7,8]. Those mediators inhibits erythropoietin which later contributes to the development of anemia. Apparently, some inflammatory mediators such as IL-10, IL-6, TNF-alpha, and sCD25 were also expressed in HL [12-14]. However, IL-6 is still believed to be the key player for anemia both in NHL and HL. There is an association between HL disease activity with the production and release of IL-6 into systemic circulation, stimulating the overproduction of hepcidin. Elevated hepcidin level will trap iron in macrophages and iron-absorbing enterocytes, as well as blocking the release of iron from the reticuloendothelial system and the liver leading to iron deficiency anemia (IDA) [8,10]. This mechanism is similar in NHL where IL-6 contributes for the changes in iron metabolism as remarked by an elevated ferritin, lower iron, reduced total iron-binding capacity, and higher serum fibrinogen level [3].
A significant association between male gender with higher hepcidin and IL-6 levels was confirmed by Tisi et al. (2014) [15]. Another study conducted by Hohaus et al. (2010) also pointed out that there was a borderline significance for higher hepcidin levels in males compared with females. We provided evidence that male patients had anemia more frequently than female. Being male also increased the risk to develop anemic condition (OR=1.65, 95% CI=1.19-2.28, P=0.002).
A study by Hohaus et al. (2010) involving patients older than 45 years old observed higher hepcidin levels related with anemia [10]. However, our study yielded no significant impact of age for anemia occurrence in lymphoma. The occurrence of anemia was more frequently observed in younger than older patients. Our finding was in accordance with a research involving DLBCL patients that found no association between age group above 60 years old with anemia (P=0.606, 95% CI=0.49-1.51) [16]. Underweight patients (body mass index ≤18.5) are often associated with nutritional insufficiency that could manifests as nutritional anemia due to the lack of iron, B12, B6, copper, zinc, and other nutrients that play roles in the hematopoietic process [17]. Therefore, underweight group has the higher tendency to develop anemia than normal, overweight, and obese groups [18]. This is in line with our findings where the underweight group showed significant increased risk of having anemia (OR=1.87, 95% CI=1.23-2.71, P=0.001).
Regardless of the type of anemia, it is usually presented with advanced disease caused by many factors. Our study demonstrated that a high prognostic score (IPI for NHL; IPS for HL) was significantly correlated with the occurrence of anemia among lymphoma patients (OR=3.16, 95% CI=1.67-5.95, P=0.000). Elevated LDH was also found to have a significant association with anemic condition (OR=2.08, 95% CI=1.12-3.87, P=0.020). One possible explanation is that in patients with higher level of LDH, there is an elevated concentration of IL-10 involved in the pathogenesis of anemia via hepcidin [19].
Patients with advanced Ann Arbor stage was also found to have association with anemia (OR=2.12, 95% CI=1.46-3.08, P=0.000). This is coherent with a study from Shen et al. (2020) that observed a strong correlation between Ann Arbor stage III and IV with anemia than Ann Arbor stage I and II with anemia (P= <0.001) [20].
Performance status by ECOG more than 1 was not associated with the incidence of anemia (P=0.184). Previous studies showed no relationships between ECOG stage 2 and above with anemia incidence, although it lead to worse progression-free survival (PFS) [15,16].
Extranodal involvement and multiple extranodal sites were not significantly associated with anemia incidence in this study. These results might be in accordance with a study that reported non significant distributions of inflammatory markers widely known to be involved in the pathogenesis of anemia namely IL-6 and IL-10 in lymphoma cases with extranodal lesion [19].
Our study obtained a significant association between anemic patients with abnormal platelet count (low <150×109/L or elevated >450×109/L). Multivariate analysis showed that abnormal platelet count was an independent predictive factor of developing anemia (P=0.044; 95% CI=1.03-8.09). Thrombocytopenia as one of the frequent finding in lymphoma may lead to a higher bleeding risk [21]. It possibly explains the association between low platelet count and anemia in lymphoma patient with naïve treatment in this study. There was an inverse relationship between ferritin and platelet count with haemoglobin level that explains the significant association between iron deficiency anemia and thrombocytosis [22,23]. Severe thrombocytosis and leukocytosis are found to be associated with IDA, as well as elevated IL-6 and low erythropoietin [24,25]. This is in concordance with our result that yields a significant association between elevated platelet and anemia.
Lymphocytopenia was one of the protective factors for anemia in lymphoma in this study (P=0.013; 95% CI= 0.00-0.54). Lymphocytopenia generally indicates weakened immune system and often found among immunocompromised patients including lymphoma [26]. Lymphocytopenia has also been reported to be an independent prognostic factor for overall and progression-free survival in several cancers including aggressive type of NHL diffuse large B cell lymphoma (DLBCL) and HL [27]. One rare case report of iron deficiency anemia accompanied with lymphocytopenia indicated a possible vulnerability to infection. Therefore, protective role of lymphocytopenia to anemia observed in this study required further exploration. It may also be that the two conditions reflected different pathogenesis aspects of lymphoma which covers various disease entities.
Our study limitations are related to its retrospective complexion, where it left us no option to inadequateness of data. Our study also came from a single care centre that prone to a referral bias. The data needs to be validated at a larger scale to make a depiction for the whole Indonesia population.
In conclusions, this study has revealed the overall prevalence of anemia across all lymphoma is 48.5%. Male sex, Ann Arbor stage III-IV, underweight, elevated LDH, abnormal platelet, leukocytosis, and high total prognostic score were correlated significantly with the incidence of anemia. Our study also demonstrated that abnormal platelet was an independent predictive factors, as lymphocytopenia is one of the protective factors. Future directions to further predict and improve the outcome of patients with lymphoma will include incorporating the causes of anemia, and also exploring the cytokines levels that contribute to the pathogenesis of anemia.
Acknowledgements
Our profound thanks were delivered to Dr. Miraz Radhea Bagaskoro who contributed in data collection.
Funding Statement
This research was supported by Hibah Dana Masyarakat (DAMAS) Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada. 2020.
References
- The Global Cancer Observatory [Fact Sheet]. Available at: https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf (Accessed 20 December 2019) GLOBOCAN . 2018.
- Medical History, Lifestyle, Family History, and Occupational Risk Factors for Peripheral T-Cell Lymphomas: The InterLymph Non-Hodgkin Lymphoma Subtypes Project Wang SS, Flowers CR, Kadin ME, et al . J Natl Cancer Inst Monogr.2014;48:pp. 66–5.
- Frequency and causes of anemia in Lymphoma patients Yasmeen Tahira, Ali Jamshed, Khan Khadeeja, Siddiqui Neelam. Pakistan Journal of Medical Sciences.2018;35(1). CrossRef
- Kadar hemoglobin awal sebagai faktor prognostik penderita limfoma non-hodgkin (LNH) yang menjalani kemoterapi Winarto Daniel, Rena Ni Made Renny A, Adnyana Wayan Losen, Dharmayuda Tjokorda Gede, Suega Ketut, Bakta I Made. Jurnal Penyakit Dalam Udayana.2018;2(2). CrossRef
- A Case Report of Classical Hodgkin’s Lymphoma Presented with Anemia of Chronic Disease as Microcytic Hypochromic Type Ratnagiri BS, Mohan MJ, Bandari S, Sairam MV. IOSR-JDMS.2015;14(6):20-22.
- Natl Med J India Ghosh J, Singh RK, Saxena R, et al . 2013;26(2):pp. 79-1.
- Penanda Biologis Limfoma Maligna Asmara I. Unram Medical Journal.2018;7(4):40-48.
- Autoimmune Hemolytic Anemia and Hodgkin’s Disease: An Unusual Pediatric Association Gomes Maria Miguel, Oliva Tereza, Pinto Armando. Case Reports in Pediatrics.2016;2016. CrossRef
- The 2016 revision of the World Health Organization classification of lymphoid neoplasms Swerdlow Steven H., Campo Elias, Pileri Stefano A., Harris Nancy Lee, Stein Harald, Siebert Reiner, Advani Ranjana, Ghielmini Michele, Salles Gilles A., Zelenetz Andrew D., Jaffe Elaine S.. Blood.2016;127(20). CrossRef
- Anemia in Hodgkin's Lymphoma: The Role of Interleukin-6 and Hepcidin Hohaus Stefan, Massini Giuseppina, Giachelia Manuela, Vannata Barbara, Bozzoli Valentina, Cuccaro Annarosa, D'Alo' Francesco, Larocca Luigi Maria, Raymakers Reinier A.P., Swinkels Dorine W., Voso Maria Teresa, Leone Giuseppe. Journal of Clinical Oncology.2010;28(15). CrossRef
- Carbone AT, Claudio C, Carmelo S, Armando G, Annunziata. (2014). The Role of Inflammation in Lymphoma. Adv Exp Med Biol, 816, pp. 315-3 . CrossRef
- Poor clinical outcome of patients with Hodgkin's Disease and elevated interleukin-10 serum levels Bohlen H., Kessler M., Sextro M., Diehl V., Tesch H.. Annals of Hematology.2000;79(3). CrossRef
- Impact of Interleukin-6 in Hematological Malignancies Burger Renate. Transfusion Medicine and Hemotherapy.2013;40(5). CrossRef
- Impact of treatment on IL-4, IL-6, IL-10 and sCD25 levels in patients with Hodgkin’s Lymphoma Silva PB, Perini GF, Cavalcante EM, et al . Clinical Lymphoma, Myeloma and Leukemia.2015;15:S222-223.
- Anemia in diffuse large B-cell non-Hodgkin lymphoma: the role of interleukin-6, hepcidin and erythropoietin Tisi Maria Chiara, Bozzoli Valentina, Giachelia Manuela, Massini Giuseppina, Ricerca Bianca Maria, Maiolo Elena, D’Alo’ Francesco, Larocca Luigi Maria, Piciocchi Alfonso, Tjalsma Harold, Swinkels Dorine W., Voso Maria Teresa, Leone Giuseppe, Hohaus Stefan. Leukemia & Lymphoma.2013;55(2). CrossRef
- Anemia associated with worse outcome in diffuse large B-cell lymphoma patients: a single-center retrospective study Matsumoto Kenji, Fujisawa Shin, Ando Taiki, Koyama Megumi, Koyama Satoshi, Ishii Yoshimi, Numata Ayumi, Yamamoto Wataru, Motohashi Kenji, Hagihara Maki, Nakajima Hideaki. Turkish Journal of Hematology.2018. CrossRef
- Anemia epidemiology, pathophysiology, and etiology in low‐ and middle‐income countries Chaparro Camila M., Suchdev Parminder S.. Annals of the New York Academy of Sciences.2019. CrossRef
- Anemia in relation to body mass index and waist circumference among chinese women Qin Yu, Melse-Boonstra Alida, Pan Xiaoqun, Yuan Baojun, Dai Yue, Zhao Jinkou, Zimmermann Michael B, Kok Frans J, Zhou Minghao, Shi Zumin. Nutrition Journal.2013;12(1). CrossRef
- Serum Levels of Interleukin-6 and Interleukin-10 in Turkish Patients with Aggressive Non-Hodgkin's Lymphoma Guney NS, Hilal B, Mert B, et al . Asian Pac J Cancer Prev.2009;10(4):669-674.
- A multicenter investigation and analysis on anemia in lymphoma patients in Shanghai Shen J, Hao SG, Chen BB, Wang C. Zhonghua xue ye xue za zhi = Zhonghua Xueyexue Zazhi.2020;41(2):123-127. CrossRef
- Thrombocytopenia in solid tumors: Prognostic significance Ghanavat Majid, Ebrahimi Mina, Rafieemehr Hassan, Maniati Mahmood, Maleki Behzad Masumeh, Shahrabi Saeid. Oncology Reviews.2019;13(1). CrossRef
- Thrombocytosis in Iron Deficiency Anemia Mhadgut Hemendra, Galadima Hadiza, Tahhan Hassan Raymond. Blood.2018;132(Supplement 1). CrossRef
- A Prognostic Score for Advanced Hodgkin's Disease Hasenclever Dirk, Diehl Volker, Armitage James O., Assouline David, Björkholm Magnus, Brusamolino Ercole, Canellos George P., Carde Patrice, Crowther Derek, Cunningham David, Eghbali Houchingue, Ferm Christophe, Fisher Richard I., Glick John H., Glimelius Bengt, Gobbi Paolo G., Holte Harald, Horning Sandra J., Lister T. Andrew, Longo Dan L., Mandelli Franco, Polliack Aaron, Proctor Stephen J., Specht Lena, Sweetenham John W., Hudson Gillian Vaughan. New England Journal of Medicine.1998;339(21). CrossRef
- Thrombocytose et hyperleucocytose sévères au cours d’une anémie par carence martiale : à propos d’un cas Bernard F., Baccini V., Bagneres D., Rossi P., Demoux A.L., Bonin-Guillaume S., Frances Y., Granel B.. La Revue de Médecine Interne.2008;29(8). CrossRef
- SEVERE THROMBOCYTOPENIA WITH IRON DEFICIENCY ANEMIA Morris Van K., Spraker Holly L., Howard Scott C., Ware Russell E., Reiss Ulrike M.. Pediatric Hematology and Oncology.2010;27(5). CrossRef
- Lymphocytopenia as a prognostic marker for diffuse large B cell lymphomas Talaulikar Dipti, Choudhury Ayesha, Shadbolt Bruce, Brown Michael. Leukemia & Lymphoma.2008;49(5). CrossRef
- Lymphopenia as a Prognostic Factor for Overall Survival in Advanced Carcinomas, Sarcomas, and Lymphomas Ray-Coquard Isabelle, Cropet Claire, Van Glabbeke Martine, Sebban Catherine, Le Cesne Axel, Judson Ian, Tredan Olivier, Verweij Jaap, Biron Pierre, Labidi Inthidar, Guastalla Jean-Paul, Bachelot Thomas, Perol David, Chabaud Sylvie, Hogendoorn Pancras C.W., Cassier Philippe, Dufresne Armelle, Blay Jean-Yves. Cancer Research.2009;69(13). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2021
Author Details