The Expression, Morphology, and Clinical Characteristics of Fibroblast Growth Factor-10 in Breast Cancer
Download
Abstract
Background: Fibroblast growth factor-10 (FGF-10) is a member of a superfamily with characteristics of epithelial cell proliferation and embryonic development and is assumed to have a role in a phenomenon called the epithelial-mesenchymal transition (EMT). The previous study has revealed the critical role of FGF-10 in type III EMT in breast cancer cell lines. The mentioned finding, demonstrates the possible role of this factor in type III EMT in cancers with different origins such as breast. The present study investigated the expression of FGF-10 as a mitotic-inducing growth factor, normally has a low expression in breast tissues amongst breast cancer patients.
Materials and Methods: 67 breast cancer tissues and 8 normal breast tissues were randomly selected from the Iran national tumor bank. The FGF-10 gene expression analysis was performed after the RNA expression using the real-time RTPCR, which was followed by the Student’s t-test statistical analysis.
Results: The findings revealed that the relative expression of FGF-10 was elevated in tumor tissues as compared with normal breast tissues, and the higher expression had a direct correlation with the progression of clinical and pathologic staging. The expression was also significantly higher in triple-negative breast cancer and p53 null tissues.
Conclusion: Taken together, it is suggested that, although in some variables it was not significant but generally the invasion and migration in tumor tissues are the same as in-vitro analysis as indicated before, and the expression has a direct relationship with the molecular presentation and clinical -pathological progression.
Introduction
Fibroblast Growth Factor (FGF), a member of a growth-inducer family, is a heparin-binding protein and plays a role in various phenomena including proliferation, development, angiogenesis, and embryonic development [1]. FGF has almost 22 members [2]. The related receptors in mammalian cells are identified as FGFR1-4 [3]. FGF-10 is a member of this family with characteristics of epithelial cell proliferation and embryonic development and is supposed to have a role in a phenomenon called the epithelial-mesenchymal transition (EMT) [4].
EMT is a harmonic orchestra that can induce mesenchymal markers and features [5]. Embryonic development is associated with type I EMT, which leads to organogenesis [6]. Several functions have been considered for FGF-10 in the early stage of the embryonic development [7, 8]. In contrast, type III EMT is accompanied by a bizarre disturbance in differentiation pathways and is finally associated with the invasion and metastasis [5]. The present researchers’ previous study has revealed the critical role of FGF-10 in type III EMT in breast cancer cell lines. The mentioned finding demonstrates the possible role of this factor in type III EMT in cancers such as breast with different origins [4]. Breast cancer is the most common cause of cancer deaths among women and has an increasing incidence rate in Iran [9]. Although recent technologies developed for screening this cancer have increased the survival rate of breast cancer, there is yet a long way ahead to manage and control the outcome of patients suffering from breast cancer [10]. Specification of the factors affecting the prognosis would be very critical to achieve the mentioned goal.
Cell morphology findings and clinical staging are two different indicators that can be employed for the prognostic estimation and clinical management of breast cancer [11]. The comparisons made between these indicators and gene profile would provide a better insight for obtaining a more precise prediction in this regard [11]. In recent years, the molecular classification has helped to identify the prognosis and outcome of breast cancer more precisely by the administration of drugs that can directly target critical molecules in different signaling pathways. In this case, the classification of molecular subtypes according to the hormone receptor expression (luminal A, luminal B, Her2-enriched, and basal-like) has become an initial routine management of breast cancer [12]. The present study has investigated the expression of FGF-10 as a mitotic-inducing growth factor, which normally has a low expression in breast tissues amongst breast cancer patients.
Materials and Methods
Patients and tumor preparation
The present cross-sectional study involved 75 patients that were randomly selected from Iran national tumor bank (Cancer institute of Iran, Tehran University of Medical Sciences, Tehran, Iran). The mentioned sample included 67 breast tumor tissues as well as 8 breast tissues from patients that referred for mammoplasty without a breast cancer history. The clinical morphologic and immunohistochemical (IHC) information was received from tumor bank data base ( including lymph node and perineural invasion, metastasis, grade, stage, estrogen, progestron, Her2 receptor and p53 expression). All fresh tissues were stored at -80 for further procedures. An informed consent was obtained from all the patients.
Demographic, morphologic, and clinical information of patients were entered into the excel file (Microsoft 2010, USA).
Gene primer design
Primers were designed using primer3 that is an online primer designer (http://bioinfo.ut.ee/ primer3-0.4.0/primer3/). Primer sequences were 5’-ATGTCCGCTGGAGAAAGCTA-3’ and 5’-CCCCTTCTTGTTCATGGCTA-3’ as forward and reverse primers, respectively. GAPDH was also designed as a housekeeping gene following the same method with 5’-TCACCAGGGCTGCTTTTAAC-3’ and 5’-GACAAGCTTCCCGTTCTCAG-3’ sequences as forward and reverse primers, respectively. Primer-blast online tool was used to confirm the specific product and predict to avoid non- specific primer-annealing products (https://www.ncbi.nlm.nih.gov/tools/primer-blast).
RNA extraction
RNA was extracted using easy-BLUE (iNtRON, South Korea) according to the manufacturer’s instruction. The quantity of the extracted RNA was analyzed using the NanoDrop spectrophotometer (Thermo, USA).
Real time RT-PCR
The cDNA was constructed based on the construction manual of cDNA synthesis kit (Takara, Japan). The 1ug of the extracted RNA was used for cDNA synthesis. The cDNA synthesis was checked by PCR for GAPDH gene, and the following procedures were performed. 2 ul of cDNA was transferred to SYBER Green Master Mix to perform the real-time PCR (Takara, Japan). Real-time PCR was performed using the Rotor-Gene Q thermocycler (Qiagen, USA), which has been described previously [4].
Data transfer and statistics
Data for the gene amplification was analyzed by Rotor-Gene Q software (Qiagen, USA). The fold change of expression was analyzed using REST software (Qiagen, USA) to perform dct and ddct calculations. To compare tumor and normal tissue gene expression dct of tumor and normal tissue was analyzed. In case of clinical and morphologic correlation, data of ddct was used. The data of the normal tissues was pooled, and the average value was calculated. The association was also addressed by calculating the p-value and employing the Student’s t-test.
Results
Demographic analysis
A total number of 67 breast cancer tumor tissues were studied for the expression of FGF-10. The mean age of patients was 47.52 with the maximum and minimum range of 74 and 31 years old, respectively. The pathologic distribution presented as the number (percentage) was 8 (11.98%), 33 (49.25%), 21 (31.34%), and 5 (7.47%) for grades I, II, III, and X (unknown), respectively. Moreover, the pathologic distribution presented as the number (percentage) was 2 (2.99%), 39 (58.22%), 25 (37.31%), and 1 (1.48%) for clinical stages I, II, III, and IV, respectively.
Expression of FGF-10 in tumor tissues
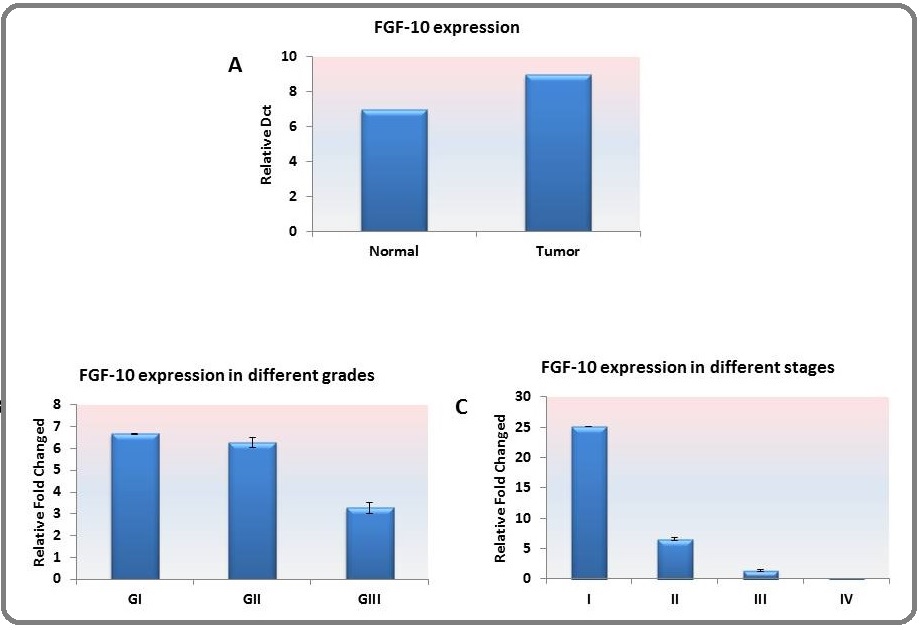
Dct comparison made between tumor tissues and pooled normal tissues revealed the higher expression of FGF-10 in tumor tissues (p=0.00008). The expression comparison is illustrated in Figure. 1. a.
Figure 1. A. The Average of the FGF-10 Expression was Significantly (*) Higher in Tumor Tissues as Compared with Pooled Normal Breast Tissues (p=0.00008). B. The expression of FGF-10 was increasing with the progression of the pathologic grade and was higher in grade II; however, there was no statistical significance in this regard. C. The same pattern reported for the grade was observed for the clinical stage and was also more evident in stage II; however, there was no statistical significance among stages.
FGF-10 expression and clinical/morphological findings
The relative expression of FGF-10 was found to increase by decreasing the differentiation of tumor. Moreover, the expression was higher in grade II as compared with grades I and III; however, it was not significant among different grades (Figure. 1. b). The same pattern was observed concerning the stages of the disease, and the expression of FGF-10 was higher in stage II as compared with the other clinical stages (Figure.1. c).
FGF-10 expression and invasion
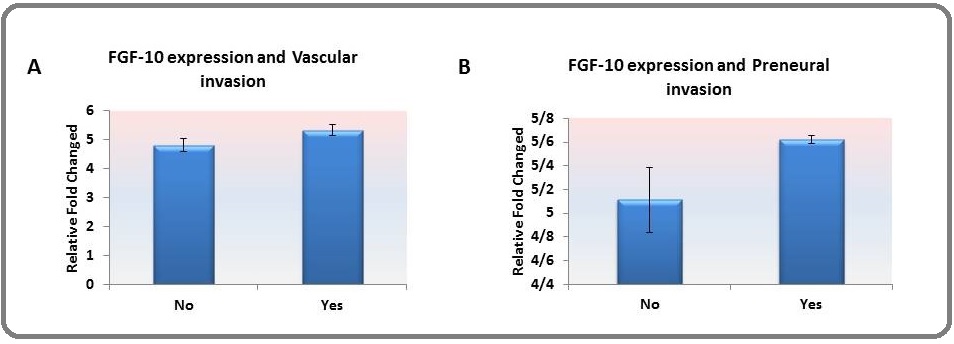
The relative expression of FGF-10 was found to be higher in tumors with perineural (Figure. 2. a) and vascular (Figure. 2. b) invasion; however, it was not significant.
Figure 2. The Relative Expression of FGF-10 was Higher among Tissues with (A) Vascular Invasion and (B) Perineural Invasion; However, This Association was not Significant.
FGF-10 expression and molecular pattern
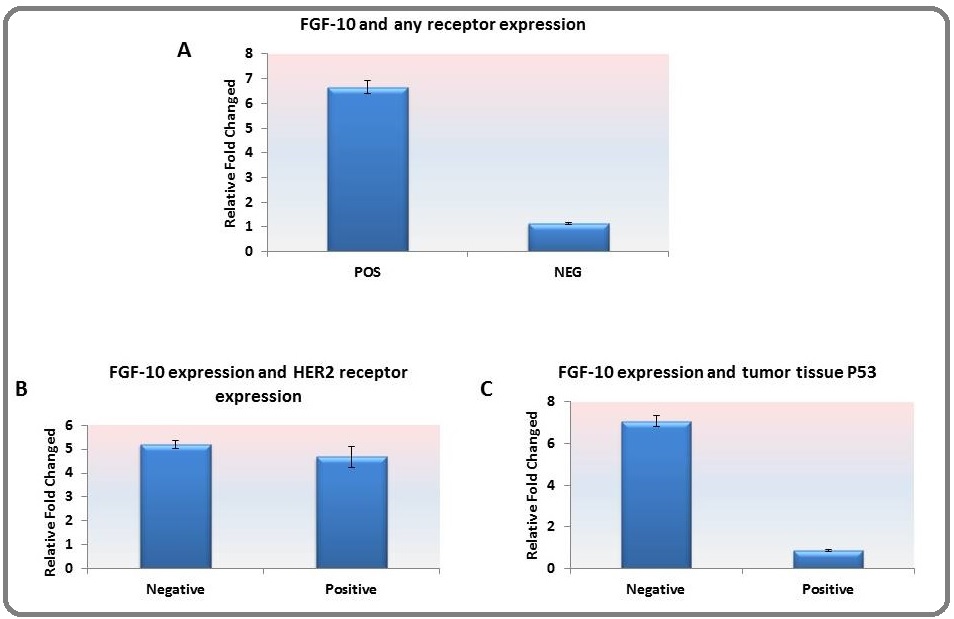
Number of 19 samples was triple negative based on immunohistochemistry report. Also 13 samples were expressing HER2 receptor expression. The findings have shown that the relative expression of FGF-10 was significantly lower in triple-negative tumor tissues as compared with tumors with at least one receptor expression (Figure. 3. a) (p=0.04). However, there was no significant difference concerning the expression of the HER-2 receptor (Figure. 3.b). In contrast, the expression of FGF-10 was significantly higher in tumor tissues with no expression of p53 (Figure. 3. c) (p=0.03).
Figure 3. A. The Relative Expression of FGF-10 was Significantly (*) Higher among Tissues with Triple-negative Receptor (p=0.04). B. Comparison made between HER2- positive and -negative tumor tissues did not show a significant difference in the relative gene expression of FGF-10. C. The relative expression of FGF-10 was higher among tumors without P53 expression (*) (p=0.03).
Discussion
The present researchers have previously demonstrated that the FGF-10 up-regulation has a positive effect on the invasion and migration in breast cancer cell lines [4]. The higher expression of FGF-10 means that the expression of this critical protein in type I EMT may have a role in a considerable number of breast cancer tumor tissues [4]. In type I EMT, the expression of FGF-10 during the gastrulation and kidney development is very critical [13]. Meanwhile, the present researchers’ previous findings have revealed that the regulation of this protein can change the cancer cell behavior [4]. The EMT is a harmonic orchestration, which is a critical process in the early stage of life [5, 14]. In this phenomenon, uniform and regular epithelial cells morphologically and functionally change to the spindle-shaped mesenchymal cells with the ability of movement and migration [14]. During the first stages of life, this phenomenon is a serious process for the development and organogenesis that is recognized as type I [6]. Although type II is also important during the inflammation, type III is known to happen during metastasis [6]. In this case, there are several studies showing that embryonic factors also have a role in the cancer progression. The mentioned finding makes the possible role of FGF-10 in breast cancer more prominent [5]
The present study revealed that the expression of FGF-10 was higher in breast cancer tumor tissues. The expression of FGF-10 was higher in grade and stage II as compared with other clinical and morphological characteristics. As we have previously indicated in colorectal carcinoma cell lines and tumor tissues, the expression of FGF-10 increased by increasing the grades and stages (specially stage III) [15].
There are several recognized signaling pathways for FGF-10 including Ras/MAPK, AKT/mTOR, TGFb, and wnt signaling pathways through the phosphorylation of GSK3b [16-19]. The invasion indexes in tumor tissues have indicated a slight increase in the expression of FGF- 10 in more invasive tumor cells. The mentioned finding has been confirmed with respect to breast cancer cell lines, as well. Concerning the correlation with FGF-10 expression and invasion in tumor tissues, one reason for insignificant results in comparison with the cell line might be because of the tumor heterogeneity [20]. There are different variables including the percentage of normal tissues or lymphocytic infiltration, the duration of tumor tissue preparation after excision, and environmental factors such as hypoxia that can affect the results of the relative gene expression. In such cases, the gene expression in tumor excision might be different from that of the community of unique cells that are derived from a cloned stable cell line [21].
The classification of breast cancer types based on immune-phenotyping (ER or Estrogen Receptor, PR or Progesterone Receptor, HER2 or Human Epidermal Growth Factor Receptor 2) helps to the better management of the disease and presentation of a breast cancer targeted therapy [12, 22]. In this case, the tumor tissues that have no immune-phenotypic feature are called triple-negative or basal-like breast cancer. The findings of the present study have shown that the expression of FGF-10 is higher in tumors with at least one receptor-positive as compared with a triple-negative. Triple-negative breast cancer that is the most aggressive type accounts for approximately 20% of breast cancer subtypes [23].
The FGF/FGFR system can be activated aberrantly in a ligand-dependent or -independent manner in breast cancer as various kinds of cancers and has a different role in invasion and drug resistance. Considering that FGF-10 usually binds to FGFR2, it has been revealed that the amplification and overexpression of FGFR2 could be detectable in only 4% of TNBC cases, which might explain the downregulation of FGF-10 expression in our study [24].
On the other hand, several studies have indicated the correlation between FGFR overexpression and hormone receptors in the cancer development and proliferation. Studies have shown the correlation between the expressions of different hormone receptors and the expression of other family members of FGF superfamily. For example, with respect to the HER2 receptor, there was a direct correlation between FGF-14 and Her2 receptor and the invasion in breast cancer [25]. Moreover, it has been already revealed that the FGF/FGFR inhibition could promote the endocrine therapy [26]. The findings of the present study did not demonstrate any significant difference among hormone receptor positive tumors and the expression of FGF-10.
The expression of FGF-10 was lower in tumor tissues without the expression of p53. Studies have shown that almost 80% of TNBC cases have the loss of p53, which correlates with the poor prognosis. In our study, 76% of TNBC cases have lost the expression of p53. Some studies have shown that p53 tumor suppressor has a role in the inhibition of other members of FGF family such as FGF-13 [27]. It can be conjectured that other members such as FGF-10 that has a different signaling pathway to the cell proliferation and invasion might have a possible correlation with the loss of p53 [28].
In conclusion, taken together, it is suggested that the invasion and migration in tumor tissues are the same as in-vitro analysis as indicated in the present researchers’ previous study, and the expression has a direct relationship with the molecular presentation and clinical-pathological progression. Investigations with sufficient tissue samples might pave the way to shed light on the mentioned difference statistically.
Acknowledgements
The study was supported by Cancer Research Center, Cancer Institute of Iran, Tehran University of Medical Sciences, Tehran, Iran.
References
- Dysregulated FGF signalling in neoplastic disorders Tanner Yasmine, Grose Richard P.. Seminars in Cell & Developmental Biology.2016;53. CrossRef
- Fibroblast Growth Factor Family in the Progression of Prostate Cancer Teishima Jun, Hayashi Tetsutaro, Nagamatsu Hirotaka, Shoji Koichi, Shikuma Hiroyuki, Yamanaka Ryoken, Sekino Yohei, Goto Keisuke, Inoue Shogo, Matsubara Akio. Journal of Clinical Medicine.2019;8(2). CrossRef
- Fibroblast Growth Factors: Biology, Function, and Application for Tissue Regeneration Yun Ye-Rang, Won Jong Eun, Jeon Eunyi, Lee Sujin, Kang Wonmo, Jo Hyejin, Jang Jun-Hyeog, Shin Ueon Sang, Kim Hae-Won. Journal of Tissue Engineering.2010;1(1). CrossRef
- FGF10: Type III Epithelial Mesenchymal Transition and Invasion in Breast Cancer Cell Lines Abolhassani Ali, Riazi Gholam Hossein, Azizi Ebrahim, Amanpour Saeid, Muhammadnejad Samad, Haddadi Mahnaz, Zekri Ali, Shirkoohi Reza. Journal of Cancer.2014;5(7). CrossRef
- Epithelial mesenchymal transition from a natural gestational orchestration to a bizarre cancer disturbance Shirkoohi Reza. Cancer Science.2012;104(1). CrossRef
- EMT in developmental morphogenesis Nakaya Yukiko, Sheng Guojun. Cancer Letters.2013;341(1). CrossRef
- Fibroblast Growth Factor-10 Promotes Cardiomyocyte Differentiation from Embryonic and Induced Pluripotent Stem Cells Chan Sunny Sun-Kin, Li Hui-Jing, Hsueh Ying-Chang, Lee Desy S., Chen Jyh-Hong, Hwang Shiaw-Min, Chen Chen-Yun, Shih Emily, Hsieh Patrick C. H.. PLoS ONE.2010;5(12). CrossRef
- Both high and low maternal salt intake in pregnancy alter kidney development in the offspring Koleganova Nadezda, Piecha Grzegorz, Ritz Eberhard, Becker Luis Eduardo, Müller Annett, Weckbach Monika, Nyengaard Jens Randel, Schirmacher Peter, Gross-Weissmann Marie-Luise. American Journal of Physiology-Renal Physiology.2011;301(2). CrossRef
- Enayatrad M, Amoori N, Salehiniya H. Epidemiology and trends in breast cancer mortality in iran. Iran J Public Health. 2015;44(3):430-1. .
- Treating Breast Cancer in the 21st Century: Emerging Biological Therapies Tinoco Gabriel, Warsch Sean, Glück Stefan, Avancha Kiran, Montero Alberto J.. Journal of Cancer.2013;4(2). CrossRef
- FGF/FGFR signaling in health and disease Xie Yangli, Su Nan, Yang Jing, Tan Qiaoyan, Huang Shuo, Jin Min, Ni Zhenhong, Zhang Bin, Zhang Dali, Luo Fengtao, Chen Hangang, Sun Xianding, Feng Jian Q., Qi Huabing, Chen Lin. Signal Transduction and Targeted Therapy.2020;5(1). CrossRef
- Molecular Classification of Breast Cancer Tsang Julia Y.S., Tse Gary M.. Advances in Anatomic Pathology.2019;27(1). CrossRef
- Kidney Development in the Absence of Gdnf and Spry1 Requires Fgf10 Michos Odyssé, Cebrian Cristina, Hyink Deborah, Grieshammer Uta, Williams Linda, D'Agati Vivette, Licht Jonathan D., Martin Gail R., Costantini Frank. PLoS Genetics.2010;6(1). CrossRef
- Echoes of the embryo: using the developmental biology toolkit to study cancer Aiello Nicole M., Stanger Ben Z.. Disease Models & Mechanisms.2016;9(2). CrossRef
- Fibroblast growth factor-10 and epithelial-mesenchymal transition in colorectal cancer Farajihaye Qazvini Fatemeh, Samadi Nasser, Saffari Mojtaba, Emami-Razavi Amir Nader, Shirkoohi Reza. EXCLI Journal; 18:Doc530; ISSN 1611-2156.2019. CrossRef
- Heparanase and Syndecan-1 Interplay Orchestrates Fibroblast Growth Factor-2-induced Epithelial-Mesenchymal Transition in Renal Tubular Cells Masola Valentina, Gambaro Giovanni, Tibaldi Elena, Brunati Anna Maria, Gastaldello Alessandra, D'Angelo Angela, Onisto Maurizio, Lupo Antonio. Journal of Biological Chemistry.2012;287(2). CrossRef
- FGF signalling through RAS/MAPK and PI3K pathways regulates cell movement and gene expression in the chicken primitive streak without affecting E-cadherin expression Hardy Katharine M, Yatskievych Tatiana A, Konieczka JH, Bobbs Alexander S, Antin Parker B. BMC Developmental Biology.2011;11(1). CrossRef
- Fibroblast growth factor and canonical WNT/β-catenin signaling cooperate in suppression of chondrocyte differentiation in experimental models of FGFR signaling in cartilage Buchtova Marcela, Oralova Veronika, Aklian Anie, Masek Jan, Vesela Iva, Ouyang Zhufeng, Obadalova Tereza, Konecna Zaneta, Spoustova Tereza, Pospisilova Tereza, Matula Petr, Varecha Miroslav, Balek Lukas, Gudernova Iva, Jelinkova Iva, Duran Ivan, Cervenkova Iveta, Murakami Shunichi, Kozubik Alois, Dvorak Petr, Bryja Vitezslav, Krejci Pavel. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2015;1852(5). CrossRef
- Phosphorylation status at Smad3 linker region modulates transforming growth factor‐β‐induced epithelial‐mesenchymal transition and cancer progression Ooshima Akira, Park Jinah, Kim Seong‐Jin. Cancer Science.2019;110(2). CrossRef
- Cancer heterogeneity is not compatible with one unique cancer cell metabolic map Strickaert A, Saiselet M, Dom G, De Deken X, Dumont J E, Feron O, Sonveaux P, Maenhaut C. Oncogene.2016;36(19). CrossRef
- Tumour sampling method can significantly influence gene expression profiles derived from neoadjuvant window studies Pearce Dominic A., Arthur Laura M., Turnbull Arran K., Renshaw Lorna, Sabine Vicky S., Thomas Jeremy S., Bartlett John M. S., Dixon J. Michael, Sims Andrew H.. Scientific Reports.2016;6(1). CrossRef
- Molecular classification of breast cancer: A retrospective cohort study Al-thoubaity Fatma Khinaifis. Annals of Medicine and Surgery.2020;49. CrossRef
- FGFR3 signaling and function in triple negative breast cancer Chew Nicole J., Nguyen Elizabeth V., Su Shih-Ping, Novy Karel, Chan Howard C., Nguyen Lan K., Luu Jennii, Simpson Kaylene J., Lee Rachel S., Daly Roger J.. Cell Communication and Signaling.2020;18(1). CrossRef
- The FGF/FGFR System in Breast Cancer: Oncogenic Features and Therapeutic Perspectives Santolla Maria Francesca, Maggiolini Marcello. Cancers.2020;12(10). CrossRef
- The Fibroblast Growth Factor–Inducible 14 Receptor Is Highly Expressed in HER2-Positive Breast Tumors and Regulates Breast Cancer Cell Invasive Capacity Willis Amanda L., Tran Nhan L., Chatigny Julie M., Charlton Nichole, Vu Hong, Brown Sharron A.N., Black Michael A., McDonough Wendy S., Fortin Shannon P., Niska Joshua R., Winkles Jeffrey A., Cunliffe Heather E.. Molecular Cancer Research.2008;6(5). CrossRef
- Targeting FGFR pathway in breast cancer Perez-Garcia J., Muñoz-Couselo E., Soberino J., Racca F., Cortes J.. The Breast.2018;37. CrossRef
- Tumor suppression by p53 involves inhibiting an enabler, FGF13 Manfredi James J.. Proceedings of the National Academy of Sciences.2017;114(4). CrossRef
- Association of p53 expression with poor prognosis in patients with triple-negative breast invasive ductal carcinoma Li Jing-ping, Zhang Xiang-mei, Zhang Zhenzhen, Zheng Li-hua, Jindal Sonali, Liu Yun-jiang. Medicine.2019;98(18). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2021
Author Details