Characteristics and Prognostic Factors in Gastric Adenocarcinoma Patients with Distant Metastasis
Download
Abstract
Objective: To explore the characteristics and prognostic factors of distant metastatic gastric adenocarcinoma (DMGA).
Methods: The data of DMGA patients who were enrolled in the SEER database from 2010 to 2017 was obtained. The Chi-squared test was performed for comparison between groups. The Kaplan-Meier method and the log-rank test was used for survival analysis. Univariate and multivariate Cox regression analysis was used to identify independent prognostic factors.
Results: A total of 2324 DMGA patients was identified. The association between different metastatic sites and clinicopathological characteristics was detected. The survival curves of patients with single and double-organ metastasis were established. The multivariate Cox analysis indicated that age, histological grade, T stage, N stage, surgery and tumor size were independent prognostic factors.
Conclusion: DMGA patients have poor outcomes, especially brain metastasis, bone metastasis, liver-brain metastasis, and lung-brain metastasis. Age <60 years old and cancer-directed surgery indicated a better prognosis, while higher T and N stage, higher grade and tumor size ≥5 cm indicated a worse prognosis.
Introduction
Gastric cancer is a common malignancy worldwide, despite recent declines in its morbidity and mortality, the morbidity rate still ranks fifth, while the mortality rate is third. Gastric adenocarcinoma (GAC) is the most prevalent histological form of all stomach cancers (approximately 95%). GAC is a heterogeneous condition with a variety of genotypes and symptoms, and infection with H. pylori as well as exposure to other carcinogens, are known to have a role in its development [1]. The American Cancer Society estimates that there will be 26560 individuals diagnosed and 11180 deaths from GAC in 2021 [2]. While early detection screening measures have been demonstrated to be effective in several East Asian countries, they are not widely adopted in the majority of the globe, resulting in poor outcomes for patients [3].
Distant metastasis is known to be an advanced status of most cancers, accounting for more than 90% of tumor- related mortality. Distant metastasis is a marker of poor prognosis in GAC patients [4-6]. Due to the complex pathogenesis and lack of specific treatments, distant metastatic gastric cancer (DMGA) is considered a great challenge for oncologists. This study aimed to explore the features and prognostic factors of DMGA, in order to provide a reference for the diagnosis and treatment of this disease.
Materials and Methods
Study Design and Patients Selection
Surveillance, Epidemiology, and End Results (SEER) program was constructed by the National Cancer Institute in 1973. It is a representative large-scale tumor incidence and death registration database in North America [7]. We screened the data of DMGA patients who were enrolled in the SEER database from 2010 to 2017.
The inclusion criteria were as follows: (1) Patients who had a known history of gastric adenocarcinoma; (2) gastric adenocarcinoma was the only diagnosed primary cancer; (3) patients aged 18 and older; (4) patients having complete metastasis information; and (5) patients with active follow-up. The exclusion criteria were as follows:
(1) patients under the age of 17; (2) patients who lacked complete information (grade unknown, T stage unknown, N stage unknown, surgery unknown, tumor size unknown, and metastatic sites unknown). The TNM staging followed the AJCC 7th edition TNM staging standard. Clinical death was the follow-up endpoint in this study, and the follow-up deadline was December 31st, 2018.
Statistical Analysis
R language was used to analyze the data. The Chi- squared test was performed for comparison between groups. The Kaplan-Meier method was used to establish the survival curve, and the log-rank test was used for univariate analysis. Univariate Cox regression analysis was performed to identify potential factors associated with prognosis, then potential factors were used to develop multivariate Cox analysis to identify independent prognostic factors. P-value <0.05 was considered to be statistically significant.
Results
Metastatic Sites and Clinicopathological Characteristics
Among the 2324 cases of DMGA, there were 365 (15.71%) cases of bone metastasis, 63 (2.71%) cases of brain metastasis, 1882 (80.98%) cases of liver metastasis, and 570 (24.53%) cases of lung metastasis, 1842 (79.26%) cases of single organ metastasis, and 482 (20.74%) cases of multiple organ metastasis.
As shown in Table 1, bone metastasis is related to age, ethnicity, histological grade, surgery, and tumor size. Brain metastasis is related to age.
| Characteristics | Total cohort | Metastatic site | |||||||
| Bone (%) | P | Brain (%) | P | Liver (%) | P | Lung (%) | P | ||
| Age | <0.001 | 0.005 | 0.445 | 0.757 | |||||
| <60 | 665 | 134 (20.15) | 28 (4.21) | 532 (80) | 166 (24.96) | ||||
| ≥60 | 1659 | 231 (13.92) | 35 (2.11) | 1350 (81.37) | 404 (24.35) | ||||
| Gender | 0.783 | 0.422 | 0.073 | 0.1 | |||||
| Female | 580 | 89 (15.34) | 13 (2.24) | 455 (78.45) | 157 (27.07) | ||||
| Male | 1744 | 276 (15.83) | 50 (2.87) | 1427 (81.82) | 413 (23.68) | ||||
| Ethnicity | 0.025 | 0.488 | <0.001 | 0.072 | |||||
| White | 1731 | 284 (16.41) | 51 (2.95) | 1391 (80.36) | 439 (25.36) | ||||
| Black | 306 | 32 (10.46) | 6 (1.96) | 270 (88.24) | 59 (19.28) | ||||
| Other | 287 | 49 (17.07) | 6 (2.09) | 221 (77.00) | 72 (25.09) | ||||
| Grade | <0.001 | 0.3 | 0.007 | 0.082 | |||||
| I | 73 | 9 (12.33) | 2 (2.74) | 54 (73.97) | 27 (36.99) | ||||
| II | 828 | 95 (11.47) | 27 (3.26) | 700 (84.54) | 200 (24.15) | ||||
| III | 1384 | 255 (18.42) | 32 (2.31) | 1098 (79.34) | 332 (23.99) | ||||
| IV | 39 | 6 (15.38) | 2 (5.13) | 30 (76.92) | 11 (28.21) | ||||
| T stage | 0.73 | 0.493 | 0.025 | 0.209 | |||||
| T0 | 7 | 1 (14.29) | 0 (0) | 4 (57.14) | 4 (57.14) | ||||
| T1 | 476 | 77 (16.18) | 13 (2.73) | 381 (80.04) | 116 (24.37) | ||||
| T2 | 90 | 15 (16.67) | 3 (3.33) | 65 (72.22) | 25 (27.78) | ||||
| T3 | 447 | 79 (17.67) | 11 (2.46) | 351 (78.52) | 97 (21.7) | ||||
| T4 | 444 | 69 (15.54) | 7 (1.58) | 363 (81.76) | 107 (24.1) | ||||
| Tx | 860 | 124 (14.42) | 29 (3.37) | 718 (83.49) | 221 (25.7) | ||||
| N stage | 0.087 | 0.163 | 0.402 | 0.279 | |||||
| N0 | 767 | 103 (13.43) | 17 (2.22) | 615 (80.18) | 178 (23.21) | ||||
| N1 | 915 | 162 (17.70) | 26 (2.84) | 755 (82.51) | 241 (26.34) | ||||
| N2 | 202 | 38 (18.81) | 2 (0.99) | 160 (79.21) | 45 (22.28) | ||||
| N3 | 189 | 27 (14.29) | 8 (4.23) | 146 (77.25) | 39 (20.63) | ||||
| Nx | 251 | 35 (13.94) | 10 (3.98) | 206 (82.07) | 67 (26.69) | ||||
| Surgery | 0.001 | 0.609 | 0.717 | <0.001 | |||||
| Yes | 328 | 31 (9.45) | 7 (2.13) | 268 (81.71) | 49 (14.94) | ||||
| No | 1996 | 334 (16.73) | 56 (2.81) | 1614 (80.86) | 521 (26.1) | ||||
| Size | 0.014 | 0.775 | <0.001 | 0.104 | |||||
| <2 cm | 127 | 30 (23.62) | 5 (3.94) | 82 (64.57) | 41 (32.28) | ||||
| 2 – 3 cm | 215 | 43 (20.00) | 7 (3.26) | 158 (73.49) | 59 (27.44) | ||||
| 3 – 5 cm | 739 | 112 (15.16) | 19 (2.57) | 601 (81.33) | 170 (23) | ||||
| ≥5 cm | 1243 | 180 (14.48) | 32 (2.57) | 1041 (83.75) | 300 (24.14) |
Liver metastasis is related to ethnicity, histological grade, T stage, and tumor size. Lung metastasis is related to surgery. Among them, the elderly with gastric adenocarcinoma were prone to bone and brain metastasis, black patients were prone to liver metastasis, while white and other races were prone to bone metastasis. Patients with poorly and undifferentiated grade as well as patients without cancer-directed surgery were prone to bone and liver metastasis. Those with larger tumor size were prone to liver metastasis, while those with smaller tumor size were prone to bone metastasis.
Survival Analysis
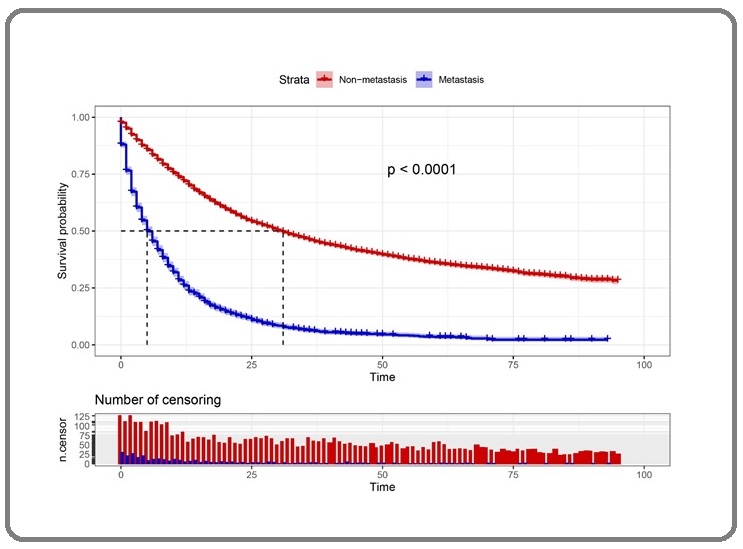
Figure 1 showed the overall survival (OS) curve of GAC patients with and without distant metastasis.
Figure 1. Kaplan-Meier Curve of GAC Patients with and without Distant Metastasis.
Among patients with single-organ metastasis, the 1-year OS of patients with bone, brain, liver, and lung metastasis were 17.14%, 15.4%, 28.68% and 33.90%,
respectively; the 2-year OS were 5.62%, 0%, 13.08% and 19.67%, respectively; and the 3-year OS were 0%, 0%, 6.97% and 10.83%, respectively. The OS of patients with brain and bone metastasis was relatively low, and the OS of those with liver and lung metastasis was better, as shown in Figure 2A.
Figure 2. Kaplan-Meier Curves of GAC Patients with Single-organ Metastasis. A, OS curve; B, GASS curve.
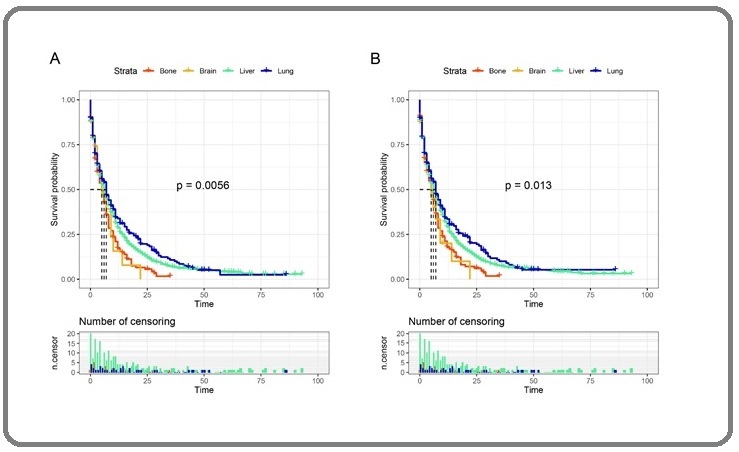
The 1-year gastric adenocarcinoma-specific survival (GASS) of patients with bone, brain, liver, and lung metastasis were 17.83%, 20.10%, 29.19% and 33.54%, respectively; the 2-year GASS were 6.13%, 0%, 13.18% and 20.26%, respectively; and the 3-year GASS were 0%, 0%, 7.32% and 9.97%, respectively. The GASS of patients with brain and bone metastasis was relatively low, and the CSS of those with liver and lung metastasis was relatively high (Figure 2B), this result was the same as the OS.
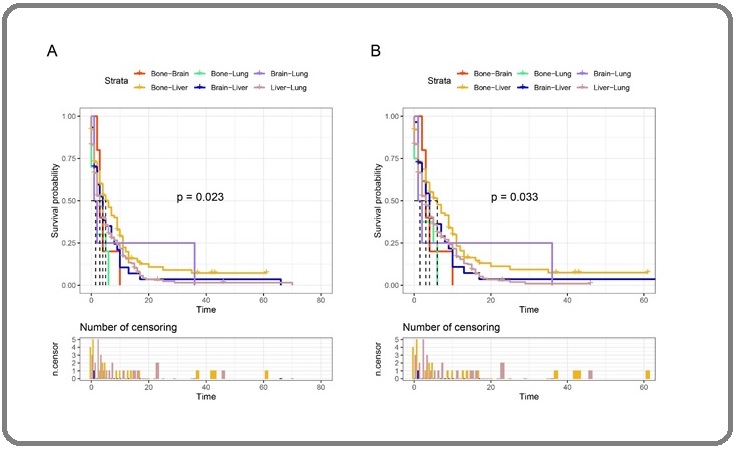
Among patients with double-organ metastasis, the 1-year OS of those with liver-lung, liver-bone, liver- brain, lung-brain, lung-bone, and bone-brain metastasis were 15.75%, 19.77%, 0%, 0%, 10.50% and 15.75%,
respectively; the 2-year OS were 3.11%, 10.85%, 0%, 0%, 3.50% and 0%, respectively; and the 3-year OS were 1.55%, 0%, 0%, 0%, 0% and 0%, respectively. The OS is relatively low in patients with liver-brain and lung-brain metastasis, while the OS in patients with liver-lung metastasis is better (Figure 3A). Figure 3B showed the GASS of patients with double-organ metastasis.
Figure 3. Kaplan-Meier Curves of GAC Patients with Double-organ Metastasis. A, OS curve; B, GASS curve.
Univariate and Multivariate Cox Regressive Analysis
Univariate Cox analysis showed that age, histological grade, T stage, N stage, surgery and tumor size were the potential risk factors that affect the prognosis of DMGA patients, as shown in Table 2.
| Characteristics | HR | 95% CI | P |
| Age | |||
| <60 | R | ||
| ≥60 | 1.21 | 1.13 - 1.30 | <0.001 |
| Gender | |||
| Female | R | ||
| Male | 0.96 | 0.89 - 1.03 | 0.272 |
| Ethnicity | |||
| White | R | ||
| Black | 1.02 | 0.92 - 1.12 | 0.749 |
| Other | 0.99 | 0.88 - 1.10 | 0.795 |
| Grade | |||
| I | R | ||
| II | 1.01 | 0.92 - 1.11 | 0.357 |
| III | 1.18 | 1.13 - 1.24 | 0.007 |
| IV | 1.23 | 1.19 - 1.26 | 0.002 |
| T stage | |||
| T0 | R | ||
| T1 | 1.03 | 0.75 - 1.42 | 0.641 |
| T2 | 1.1 | 0.84 - 1.43 | 0.354 |
| T3 | 1.11 | 1.04 - 1.19 | 0.022 |
| T4 | 1.22 | 1.09 - 1.36 | 0.034 |
| Tx | 1.08 | 1.01 - 1.15 | 0.043 |
| N stage | |||
| N0 | R | ||
| N1 | 1.09 | 0.97 - 1.22 | 0.143 |
| N2 | 1.25 | 1.10 - 1.41 | 0.001 |
| N3 | 1.23 | 1.20 - 1.26 | <0.001 |
| Nx | 1.03 | 0.90 - 1.18 | 0.674 |
| Surgery | |||
| Yes | R | ||
| No | 1.28 | 1.17 - 1.40 | <0.001 |
| Size | |||
| <2 cm | R | ||
| 2 – 3 cm | 0.95 | 0.84 - 1.07 | 0.41 |
| 3 – 5 cm | 1.1 | 0.97 - 1.26 | 0.138 |
| ≥5 cm | 1.15 | 1.00 - 1.32 | 0.047 |
R, reference; HR, hazard ratio; CI, confidence interval.
Then multivariate analysis was conducted, the analysis indicated that age, histological grade, T stage, N stage, surgery and tumor size were independent prognostic factors (Table 3).
| Characteristics | HR | 95% CI | P |
| Age | |||
| <60 | R | ||
| ≥60 | 1.24 | 1.16 - 1.33 | <0.001 |
| Grade | |||
| I | R | ||
| II | 1.03 | 0.94 - 1.13 | 0.253 |
| III | 1.16 | 1.08 - 1.25 | 0.017 |
| IV | 1.25 | 1.22 - 1.28 | <0.001 |
| T stage | |||
| T0 | R | ||
| T1 | 1.07 | 0.70 - 1.63 | 0.792 |
| T2 | 1.06 | 0.82 - 1.37 | 0.537 |
| T3 | 1.14 | 0.83 - 1.57 | 0.576 |
| T4 | 1.19 | 1.16 - 1.22 | <0.001 |
| Tx | 1.04 | 1.01 - 1.07 | 0.035 |
| N stage | |||
| N0 | R | ||
| N1 | 1.09 | 0.97 - 1.23 | 0.132 |
| N2 | 1.1 | 1.01 - 1.20 | 0.014 |
| N3 | 1.26 | 1.18 - 1.35 | <0.001 |
| Nx | 1.04 | 0.91 - 1.20 | 0.577 |
| Surgery | |||
| Yes | R | ||
| No | 1.26 | 1.13 - 1.40 | <0.001 |
| Size | |||
| <2 cm | R | ||
| 2 – 3 cm | 0.96 | 0.85 - 1.09 | 0.566 |
| 3 – 5 cm | 1.11 | 0.97 - 1.27 | 0.133 |
| ≥5 cm | 1.18 | 1.03 - 1.37 | 0.021 |
R, reference; HR, hazard ratio; CI, confidence interval.
Among them, age <60 years and cancer- directed surgery indicated a better prognosis, while higher T and N stage, higher grade and tumor size ≥5 cm indicated a worse prognosis.
Discussion
Gastric adenocarcinoma is the most common malignant tumor of the digestive tract. Because of its insidious onset and lack of specific symptoms at the initial stage, many patients have already developed distant metastasis at the time of diagnosis, and have lost the optimum treatment opportunity and have a poor prognosis [8]. Based on the clinical data of the SEER database, this study explored the prognostic factors of DMGA and provided a reference for the clinical treatment of this condition.
In recent years, clinical studies have reported that the incidence of bone metastasis in gastric cancer patients is 1.8% – 10%, which is a low incidence, but the prognosis is extremely poor, with a median survival time of 3.6 months [9, 10]. Brain metastasis is the advanced stage of gastric cancer, and reports on brain metastasis are rare. Despite the fact that brain metastases only occur in around 4% of all gastrointestinal (GI) malignancies, the incidence of GI brain metastasis is on the rise, in part due to more effective systemic therapies and longer survival of GI cancer patients [11]. Liver is the most common metastatic site for gastric cancer. A research summarized 67 randomized clinical trials, among 12656 patients with advanced gastric cancer, the liver metastasis rate was 44% [12]. It was reported that the median survival time of liver metastatic gastric cancer is 11 – 38 months [13, 14], which is consistent with our results. Regarding lung metastasis, Kong JH et al. [15] revealed that the median survival time is 4 – 7 months. We could find that the prognosis of patients with liver and lung metastasis is better than that with bone and brain metastasis. The prognosis of liver- lung metastasis is better in patients with double-organ metastasis in the present study, and the prognosis of bone-brain and lung-brain metastasis is worse. Overall, the survival rates of gastric adenocarcinoma patients with various metastatic sites were very low, and early diagnosis should be actively carried out to seize the opportunity for treatment; especially for patients with brain metastasis, they should receive sufficient attention from clinicians, and active treatment measures should be taken to prolong their survival.
There were many factors that affect the prognosis of DMGA patients. This study used univariate and multivariate Cox regression analysis to conclude that age, histological grade, T stage, N stage, surgery and tumor size were independent prognostic factors affecting the survival of DMGA patients. Among them, age <60 years and cancer-directed surgery indicated a better prognosis, while higher T and N stage, higher grade and tumor size ≥5 cm indicated a worse prognosis.
The biological functions deteriorate and more accompanying diseases occur as people get older, which is unfavorable for the prognosis of the elderly with gastric cancer. This study indicated that age was an independent factor affecting prognosis, and the risk of death for patients
≥60 years old was 1.21 times that of patients <60 years old. Studies have also pointed out that with age, postoperative complications and mortality gradually increase, early detection, early diagnosis and early surgical treatment are important for improving the prognosis of patients. In a meta-analysis study [16], primary surgery had a significant survival benefit for patients with metastatic gastric cancer (HR = 0.62; 95% CI: 0.49 – 0.78; P < 0.001). Regarding surgery of gastric cancer, negative margin resection and clearance of nodal disease have great impact on locoregional control of disease [17]. In this study, cancer-directed surgery was a protective factor for the prognosis of DMGA patients. The risk of death for patients without surgery was 1.28 times that of patients with surgery. Early surgery could improve the prognosis and bring great benefits for patients.
Many studies have confirmed that TNM stage was of great significance in assessing the prognosis of patients with gastric cancer [18-20]. It is well known that patients with high TNM stage have a poor prognosis, which is consistent with the poor prognosis conclusions drawn in this study for patients with higher T and N stage. In this study, the survival time of patients with higher histological grade was significantly worse. As the level of differentiation progresses, the more poorly differentiated patients become more severe. As far as we know, compared with moderately and well differentiated gastric cancer, patients with distant metastasis with poorly and undifferentiated gastric cancer have a shorter survival time, which is consistent with the prior results [21].
Many studies have evaluated the correlation between tumor size and prognosis. Tumor size was found to be a significant predictor of OS in young individuals with stomach cancer [22]. Another study also revealed that tumor size was an independent prognostic factor of OS and cancer-specific survival in patients with gastric cardia cancer [23]. In addition, Liao F et al. recognized that tumor size >1 cm was one of the most informative factors correlated with poor prognosis via the LASSO Cox analysis [24]. The present study indicated that DMGA patients with tumor size ≥5 cm had relatively poor survival.
This study initially explored the relationship between the distant metastatic sites of gastric cancer and the prognosis of patients, as well as analyzed related prognostic factors. However, there are still some limitations. Firstly, this study was a retrospective study, and the SEER database only provided the data of patients with bone, liver, lung, and brain metastasis, while other metastatic sites, such as the peritoneum, adrenal gland, was not given. Secondly, there is no more detailed information about surgery reported in the SEER database. Finally, SEER did not include detailed descriptions of distant metastasis.
In conclusion, DMGA patients had a poor prognosis and various risk factors. Age, histological grade, T stage, N stage, surgery and tumor size are prognostic factors that affect patients’ survival. Clinically, we should attach great importance to regular follow-up of DMGA patients with high-risk factors, especially those with bone metastasis, brain metastasis and multiorgan metastasis. DMGA patients could benefit from surgery of primary tumor, so active treatment is needed to improve the patients’ prognosis.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for- profit sectors. The authors declare no conflict of interest.
References
- Gastric adenocarcinoma Ajani Jaffer A., Lee Jeeyun, Sano Takeshi, Janjigian Yelena Y., Fan Daiming, Song Shumei. Nature Reviews. Disease Primers.2017;3. CrossRef
- Cancer Statistics, 2021 Siegel Rebecca L., Miller Kimberly D., Fuchs Hannah E., Jemal Ahmedin. CA: a cancer journal for clinicians.2021;71(1). CrossRef
- Global Cancer Incidence and Mortality Rates and Trends--An Update Torre Lindsey A., Siegel Rebecca L., Ward Elizabeth M., Jemal Ahmedin. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology.2016;25(1). CrossRef
- Jin X, Zhu Z, Shi Y. Metastasis mechanism and gene/protein expression in gastric cancer with distant organs metastasis. Bull Cancer. 2014;101(1):E1-12 .
- Liu J, Chen L. Current status and progress in gastric cancer with liver metastasis. Chin Med J (Engl). 2011;124(3):445-56. .
- Metastatic gastric cancer: Does the site of metastasis make a difference? Tan Hwee Leong, Chia Claramae Shulyn, Tan Grace Hwei Ching, Choo Su Pin, Tai David Wai-Meng, Chua Clarinda Wei Ling, Ng Matthew Chau Hsien, Soo Khee Chee, Teo Melissa Ching Ching. Asia-Pacific Journal of Clinical Oncology.2019;15(1). CrossRef
- Practical Guide to Surgical Data Sets: Surveillance, Epidemiology, and End Results (SEER) Database Doll Kemi M., Rademaker Alfred, Sosa Julie A.. JAMA surgery.2018;153(6). CrossRef
- Advanced gastric adenocarcinoma: optimizing therapy options Mizrak Kaya Dilsa, Harada Kazuto, Shimodaira Yusuke, Amlashi Fatemeh G., Lin Quan, Ajani Jaffer A.. Expert Review of Clinical Pharmacology.2017;10(3). CrossRef
- Bone recurrence after curative resection of gastric cancer Park Jae Myung, Song Kyo Young, O Joo Hyun, Kim Won Chul, Choi Myung-Gyu, Park Cho Hyun. Gastric Cancer: Official Journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association.2013;16(3). CrossRef
- Natural history of malignant bone disease in gastric cancer: final results of a multicenter bone metastasis survey Silvestris Nicola, Pantano Francesco, Ibrahim Toni, Gamucci Teresa, De Vita Fernando, Di Palma Teresa, Pedrazzoli Paolo, Barni Sandro, Bernardo Antonio, Febbraro Antonio, Satolli Maria Antonietta, Bertocchi Paola, Catalano Vincenzo, Giommoni Elisa, Comandone Alessandro, Maiello Evaristo, Riccardi Ferdinando, Ferrara Raimondo, Trogu Antonio, Berardi Rossana, Leo Silvana, Bertolini Alessandro, Angelini Francesco, Cinieri Saverio, Russo Antonio, Pisconti Salvatore, Brunetti Anna Elisabetta, Azzariti Amalia, Santini Daniele. PloS One.2013;8(10). CrossRef
- Gastrointestinal cancer and brain metastasis: a rare and ominous sign Go Pauline H., Klaassen Zachary, Meadows Michael C., Chamberlain Ronald S.. Cancer.2011;117(16). CrossRef
- Reporting patient characteristics and stratification factors in randomized trials of systemic chemotherapy for advanced gastric cancer Shitara Kohei, Ikeda Junko, Kondo Chihiro, Takahari Daisuke, Ura Takashi, Muro Kei, Matsuo Keitaro. Gastric Cancer: Official Journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association.2012;15(2). CrossRef
- Surgical treatment of liver metastases of gastric cancer: Is local treatment in a systemic disease worthwhile? Garancini Mattia, Uggeri Fabio, Degrate Luca, Nespoli Luca, Gianotti Luca, Nespoli Angelo, Uggeri Franco, Romano Fabrizio. HPB : the official journal of the International Hepato Pancreato Biliary Association.2012;14. CrossRef
- Hepatic resection for synchronous hepatic metastasis from gastric cancer Qiu J.-L., Deng M.-G., Li W., Zou R.-H., Li B.-K., Zheng Y., Lao X.-M., Zhou K., Yuan Y.-F.. European Journal of Surgical Oncology: The Journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology.2013;39(7). CrossRef
- Lung metastases in metastatic gastric cancer: pattern of lung metastases and clinical outcome Kong Jee Hyun, Lee Jeeyun, Yi Chin-A., Park Se Hoon, Park Joon Oh, Park Young Suk, Lim Ho Yeong, Park Keon Woo, Kang Won Ki. Gastric Cancer: Official Journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association.2012;15(3). CrossRef
- Clinical significance of palliative gastrectomy on the survival of patients with incurable advanced gastric cancer: a systematic review and meta-analysis Sun Jingxu, Song Yongxi, Wang Zhenning, Chen Xiaowan, Gao Peng, Xu Yingying, Zhou Baosen, Xu Huimian. BMC cancer.2013;13. CrossRef
- Management of Gastric Adenocarcinoma for General Surgeons Hoshi Hisakazu. The Surgical Clinics of North America.2020;100(3). CrossRef
- Comparison of the 7th and 8th editions of the American joint committee on cancer TNM classification for patients with stage III gastric cancer Lu Jun, Zheng Chao-Hui, Cao Long-Long, Li Ping, Xie Jian-Wei, Wang Jia-Bin, Lin Jian-Xian, Chen Qi-Yue, Lin Mi, Tu Ru-Hong, Huang Chang-Ming. Oncotarget.2017;8(48). CrossRef
- TNM and Japanese staging systems for gastric cancer: how do they coexist? Sayegh Mazin E., Sano Takeshi, Dexter Simon, Katai Hitoshi, Fukagawa Takeo, Sasako Mitsuru. Gastric Cancer: Official Journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association.2004;7(3). CrossRef
- Pang L, Wang J, Fan Y, Xu R, Bai Y, Bai L. Correlations of TNM staging and lymph node metastasis of gastric cancer with MRI features and VEGF expression. Cancer Biomark. 2018;23(1):53-9. https://doi.org/10.3233/cbm-181287 .
- The Prognostic Value of Signet-Ring Cell Histology in Resected Gastric Adenocarcinoma Postlewait Lauren M., Squires Malcolm H., Kooby David A., Poultsides George A., Weber Sharon M., Bloomston Mark, Fields Ryan C., Pawlik Timothy M., Votanopoulos Konstantinos I., Schmidt Carl R., Ejaz Aslam, Acher Alexandra W., Worhunsky David J., Saunders Neil, Swords Douglas, Jin Linda X., Cho Clifford S., Winslow Emily R., Cardona Kenneth, Staley Charles A., Maithel Shishir K.. Annals of Surgical Oncology.2015;22 Suppl 3. CrossRef
- Development and validation of prognostic nomogram for young patients with gastric cancer Yu Chaoran, Zhang Yujie. Annals of Translational Medicine.2019;7(22). CrossRef
- Development and validation of a nomogram to predict the prognosis of patients with gastric cardia cancer Shi Xiuquan, Xu Lijun, Ma Bingwei, Wang Siben. Scientific Reports.2020;10(1). CrossRef
- A validated survival nomogram for early-onset diffuse gastric cancer Liao Fei, Guo Xufeng, Lu Xiaohong, Dong Weiguo. Aging.2020;12(13). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2022
Author Details