Significance of CD133 Expression in Invasive Ductal Carcinoma of the Breast
Download
Abstract
Background and Aim: Cancer stem cells (CSCs) are thought to be responsible for tumor initiation, progression, and resistance to chemotherapy and radiotherapy. CD133 is a trans-membrane glycoprotein which is considered as a putative CSCs marker. Emerging evidence suggests that CD133 may be a critical factor in tumor development, progression and metastasis. The aim of this study was to evaluate the expression of CD133 in mammary infiltrating ductal carcinoma (IDC), and to correlate its expression with some known clinicopathological parameters.
Methods: Fifty patients with mammary IDC who underwent modified radical mastectomy were included in this study. From each specimen, two tissue sections were obtained; one was stained by hematoxylin and eosin (H&E) stain to determine the histologic subtype, grade and indicators of local aggressiveness. The second tissue section was immunohistochemically stained by anti-human CD133 antibody.
Results: The study revealed statistically significant associations between CD133 expression and poorly differentiated, advanced stage tumors with poor Nottingham Prognostic Index (NPI), triple negative phenotype, lymphovascular invasion (LVI) and lymph node metastasis (LNM).
Conclusion: The current study revealed that CD133 is strongly associated with poor prognostic indices; it is positively correlated to poorly-differentiated tumors with high histologic grade and advanced stage
Introduction
Breast carcinoma is a major public health problem worldwide. It is the most frequent malignant tumor in women worldwide comprising 30% on average of all cancers and the most likely cause of cancer-related deaths among women worldwide [1]. In Egypt, breast carcinoma is the most prevalent cancer among women representing 32% of total cancer cases [2].
Breast carcinoma is currently regarded as a heterogeneous group of tumors with diverse morphology, behavior, outcome and response to therapy. In spite of advances in diagnosis and treatment of breast carcinoma, the clinical outcome remains unsatisfactory due to recurrence, metastasis or chemotherapy-resistance [3].
Cancer stem cells are recognized as a subpopulation of cancer cells that show the characteristics of normal stem cells. They are believed to possess the capacity to self-renewal and are responsible for tumor formation and progression. CSCs also promote tumor cell heterogeneity and metastasis. In addition, CSCs are thought to be more resistant to chemotherapy and radiotherapy [4]. Given the significance of CSCs in tumor development, the identification and characterization of CSCs could lead to the development of directed therapies against these aggressive cells, hence more effective treatments for cancer [5].
CSCs have been described in different types of cancers including breast cancer. Breast cancer stem cells (BCSCs) subpopulation was shown to express higher level of pro- invasive genes and had highly invasive properties. With evidence forthcoming regarding the effects of the stroma and the microenvironment in breast tumor progression, several genes have also been reported to be associated with BCSCs [6].
CD133, also known as prominin-1, is a member of pentaspan trans-membrane glycoproteins. It contains an extracellular N-terminal domain, five trans-membrane segments which separate two small intracellular loops and two large extracellular loops, and an intracellular C-terminal domain. It is a 120 kDa protein that comprises 865 amino acids. The gene encoding for human CD133 is located on chromosome 4 [7]. It is frequently expressed on multipotent progenitor cells, including immature hematopoietic stem and progenitor cells. CD133 is a putative CSCs marker; it has been extensively used as a stem cell marker for normal and cancerous tissues. CD133 expressing cells possessing stem cell-like characteristics including self-renewal, high proliferation and drug resistance substantiating a tumorigenic role of CD133- expressing cells. It also plays a role in cell differentiation, proliferation and apoptosis [8].
Emerging evidences suggest that CD133 may be a critical factor in tumor development, progression and metastasis. CD133-positive CSC showed increased expression levels of CXCR4 which is a critical protein for the adhesion and/or migration of tumor cells, indicating an important role of CD133 in tumor cells migration and tumor invasion [9]. CD133 is used as a diagnostic and prognostic marker which is overexpressed in various neoplasms. In breast cancer, its overexpression confers a poor prognosis. Increasing clinical evidence has confirmed that it is involved in breast cancer progression. CD133 is a promising marker for the identification of CSC in breast cancer subtypes including aggressive HER2+ and triple- negative phenotypes [10].
The aim of this study was to investigate the immunohistochemical expression of CD133 in mammary IDC and to correlate its expression to different clinicopathological parameters of the studied cases.
Materials and Methods
Clinical data and specimens’ collection
The current study enrolled fifty patients suffered from mammary IDCs. The patients admitted to the General Surgery Department of Sohag University Hospital from June 2020 to June 2021. Modified radical mastectomy operations were done. Inclusion criterion included patients with mammary IDC who underwent modified radical mastectomy, while exclusion criteria included patients who received pre-operative chemo or radiotherapy, and patients with insufficient clinical data. All patients gave their informed written consents, and the study was approved by the Ethical Committee of Sohag University (Soh-Med-21-04-32). The specimens of mammary IDC were sent to Pathology Department, Sohag University Hospital. From each specimen; formalin-fixed and paraffin-embedded tissue blocks were prepared. From each block; two tissue sections were obtained, one was stained by H&E stain and the other was subjected to immunohistochemical staining by anti-human CD133 antibody. H&E stained sections were evaluated for the following parameters: Histological subtype, tumor grade, invasion of tumor resection margins, and LVI. LNM and pathological stage of the tumor were also assessed whenever possible. Clinical data of the investigated cases were obtained from patients’ hospital reports. These data included patients’ age, tumor size and laterality. Hormonal status reports as regard Estrogen receptors (ER), Progesterone receptors (PR) and HER2/neu expression were also included.
Immunohistochemical staining of CD133
A streptavidin-biotin immunoperoxidase complex procedure was used for staining. 4μm thick sections were deparaffinized by being placed in a hot xylene for 10 minutes. Tissue sections were re-hydrated by descending grades of alcohol. Endogenous peroxidase activity was blocked by incubation in 3% H2O2 for 10 minutes at room temperature followed by washing in two changes of phosphate buffer solution (PBS). Antigen retrieval was achieved by using 0.01 mmol/L Citrate buffer fluid at 92oC for 20 minutes. Sections were incubated with primary antibody overnight at room temperature with 1:50 dilution of a mouse monoclonal Anti-CD133 (Prominin-1) (Catalog # 030034, Clone No. 13B112, USBIOLOGICAL,
life sciences, USA). Tissue sections were incubated with goat serum secondary antibody followed by streptavidin biotin for ten minutes, separated by washing in PBS for five minutes after each step. The reaction products were visualized by immersing the sections in diaminobenzidine (DAB) for 15 minutes at room temperature (ScyTek, P.O. Box 3286- Logan, Utah 84323, USA). Slight nuclear counterstaining was done by immersion in Harris’ Hematoxylin for few seconds, followed by rapid washing in tap water to remove extra dye. Sections were dehydrated by ascending grades of alcohol. Clearance was done by xylene. Each staining run included positive and negative control sections to confirm that staining systems were working accurately and the positive signals were specific. The positive control tissue sections were prepared from human normal kidney tissue as recommended in the data sheet. Negative control is obtained by omission of the primary antibody from the staining procedure and adding PBS instead of the primary antibody.
Evaluation of CD133 immunostaining
Staining degree was scored and the staining pattern; membranous and/or cytoplasmic reactivity of the cells was noted. Both cytoplasmic and membranous staining were considered as positive. The immunostaining scores criteria were defined as the cell staining intensity (0 = nil; 1 = weak; 2 = moderate; and 3 = strong). The percentage of positive cells was calculated as follows: 1 when < 10%, score 2 (11– 50 %), score 3 (51- 75%), and score 4 >75%. The final score was calculated by multiplying intensity of CD 133 staining and the extent of positivity. The final score ranged between 0 to 12 and the score ≥3 was considered as positive for CD133 expression [11].
Statistical analysis
Statistical analysis was conducted using Statistical Software Package for Social Science (SPSS software version 20). Quantitative data was presented as mean, median and range. Student t-test was used to compare means of two groups. x2 test was used to compare the expression rate between the categories. p was considered statistically significant if < 0.05.
Results
Patients’ characteristics
The current study included 50 specimens of mammary IDC. The patients’ age ranged from 30 to 70 years (mean 50.3). The size of the resected tumors ranged from 1 to 10 cm. (mean 5.4). The tumors were localized in the left breast in 30 (60%) out of 50 patients. Table 1 describes the clinical parameters of the studied cases.
| Variable | No. of cases |
| Age/ Year | |
| Range | 30-70 years. |
| Mean | 50.3 |
| Tumor size/cm. | |
| Range | 1-10 cm. |
| Mean | 5.4 |
| Laterality | |
| Right breast | 20 |
| Left breast | 30 |
Among the investigated IDC specimens, 5 (10%) of tumors were grade I, 26 (52%) were grade II and 19 (38%) tumors were grade III. Histologically, the tumors were classified as infiltrating ductal carcinoma of no specific type (IDC-NST) in 39 (78%) and rare special subtypes in 11 (22%) including: medullary, sarcomatoid, micropapillary, mucinous and cribriform carcinomas in 4, 2, 2, 2, and 1 case, respectively. Histologically normal mammary tissue and ductal carcinoma insitu (DCIS) were found adjacent to the invasive tumor in 18 (36%) and 26 (52%) cases, respectively.
Data about hormone receptor status; ER, PR and HER2/ neu expression were available for all included cases. 15/50 (30%) of specimens were triple negative breast cancer (TNBC). Table 2 describes the histopathological findings of the studied cases.
| Variable | No. of cases (%) |
| Histological subtype | |
| IDC-NST | 39 (78) |
| Rare special subtypes | 11 (22) |
| Tumor grade | |
| Grade I | 5 (10) |
| Grade II | 26 (52) |
| Grade III | 19 (38) |
| Tumor size | |
| T1 (< 2 cm) | 6 (12) |
| T2 (2-5 cm) | 24 (48) |
| T3 (> 5 cm) | 20 (40) |
| Lymph node status | |
| N0 (0) | 16 (32) |
| N1 (1-3) | 13 (26) |
| N2 (4-9) | 12 (24) |
| N3 (> 9) | 9 (18) |
| AJCC stage | |
| I | 6 (12) |
| II | 20 (40) |
| III | 24 (48) |
| NPI | |
| Good | 11 (22) |
| Moderate | 21 (42) |
| Poor | 18 (36) |
| Tumor margins | |
| Free | 33 (66) |
| Infiltrated | 17 (34) |
| LVI | |
| Present | 12 (24) |
| Absent | 38 (76) |
| ER | |
| Positive | 27 (54) |
| Negative | 23 (46) |
| PR | |
| Positive | 27 (54) |
| Negative | 23 (46) |
| HER2/neu | |
| Positive | 13 (26) |
| Negative | 37 (74) |
| TNBC | |
| Yes | 16 (32) |
| No | 34 (68) |
Immunohistochemical detection of CD133
On applying the semi-quantitative scoring method; CD133 positive expression (score ≥3) was detected in 34 (68%) of cases, while 16 (32%) cases were scored as negative for CD133 expression. Regarding subcellular localization, membranous and cytoplasmic reactivity of the cells was detected and both considered as positive. Nuclear staining was not recorded in any of the investigated cases, Figure 1.
Figure 1. H&E Staining of IDC-NST (A), Strong Membranous and Cytoplasmic Immunostaining of CD133 (B), Immunostaing of CD133 Showing Moderate Membranous Staining (C), Negative CD133 Expression Detected in Histologically Normal Mammary Tissue Adjacent to Invasive Tumor (D). X400.
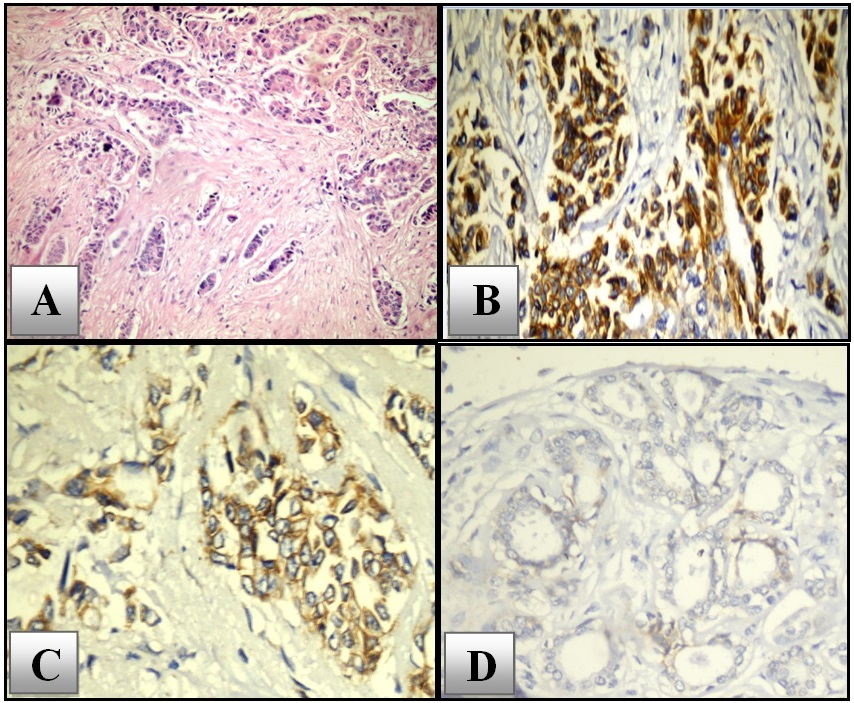
The statistical evaluation of CD133 immunostaining findings was compared with the studied clinical and histopathological parameters. Statistically, Increased CD133 expression was positively correlated with less differentiated tumors (p= 0.008), advanced stage (p=0.002), and poor NPI (p=0.001). The current study also identified a significant positive correlation of CD133 overexpression with negative hormone status and triple negative phenotype (p= 0.003). The correlation of CD133 expression with invasive potential of tumor cells was also studied; there was a steady increase of CD133 expression level as the tumor becomes more invasive. A statistically significant positive association was found between CD133 expression with infiltration of surgical resection margins (p=0.041), LVI (p=0.007), and LNM (p=0.011), Table 3.
| Histopathological Parameter | CD133 Expression | |||
| Positive (%) | Negative (%) | p value | ||
| Histologic subtype | ||||
| IDC-NST | 27 (69) | 12 (31) | 0.495 | |
| Other subtypes | 7 (64) | 4 (36) | ||
| Tumor grade | ||||
| I | 1 (20) | 4 (80) | ||
| II | 16 (62) | 10 (38) | 0.008 | |
| III | 15 (79) | 4 (21) | ||
| LN status | ||||
| N0 | 4 (25) | 12 (75) | ||
| N1 | 8 (62) | 5 (38) | 0.011 | |
| N2 | 9 (75) | 3 (25) | ||
| N3 | 8 (89) | 1 (11) | ||
| AJCC stage | ||||
| I | 1 (17) | 5 (83) | ||
| II | 9 (45) | 11 (55) | 0.002 | |
| III | 19 (79) | 5 (21) | ||
| NPI | ||||
| Good | 2 (18) | 9 (82) | ||
| Moderate | 11 (52) | 10 (48) | 0.001 | |
| Poor | 16 (89) | 2 (11) | ||
| Tumor margins | ||||
| Free | 10 (30) | 23 (70) | 0.041 | |
| Infiltrated | 12 (71) | 5 (29) | ||
| LVI | ||||
| Present | 10 (83) | 2 (17) | 0.007 | |
| Absent | 18 (47) | 20 (53) | ||
| ER | ||||
| Positive | 10 (37) | 17 (63) | 0.005 | |
| Negative | 18 (78) | 5 (22) | ||
| PR | ||||
| Positive | 9 (33) | 18 (67) | 0.005 | |
| Negative | 16 (70) | 7 (30) | ||
| HER2/neu | ||||
| Positive | 10 (77) | 3 (23) | 0.001 | |
| Negative | 12 (32) | 25 (68) | ||
| TNBC | ||||
| Yes | 11 (69) | 5 (31) | 0.003 | |
| No | 13 (38) | 21 (62) |
However, statistical evaluation of CD133 expression in relation to histologic subtype, patients’ age, tumor laterality, and tumor size showed no statistically significant difference, Table 4.
| Variable | CD133 Expression | p- value | |
| Positive (%) | Negative (%) | ||
| Age | |||
| ≤ 50 | 26 (70) | 11 (30) | 0.496 |
| >50 | 8 (62) | 5 (38) | |
| Tumor Size(cm) | |||
| T1 (<2) | 4 (67) | 2 (33) | 0.187 |
| T2 (2 - 5) | 15 (63) | 9 (37) | |
| T 3 (>5) | 9 (45) | 11 (55) | |
| Laterality | |||
| Right breast | 15 (75) | 5 (25) | 0.872 |
| Left breast | 19 (63) | 11 (37) |
Discussion
Breast carcinoma is a life threatening malignant tumor of females with an increasing incidence worldwide. Despite of many chemotherapeutics agents, the invasion and metastasis of breast carcinoma always impair the curative effect and remain the main cause for relapse and mortality [12]. BCSCs were shown to express higher level of pro-invasive genes and had highly invasive properties [13]. CD133 expression has received increasing attention as CSCs marker and as potential prognostic factor. It promotes cancer cell proliferation, migration, and invasiveness. Previous data showed that aberrant expression of CD133 in tumor cells was associated with different progressive properties in breast carcinoma [14].
In our study, we evaluated the expression of CD133 in 50 specimens of mammary IDC, and such expression was correlated with different clinical and histopathological parameters of the studied cases. CD133 immunoreactivity was detected in membrane and/or cytoplasm of tumor cells. By contrast, there was no detectable CD133 expression in histologically normal mammary tissue adjacent to invasive tumor; the expression rate increased with progression from normal to non-invasive to invasive carcinoma. These findings were consistent with the pattern observed in previous reports [15].
CD133 positive expression rate (score ≥3) was detected in 34 (68%) of cases. The current study identified that increased CD133 expression was significantly correlated with high grade, advanced stage and poor NPI. These findings were consistent with previous findings [16]. The association between CD133 expression and poorly differentiated IDC tumors with advanced stages may have a promising hope in developing novel anti- cancer therapeutic agents that target CD133 [17] .
More importantly tumors with CD133 overexpression had a significantly higher potential to invade lymphovascular spaces and to form tumor emboli by the process of epithelial mesenchymal transition. This is proved by positive significant association between CD133 expression and LVI and LNM, these findings are consistent with some previous reports [18].
High CD133 expression showed significant association with negative ER/PR status, CD133 was also highly expressed in TNBC and HER2+ tumors; this was in concordance with findings of previous studies [19]. There was no significant correlation between CD133 expression and some clinicopathological parameters such as histological subtype, age, tumor Laterality and tumor size as reported by previous studies [20].
Previous reports indicated that the overexpression of CD133 in breast carcinoma was associated with tumor invasiveness and poor prognosis. This proves the aggressive nature of the tumor that makes the tumor metastasize and CD 133 positive cells may play an important role in developing metastatic potential [21].
In conclusion, CD133 may play an important role in development and progression of mammary IDC. CD133 up-regulation was strongly associated with poor prognostic indices and with different indicators local aggressiveness of IDC. As result, targeting this molecule in therapeutic approaches may be a useful strategy to reduce breast carcinoma invasion and metastasis.
Limitations of the study
The sample size was not large enough and the number of included some histologic subtypes is very small which in turn weakened the statistical power of the results.
Recommendations
Studying CD133 expression on a large number of cases of different breast lesions including larger number of different histological and molecular subtypes is recommended. Follow up of patients to emphasize the correlation between CD133 expression with patient survival and disease outcome.
Acknowledgements
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
BCSCs, Breast cancer stem cells; PBS, Phosphate buffer solution; CSCs, Cancer stem cells; DCIS, Ductal carcinoma insitu; ER, Estrogen receptors; H&E, hematoxylin and eosin; IDC, Infiltrating ductal carcinoma; IDC-NST, Infiltrating ductal carcinoma of no specific type; LNM, Lymph node metastasis; LVI, lymphovascular invasion; NPI, Nottingham prognostic index; PR, Progesterone receptors; TNBC, Triple- negative breast cancer.
References
- Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D. M., Piñeros M., Znaor A., Bray F.. International Journal of Cancer.2019;144(8). CrossRef
- Clinico-Epidemiological Study of Elderly Breast Cancer in a Developing Country: Egypt Ibrahim Noha. Journal of Cancer Treatment and Research.2019;7. CrossRef
- Breast cancer statistics, 2019 DeSantis Carol E., Ma Jiemin, Gaudet Mia M., Newman Lisa A., Miller Kimberly D., Goding Sauer Ann, Jemal Ahmedin, Siegel Rebecca L.. CA: A Cancer Journal for Clinicians.2019;69(6). CrossRef
- Cancer Stem Cells: The Architects of the Tumor Ecosystem Prager Briana C., Xie Qi, Bao Shideng, Rich Jeremy N.. Cell Stem Cell.2019;24(1). CrossRef
- Cancer Stem Cells and Their Microenvironment: Biology and Therapeutic Implications Lau Eunice Yuen-Ting, Ho Nicole Pui-Yu, Lee Terence Kin-Wah. Stem Cells International.2017;2017. CrossRef
- Breast Cancer Stem Cells Crabtree Judy S., Miele Lucio. Biomedicines.2018;6(3). CrossRef
- CD133: beyond a cancer stem cell biomarker Barzegar Behrooz Amir, Syahir Amir, Ahmad Syahida. Journal of Drug Targeting.2019;27(3). CrossRef
- Cancer stem cells revisited Batlle Eduard, Clevers Hans. Nature Medicine.2017;23(10). CrossRef
- The role of CD133 in cancer: a concise review Glumac Paige M., LeBeau Aaron M.. Clinical and Translational Medicine.2018;7(1). CrossRef
- Overexpression of the cancer stem cell marker CD133 confers a poor prognosis in invasive breast cancer Joseph Chitra, Arshad Maariya, Kurozomi Sasagu, Althobiti Maryam, Miligy Islam M., Al-Izzi Sara, Toss Michael S., Goh Fang Qin, Johnston Simon J., Martin Stewart G., Ellis Ian O., Mongan Nigel P., Green Andrew R., Rakha Emad A.. Breast Cancer Research and Treatment.2019;174(2). CrossRef
- Clinicopathological significance of CD133 and CD44 expression in infiltrating ductal carcinoma and their relationship to angiogenesis Han Zhengquan, Chen Zhendong, Zheng Rongsheng, Cheng Zenong, Gong Xiaomeng, Wang Danna. World Journal of Surgical Oncology.2015;13. CrossRef
- Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis Feng Yixiao, Spezia Mia, Huang Shifeng, Yuan Chengfu, Zeng Zongyue, Zhang Linghuan, Ji Xiaojuan, Liu Wei, Huang Bo, Luo Wenping, Liu Bo, Lei Yan, Du Scott, Vuppalapati Akhila, Luu Hue H., Haydon Rex C., He Tong-Chuan, Ren Guosheng. Genes & Diseases.2018;5(2). CrossRef
- Signaling pathways governing breast cancer stem cells behavior Song Kai, Farzaneh Maryam. Stem Cell Research & Therapy.2021;12(1). CrossRef
- Clinicopathological characteristics and prognostic value of cancer stem cell marker CD133 in breast cancer: a meta-analysis Li Zhan, Yin Songcheng, Zhang Lei, Liu Weiguang, Chen Bo, Xing Hua. OncoTargets and Therapy.2017;10. CrossRef
- CD133 expressionand clinicopathologic significance in benign and malignant breast lesions Liu Ting Ting, Li Xue Feng, Wang Li, Yang Ju Lun. Cancer biomarkers.2020;28(3). CrossRef
- CD133 as a regulator of cancer metastasis through the cancer stem cells Liou Geou-Yarh. The International Journal of Biochemistry & Cell Biology.2019;106. CrossRef
- Prognostic Impact and Clinicopathological Correlation of CD133 and ALDH1 Expression in Invasive Breast Cancer Kim Sung Jeep, Kim Yong Seok, Jang Eun Duok, Seo Kyung Jin, Kim Jeong Soo. Journal of Breast Cancer.2015;18(4). CrossRef
- Expression of CD 133 in Invasive Ductal Carcinoma of Breast Utnal Preeti Ashok, A Hemalatha, Pn Sreeramulu, Gn Manjunath. Asian Pacific journal of cancer prevention: APJCP.2020;21(10). CrossRef
- Prognostic Value of Cancer Stem Cells Markers in Triple-Negative Breast Cancer Collina Francesca, Di Bonito Maurizio, Li Bergolis Valeria, De Laurentiis Michelino, Vitagliano Carlo, Cerrone Margherita, Nuzzo Francesco, Cantile Monica, Botti Gerardo. BioMed Research International.2015;2015. CrossRef
- CD133 in breast cancer cells and in breast cancer stem cells as another target for immunotherapy Tume Luis, Paco Karen, Ubidia-Incio Roberto, Moya Jeel. Gaceta Mexicana de Oncología.2016;15(1). CrossRef
- CD133 in Breast Cancer Cells: More than a Stem Cell Marker Brugnoli Federica, Grassilli Silvia, Al-Qassab Yasamin, Capitani Silvano, Bertagnolo Valeria. Journal of Oncology.2019;2019. CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2022
Author Details