Prognostic Impact on Survival of Early Relapse after Autologous Stem Cell Transplantation with Non-cryopreserved Stem Cells for Multiple Myeloma in Real Life: A Single-center Cohort Study from Oran (Algeria)
Download
Abstract
Background: The aim of this retrospective study was to analyze early relapse in multiple myeloma (MM) in real life and to evaluate its impact on overall survival (OS) and progression-free survival (PFS).
Methods: Two groups of patients were identified according to the date of occurrence of relapse after autologous transplantation, within less than 24 months, defining early relapse (G1), or after more than 24 months, defining late relapse (G2).
Results: A total of 307 patients with MM were enrolled, including 93 patients (30%) who had experienced relapse. There were 56 early relapses (18%) and 37 late relapses (12%). In G1 the median follow-up was 19.5 months (3-93), as compared to59 months (24-117) in G2. The median of PFS was 18 months (14.8-21.14) in G1 and was not attained in G2 (p=0.0001). The median of OS was 29 months (18.2-39.7) in G1 and was not attained in G2 (p=0.0001). In a univariate analysis, age>60 years (p=0.003), performance status>1 (p=0.036), LDH>normal (p=0.002), ISS III (p=0.0002) and an absence of maintenance therapy (p=0.002) were found to be predictive factors for early relapse. In a multivariate analysis, only a delay from the initiation of treatment to ASCT of>12 months (p=0.02) and an absence of maintenance therapy (p=0.002) were predictive of early relapse.
Conclusion: The predictive factors identified here should allow us to adapt the therapeutic strategy.
Introduction
Considerable progress has been made in improving response and survival in multiple myeloma (MM) in the era of novel agents (proteasome inhibitors, immuno- modulators, monoclonal antibodies [1, 2], associated or not with autologous stem cell transplantation (ASCT) [3], but unfortunately, to date, relapse remains inevitable. As a result, numerous studies have focused on the treatment of relapse and its prognostic factors [4], including prognostic stratifications like the R-ISS score or cytogenetic anomalies at diagnosis such as del (17p) or t (4;14) [5].
However, few reports have considered the chronology of relapse and in particular the influence of early relapse [6]. The aim of this study was to analyze early relapse with non-cryopreserved stem cells in real life and to determine predictive factors and their impacts on overall survival (OS) and progression-free survival (PFS) within the Department of Hematology and Cell Therapy of the EHU 1er Novembre, Oran.
Materials and Methods
This was a retrospective study, covering a period of 10 years (2009-2017), in patients with MM having experienced relapse after ASCT. All patients had received induction therapy of the type bortezomib-dexamethasone (VD, n=61), bortezomib-cyclophosphamide-dexamethasone (VCD, n=95), bortezomib-thalidomide-dexamethasone (VTD, n=135), bortezomib-lenalidomide-dexamethasone (VRD, n=7) or cyclophosphamide-thalidomide-dexamethasone (CTD, n=9), followed by ASCT, as indicated in patients having achieved at least partial remission. Hematopoietic stem cells (HSC) were mobilized by administering G-CSF alone at a dose of 10 µg/kg/day for 5 days. After collection, the HSC were stored in a refrigeratorat+4°C for 24 to 48 hours. Intensification was obtained with melphalan at a myeloablative dose, 200 mg/m2 or 140 mg/m2 in the case of known renal insufficiency.
The aim of this retrospective study was to analyze early as compared to late relapse in autologous transplant with non-cryopreserved stem cells, on the basis of OS and PFS as discriminating factors. Thus, the patients were divided into two groups according to the date of occurrence of relapse following ASCT, within less than 24 months, defining early relapse (G1) or after more than 24 months, defining late relapse (G2).The comparison of the two groups G1 and G2 comprised the analysis of and search for factors predicting early relapse, such as age, sex, performance status (PS), ISS score, serum lactate dehydrogenase (LDH), the time from the initiation of treatment to transplantation and maintenance therapy. The del 17p and t (4,14) are cytogenetic abnormalities with poor prognosis and are responsible for early relapses and resistance to treatment in MM. The del 17p and the t (4,14) have not been studied because these examinations are not carried out in all patients in Algeria. This is why they were not included in the analysis of prognostic factors for relapses. The closing date of the study was the 12/31/2019.
Statistics
PFS was calculated from ASCT until the first evidence of disease progression or the date of the last follow-up evaluation. OS was calculated from ASCT until death from any cause or the date of the last contact. The follow up after ASCT was 37 months (range, 3-117) for all patients. Univariate and multivariate analyses of predictive factors for PFS and OS (age, sex, myeloma isotype, ISS score, time from diagnosis to transplantation, maintenance post-ASCT or not) were performed using Fisher’s exact test and exact logistic regression, respectively.
Results
Over a period of 10 years, 307 patients suffering from MM had been registered and were suitable for the study, including 93 patients (30%) who had experienced relapse. There were 56 early relapses (18%), representing group G1and 37 late relapses (12%), representing group G2, while 61 patients (66%) had received maintenance therapy. The clinical and biological characteristics of the two groups are presented in Table 1.
| Characteristics | Entire population (N=307) | G1 Early relapse (N=56) | G2 Late relapse (N=37) | P-value |
| Median age (years) | 54 | 55 | 50.2 | 0.004 |
| (minimum-maximum) | (35-67) | (41-67) | (35-65) | |
| Sex | N=307 (%) | N=56 (%) | N=37 (%) | |
| Male | 192 (62, 5) | 36 (64) | 24 (65) | 0.95 |
| Female | 115 (37, 5) | 20 (36) | 13 (35) | |
| Performance status | N=307 (%) | N=56 (%) | N=37 (%) | |
| ≤1 | 203 (66) | 27 (48) | 29 (78) | 0.025 |
| >1 | 104 (34) | 29 (52) | 8 (22) | |
| ISS score | N=148 (%) | N=46 (%) | N=22 (%) | |
| ISS I | 34 (23) | 8 (17) | 5 (23) | |
| ISS II | 39 (26) | 13 (28) | 7 (32) | 0.77 |
| ISS III | 75 (51) | 25 (55) | 10 (45) | |
| Not determined | 159 (52) | |||
| Hemoglobin (g/dL) | 10 | 9.2 | 8.6 | 0.21 |
| LDH (IU/L) | 434.5 | 946 | 512 | 0.07 |
| Albumin (g/L) | 35 | 32.8 | 33.2 | 0.85 |
| Paraprotein (g/L) | 43 | 44 | 42.5 | 0.78 |
| Monoclonal component | N= 307 (%) | N=56 (%) | N=37 (%) | |
| IgG | 175 (57) | 36 (64) | 27 (73) | |
| IgA | 43 (14) | 10 (18) | 6 (16) | 0.53 |
| Light chain | 62 (29) | 10 (18) | 4 (11) | |
| NA | 27 | 0 | 0 | |
| Beta-2 microglobulin (mg/L) | 7 | 7.4 | 6.9 | 0.9 |
| Response end of induction | N=307 (%) | N=56 (%) | N=37 (%) | |
| CR | 92 (30) | 17 (30) | 16 (43) | |
| VGPR | 147 (48) | 24 (43) | 15 (40, 5) | 0.3 |
| PR | 65 (21) | 13 (23) | 6 (16, 5) | |
| Stable disease | 3 (1) | 2 (4) | 0 | |
| Response after ASCT day 100 | N=307 (%) | N=56 (%) | N=37 (%) | |
| Death | 10 (3, 2) | 0 | 0 | |
| CR | 184 (60) | 35 (62, 5) | 29 (78) | 0.16 |
| VGPR | 107 (35) | 19 (34) | 8 (22) | |
| PR | 6 (1, 8) | 2 (3, 5) | 0 (0) | |
| Number of CD34+cells reinjected (x106/kg) | 3.34 | 3.9 | 3.6 | 0.39 |
| Conditioning | N=307 (%) | N=56 (%) | N=37 (%) | |
| Melphalan (200 mg/m2) | 267 (87) | 50 (89) | 34 (92) | 0.53 |
| Melphalan (140 mg/m2) | 40 (13) | 6 (11) | 3 (8) | |
| Delay from initiation of treatment to ASCT | N=307 (%) | N=56 (%) | N=37 (%) | |
| ≤ 12 months | 253 (82) | 41 (73) | 30 (81) | 0.76 |
| >12 months | 54 (18) | 15 (27) | 7 (19) | |
| Maintenance therapy | N=307 (%) | N=56 (%) | N=37 (%) | |
| Yes | 227 (74) | 38 (68) | 28 (76) | 0.12 |
| No | 80 (26) | 18 (32) | 9 (24) |
Results are median values for continuous variables; ISS, International Staging System; LDH, lactate dehydrogenase; CR, complete remission; VGPR, very good partial remission; PR, partial remission; ASCT, autologous stem cell transplantation.
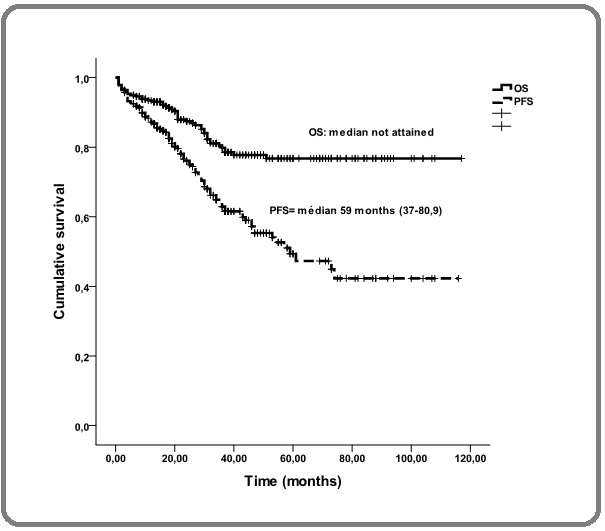
In the overall population, the median follow-up was 37 months (range, 3-117), the median PFS was 59 months (range, 37-80.9) and the median OS was not attained (range, 51% at 117 months) (Figure 1).
Figure 1. Overall Survival and Progression-free Survival in the Entire Cohorte.
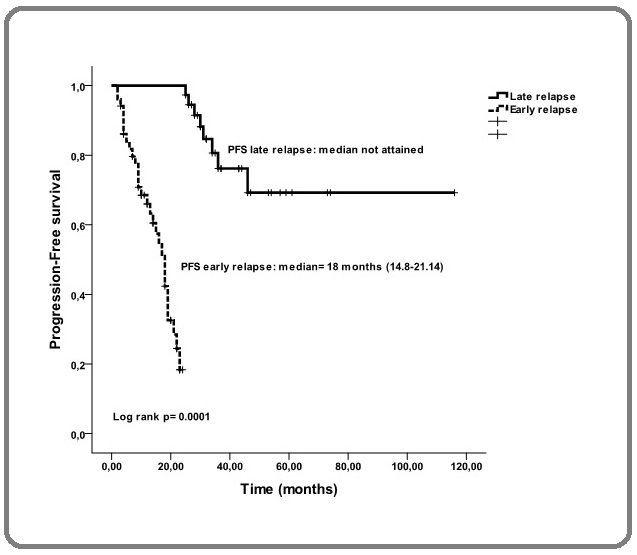
In group G1, the median duration of follow-up was 19.5 months (range, 3-93), as compared to 59 months (range, 24-117) in group G2. The median duration of PFS was18 months (range, 14.8-21.14) in group G1 and was not reached in group G2 (p=0.0001) (Figure 2).
Figure 2. Progression-free Survival in Paients Experiencing Early or Late Relapse after ASCT.
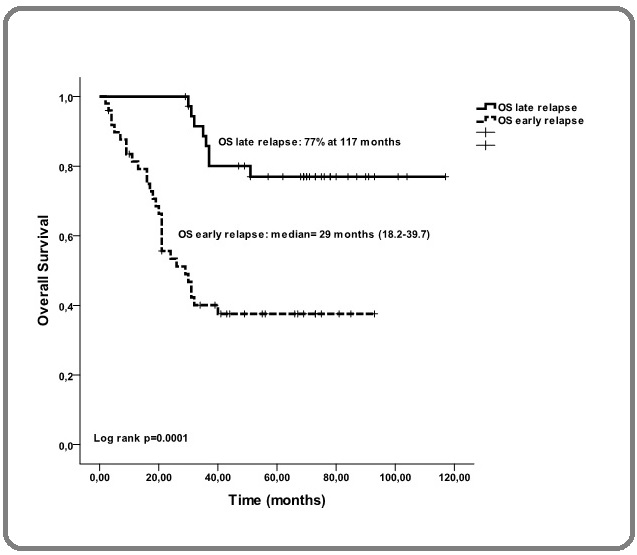
The median duration of OS was 29 months (range, 18.2-39.7) in group G1 and was not attained in group G2 (p=0.0001) (Figure 2). In a univariate analysis, age>60 years (p=0.003), PS>1 (p=0.036), LDH>normal (p=0.002), ISS III (p=0.0002) and an absence of maintenance therapy (p=0.002) were found to be predictive factors for early relapse (Table 2).
| Univariate analysis | |||
| HR | CI 95% | P-value | |
| Age>60 years | 1.05 | 1.02- 1.09 | 0.003 |
| Sex: M/F | 1.01 | 0.8-1.3 | 0.94 |
| PS > 1 | 0.73 | 0.6-0.98 | 0.036 |
| Plasmocytes | 0.99 | 0.98-1.01 | 0.82 |
| Salmon-Durie stage | 0.97 | 0.7-1.4 | 0.89 |
| A/B | |||
| Hemoglobin | 1.1 | 0.9-1.2 | 0.19 |
| LDH> normal | 1 | 1-1.01 | 0.002 |
| Albumin | 0.99 | 0.97-1.03 | 0.77 |
| Paraprotein | 1 | 0.98-1.01 | 0.88 |
| Monoclonal component | |||
| IgG | 1 | ||
| IgA | 1.04 | 0.6-1.7 | 0.97 |
| Light chain | 0.78 | 0.5-1.2 | 0.31 |
| Beta-2 microglobulin | 1 | 0.95-1.06 | 0.82 |
| ISS score | |||
| ISS I | 0.93 | 0.5-1.6 | 0.8 |
| ISS II | 0.88 | 0.5-1.4 | 0.6 |
| ISS III | 1 | ||
| Response end of induction | |||
| CR | 1 | ||
| VGPR | 0.87 | 0.5-1.5 | 0.61 |
| PR | 1.01 | 0.6-1.7 | 0.96 |
| Stable disease | 1.7 | 0.6-5.1 | 0.29 |
| Number of CD34+ cells | 1 | 0.9-1.15 | 0.68 |
| Conditioning (melphalan 200/140 mg/m2) | 1.55 | 1.1-2.2 | 0.059 |
| Delay from initiation of treatment to ASCT ≤ 12 months | 0.96 | 0.9-1.006 | 0.09 |
| No maintenance | 1.4 | 1.05-1.8 | 0.019 |
HR, hazard ratio; CI 95%, 95% confidence interval; PS, performance status.
In a multivariate analysis, only a delay between the initiation of treatment and ASCT of>12 months (p=0.02) and an absence of maintenance therapy (p=0.002) were predictive of early relapse (Figure 3).
Figure 3. Overall Survival in Patients Experiencing Lata or Early Relapse after ASCT.
Discussion
There exist few data on MM in Algeria and in particular few therapeutic data with non-cryopreserved stem cells. Our work, although retrospective, shows an overall rate of relapse following ASCT of 30% with a median follow-up of 37 months, close to that of the literature [7], in real life and in the era of novel agents, together with a rate of early relapse similar to that reported in clinical trials, 18% as compared to 16% [8]. These early relapses compared to late relapse (median durations of PFS and OS are not reached) are characterized by median durations of PFS of 18 months and of OS of 29 months, which are short and represent an unfavorable clinical prognostic factor (p=0.0001). Comparable results were obtained by Majithia et al [8], with a PFS of 8 months and an OS of 21 months (p=0.001),as likewise by Kumar et al [9] of the CIBMTR, where among 3,256 patients, 35% presented early relapse and a short OS (p<0.0001). Similarly, Jimenez-Zepeda et al [10] found a rate of early relapse of 36% (27 patients) among 75 patients having relapsed, with a median PFS of 17.2 months and a median OS of 20 months (p=0.001) in the group displaying early relapse. Lee et al [11] reported a similar rate of early relapse (13.6%), with a short median OS after early relapse (17.8 months) as compared to late relapse (p=0.0001).
Cytogenetic factors were not analyzed in this work as such tests cannot be performed in routine practice. Thus, in a univariate analysis, PS>1 (p=0.036), LDH > normal (p=0.002), a delay from the beginning of treatment to ASCT exceeding 12 months (p=0.02) and an absence of maintenance therapy (p=0.002) were found to be factors predictive of early relapse in our study. Concerning the interval between the initiation of treatment and autologous transplantation, Jain et al [12] observed a short PFS when ASCT was carried out later as compared to within 12 months (p=0.001), but a similar OS. Meanwhile, in the literature, the factors reported to predict relapse are an ISS stage III [13], the time from the initiation of treatment to ASCT and a lack of maintenance therapy [14]. On the other hand, in our multivariate analysis, the common factor predictive of early relapse was an absence of maintenance therapy (p=0.002). In fact, maintenance therapy is today considered to be indispensable in the management of MM in patients eligible for transplantation, affording in particular with lenalidomide, a prolongation of the PFS and a reduction of the rate of relapse [15]. Overall, early relapse is a major independent clinical prognostic factor for PFS and OS [16-17].
Finally, the present work has limitations, such as the retrospective nature of the study, the heterogeneity of the induction therapy, the lack of cytogenetic studies, or the absence of evaluation of residual disease. It is nevertheless of interest in that early relapse was found to be associated with a short OS and PFS and thereby defines a category of patients at high risk, requiring a particular biological approach and specific therapeutic management. In conclusion, in real life as in academic clinical trials, early relapse has a negative impact on PFS and OS in MM. Apart from the cytogenetic factors of poor prognosis which have not been studied in our work, we found age, performans status, LDH and ISS III as being independent factors of poor prognosis and early relapse. Performing ASCT within 12 months of the initiation of treatment and administering maintenance therapy after transplantation improve the progression-free and overall survival and are responsible for late relapses. The predictive factors identified here should allow us to adapt the therapeutic
strategy.
Acknowledgments
I thank the patients and the families of the patients.
Authorship Contributions
Medical Practices: BMA; MB; OH; BSa: CL; EB; YN
Concept: BMA; LG
Design: BMA; LG
Data Collection or processing: BB
Analysis or Interpretation: BMA; LG
Literature Search: BMA; LG; BSo
Writing: BMA; LG
Conflict of interest
The authors of this study have no conflicts of interest, including specific financial interest, relationships, and/ or affiliations relevant to the subject matter or materials included.
References
- Improved survival in multiple myeloma and the impact of novel therapies Kumar Shaji K., Rajkumar S. Vincent, Dispenzieri Angela, Lacy Martha Q., Hayman Suzanne R., Buadi Francis K., Zeldenrust Steven R., Dingli David, Russell Stephen J., Lust John A., Greipp Philip R., Kyle Robert A., Gertz Morie A.. Blood.2008;111(5). CrossRef
- Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management Rajkumar S. Vincent. American Journal of Hematology.2016;91(7). CrossRef
- Multiple myeloma: Role of autologous transplantation Ntanasis-Stathopoulos Ioannis, Gavriatopoulou Maria, Kastritis Efstathios, Terpos Evangelos, Dimopoulos Meletios A.. Cancer Treatment Reviews.2020;82. CrossRef
- [Management of multiple myeloma in the relapsed/refractory patient] Tabayashi Takayuki. [Rinsho Ketsueki] The Japanese Journal of Clinical Hematology.2019;60(9). CrossRef
- Role of additional chromosomal changes in the prognostic value of t(4;14) and del(17p) in multiple myeloma: the IFM experience Hebraud Benjamin, Magrangeas Florence, Cleynen Alice, Lauwers-Cances Valerie, Chretien Marie-Lorraine, Hulin Cyrille, Leleu Xavier, Yon Edwige, Marit Gerald, Karlin Lionel, Roussel Murielle, Stoppa Anne-Marie, Belhadj Karim, Voillat Laurent, Garderet Laurent, Macro Margaret, Caillot Denis, Mohty Mohamad, Facon Thierry, Moreau Philippe, Attal Michel, Munshi Nikhil, Corre Jill, Minvielle Stephane, Avet-Loiseau Herve. Blood.2015;125(13). CrossRef
- Relapse after complete response in newly diagnosed multiple myeloma: implications of duration of response and patterns of relapse Sidana Surbhi, Tandon Nidhi, Dispenzieri Angela, Gertz Morie A., Buadi Francis K., Lacy Martha Q., Dingli David, Fonder Amie L., Hayman Suzanne R., Hobbs Miriam A., Gonsalves Wilson I., Warsame Rahma M., Kourelis Taxiarchis, Hwa Yi Lisa, Kapoor Prashant, Kyle Robert A., Leung Nelson, Go Ronald S., Rajkumar S. Vincent, Kumar Shaji K.. Leukemia.2019;33(3). CrossRef
- Multiple Myeloma Treatment in Real-world Clinical Practice: Results of a Prospective, Multinational, Noninterventional Study Mohty Mohamad, Terpos Evangelos, Mateos Maria-Victoria, Cavo Michele, Lejniece Sandra, Beksac Meral, Bekadja Mohamed Amine, Legiec Wojciech, Dimopoulos Meletios, Stankovic Svetlana, Durán Maria Soledad, De Stefano Valerio, Corso Alessandro, Kochkareva Yulia, Laane Edward, Berthou Christian, Salwender Hans, Masliak Zvenyslava, Pečeliūnas Valdas, Willenbacher Wolfgang, Silva João, Louw Vernon, Nemet Damir, Borbényi Zita, Abadi Uri, Pedersen Robert Schou, Černelč Peter, Potamianou Anna, Couturier Catherine, Feys Caroline, Thoret-Bauchet Florence, Boccadoro Mario. Clinical Lymphoma, Myeloma & Leukemia.2018;18(10). CrossRef
- Early relapse following initial therapy for multiple myeloma predicts poor outcomes in the era of novel agents Majithia N., Rajkumar S. V., Lacy M. Q., Buadi F. K., Dispenzieri A., Gertz M. A., Hayman S. R., Dingli D., Kapoor P., Hwa L., Lust J. A., Russell S. J., Go R. S., Kyle R. A., Kumar S. K.. Leukemia.2016;30(11). CrossRef
- Early relapse after autologous hematopoietic cell transplantation remains a poor prognostic factor in multiple myeloma but outcomes have improved over time Kumar S. K., Dispenzieri A., Fraser R., Mingwei F., Akpek G., Cornell R., Kharfan-Dabaja M., Freytes C., Hashmi S., Hildebrandt G., Holmberg L., Kyle R., Lazarus H., Lee C., Mikhael J., Nishihori T., Tay J., Usmani S., Vesole D., Vij R., Wirk B., Krishnan A., Gasparetto C., Mark T., Nieto Y., Hari P., D'Souza A.. Leukemia.2018;32(4). CrossRef
- Early relapse after single auto-SCT for multiple myeloma is a major predictor of survival in the era of novel agents Jimenez-Zepeda V. H., Reece D. E., Trudel S., Chen C., Tiedemann R., Kukreti V.. Bone Marrow Transplantation.2015;50(2). CrossRef
- Early Relapse for Multiple Myeloma Patients Undergoing Single Autologous Stem Cell Therapy: A Single-center Experience Lee Holly, Duggan Peter, Chaudhry Ahsan, Neri Paola, Tay Jason, Rashid-Kolvear Fariborz, Bahlis Nizar J., Jimenez-Zepeda Victor H.. Clinical Lymphoma, Myeloma & Leukemia.2018;18(1). CrossRef
- High-Dose Chemotherapy with Early Autologous Stem Cell Transplantation Compared to Standard Dose Chemotherapy or Delayed Transplantation in Patients with Newly Diagnosed Multiple Myeloma: A Systematic Review and Meta-Analysis Jain Tania, Sonbol Mohamad Bassam, Firwana Belal, Kolla Kantha R., Almader-Douglas Diana, Palmer Jeanne, Fonseca Rafael. Biology of Blood and Marrow Transplantation: Journal of the American Society for Blood and Marrow Transplantation.2019;25(2). CrossRef
- Early relapsed disease of multiple myeloma following up-front HDM-ASCT: a study based on the Danish Multiple Myeloma Registry in the period 2005 to 2014 Helm-Petersen Sissel, Sørrig Rasmus, Klausen Tobias, Preiss Birgitte, Frølund Ulf, Helleberg Carsten, Breinholt Marie, Andersen Mette, Abildgaard Niels, Gimsing Peter, Vangsted Annette. Leukemia.2018;32. CrossRef
- Lenalidomide maintenance versus observation for patients with newly diagnosed multiple myeloma (Myeloma XI): a multicentre, open-label, randomised, phase 3 trial Jackson Graham H., Davies Faith E., Pawlyn Charlotte, Cairns David A., Striha Alina, Collett Corinne, Hockaday Anna, Jones John R., Kishore Bhuvan, Garg Mamta, Williams Cathy D., Karunanithi Kamaraj, Lindsay Jindriska, Jenner Matthew W., Cook Gordon, Russell Nigel H., Kaiser Martin F., Drayson Mark T., Owen Roger G., Gregory Walter M., Morgan Gareth J.. The Lancet. Oncology.2019;20(1). CrossRef
- Lenalidomide maintenance after stem-cell transplantation for multiple myeloma Attal Michel, Lauwers-Cances Valerie, Marit Gerald, Caillot Denis, Moreau Philippe, Facon Thierry, Stoppa Anne Marie, Hulin Cyrille, Benboubker Lofti, Garderet Laurent, Decaux Olivier, Leyvraz Serge, Vekemans Marie-Christiane, Voillat Laurent, Michallet Mauricette, Pegourie Brigitte, Dumontet Charles, Roussel Murielle, Leleu Xavier, Mathiot Claire, Payen Catherine, Avet-Loiseau Hervé, Harousseau Jean-Luc. The New England Journal of Medicine.2012;366(19). CrossRef
- Early relapse after autologous transplant for myeloma is associated with poor survival regardless of cytogenetic risk Corre Jill, Montes Lydia, Martin Elodie, Perrot Aurore, Caillot Denis, Leleu Xavier, Belhadj Karim, Facon Thierry, Hulin Cyrille, Mohty Mohamad, Fontan Jean, Macro Margaret, Brechignac Sabine, Jaccard Arnaud, Stoppa Anne-Marie, Orsini-Piocelle Frederique, Adiko Didier, Voillat Laurent, Keddar Faiza, Barry Marly, Demarquette Helene, Certain Marie-Noelle, Plantier Isabelle, Roussel Murielle, Hébraud Benjamin, Filleron Thomas, Attal Michel, Avet-Loiseau Hervé. Haematologica.2020;105(9). CrossRef
- Early relapse post autologous transplant is a stronger predictor of survival compared with pretreatment patient factors in the novel agent era: analysis of the Singapore Multiple Myeloma Working Group Ong S. Y., Mel S., Chen Y. X., Ooi M. G., Surendran S., Lin A., Koh L. P., Linn Y. C., Ho A. Y. L., Hwang W. Y. K., Phipps C., Loh S. M. Y., Goh Y. T., Tan D., Chng W. J., Gopalakrishnan S. K.. Bone Marrow Transplantation.2016;51(7). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2022
Author Details