Diagnostic and Prognostic Values of PTEN Expression in Functional and Pathological Endometrial Biopsies
Download
Abstract
Background: Endometrial carcinoma is the most common gynecological malignancy in developed countries. About 80% of endometrial carcinomas are preceded by atypical endometrial hyperplasia. PTEN is a tumor suppressor gene involved in many vital intracellular biological processes. PTEN is altered in more than 45% of endometrial carcinomas. The aim of this study was to evaluate the value immunohistochemical expression of PTEN in normal, hyperplastic and neoplastic endometrial tissues.
Methods: Ninety-two endometrial samples were enrolled in this study. They were classified into normal (n=6), hyperplastic (n=54) and neoplastic (n=32) endometrial tissues. Formalin-fixed and paraffin-embedded tissue blocks were prepared from each specimen. Tissue sections were immunohistochemically stained by anti-PTEN antibodies.
Results: In our study; PTEN was strongly expressed in all normal and hyperplastic endometrial tissues without atypia. Staining intensity was decreased in atypical endometrial hyperplasia and endometrial carcinoma (p<0.0001). we also detected an inverse relationship between PTEN expression on one side and tumor grade (p=0.006), stage (p< 0.0001) and myometrial invasion (p=0.001) on the other side.
Conclusions: Our study proved that immunohistochemical expression of PTEN is down-regulated in atypical hyperplastic and neoplastic endometrial tissues. Evaluation of PTEN expression can be useful as a screening method to detect the potentially precancerous hyperplastic lesions.
Introduction
Endometrial carcinoma (EC) is the most common gynecological malignancy in developed countries. It is the 4th most common cancer in women after breast, colon and lung carcinomas. EC typically occurs in pre-menopausal and post-menopausal women. However, no age group is immune [1]. A literature reported the occurrence of EC in association with intrauterine pregnancy in five women, raising the importance of studying the molecular alterations and genetic mutations of EC [2]. Based on light microscopic appearance and clinical behavior; ECs were classified into two main categories, types I and II [3]. Type I is the most common, representing more than 80% of cases. Most cases occur in pre-menopausal women. It is a low grade carcinoma that may occur in hyper-estrogenic status and develops against a background of endometrial hyperplasia and carries a more favorable prognosis [4]. On the contrary, type II occurs almost exclusively in post-menopausal women. It develops de novo, independent of estrogenic stimulation and has an aggressive behavior [5]. On the molecular basis, low numbers of somatic copy number alterations and wild type expression pattern of P53 were observed only in EC, type I [6].
Endometrial hyperplasia (EH) is defined as increase endometrial glands/ stroma ratio, coupled with glandular architectural irregularity. Different classification systems for EH have been developed over the years. Most of these classifications were based on architectural in addition to cytological features. There were numerous inter and intra-observer variations in previous classifications of EH, especially as regards to the architectural component [7]. In 2014, WHO has adopted a new classification system for EH, it was based on the cytological atypical features which are considered as a more reliable component. Such recent WHO-adopted classification system has categorized EH into two main categories according to presence or absence of cytological atypia; EH without atypia and atypical EH [8]. The latter is a precursor lesion for EC, type I. Both atypical EH and EC, type I share a variety of genetic alterations as microsatellite instability (MSI), mutations of K-ras, ᵦ- catenine and phosphatase and tensin homolog (PTEN) [1].
PTEN was firstly identified in 1997 as a tumor suppressor gene, located on chromosome 10 (10q23). The protein encoded by this gene is a 55- kDa protein composed of 403 amino acids [9]. As a tumor suppressor gene, PTEN suppresses both cellular proliferation and differentiation by antagonizing intracellular signaling pathways evoked by different growth factors [10]. PTEN has an active role in apoptosis of damaged and/ or injured cells by two efficient mechanisms. The first mechanism is that the overexpression of PTEN is invariably associated with increased p27 expression. The latter causes cell cycle arrest in G1 phase [11]. Secondly, PTEN promotes p53 functions through restricting murine double minute 2 (mdm2) to the cytoplasm [12]. So, it is clear that deregulated PTEN expression contributes to tumorigenesis by preventing apoptosis and enhances the intracellular signaling pathways that promote cellular proliferation. Deregulated PTEN expression has been detected in various human malignant neoplasms as glioma, melanoma, prostatic, mammary and bronchogenic carcinomas [13-17].
The aim of this study was to evaluate the value of immunohistochemical expression of PTEN in normal, hyperplastic and neoplastic endometrial tissues, in order to establish any potential benefit of PTEN immunostaining of endometrial hyperplastic tissue specimens. We also aimed to correlate the expression of PTEN in studied endometrial carcinoma specimens to different tumor parameters as tumor grade, stage and state of myometrium invasion.
Materials and Methods
Clinical data and specimens collection
A prospective study included 92 women. Eighty- six women suffered from pre-menopausal and/or post-menopausal bleeding admitted to the Gynecology& Obstetrics Department of Sohag University Hospital from January, 2020 to December of the same year. Endometrial specimens were obtained from all patients by dilatation and curettage (D&C). The specimens were sent to Pathology Department, Sohag University. Specimens were histopathologically diagnosed as either endometrial hyperplasia (EH) or endometrial carcinoma (EC). Additional 6 normal endometrial tissue specimens were obtained from hysterectomy operations done for patients suffered from uterine prolapses and multiple uterine leiomyomas in 2 and 4 patients, respectively. Cases of EH were classified, according to their cytological atypical features, into EH without atypia and atypical EH. Cases diagnosed as ECs have undergone hysterectomy operations and the excised uteri were evaluated for status of muscle invasion and tumor stage. The inclusion criteria included all the endometrial biopsies and hysterectomy specimens diagnosed as endometrial hyperplasia and/ or primary endometrial carcinomas. The exclusion criteria included endometrial specimens with extensive autolysis, very tiny endometrial samples that were unfit for diagnosis, specimens formed predominantly of blood clots or endometrial samples showed any evidence of endometritis. All patients gave their written informed consents and the study was approved by the Ethical Committee of Sohag University (Registration number: Soh-Med-21-04-29) and registered in Clinical Trials. gov PRS (Clinical Trials.gov ID: NCT04873206). From each endometrial specimen; formalin-fixed and paraffin- embedded tissue blocks were prepared. Two tissue sections were obtained from each block, one was stained by H&E to establish the diagnosis, and the other was stained immunohistochemically by anti-human PTEN antibody.
Immunohistochemical staining of PTEN
Formalin-fixed and paraffin-embedded endometrial tissue blocks were sectioned into 4μm thick slices. Sections were deparaffinized by being placed in a hot xylene for 10 minutes. Tissue sections were re-hydrated by descending grades of alcohol. In order to block endogenous peroxidase activity; tissue sections were incubated in 3% H O for 10 minutes at room temperature.
Antigen retrieval was achieved by using 0.01 mmol/L Citrate buffer fluid at 92oC. for 20 minutes. Sections were incubated with primary antibody overnight at room temperature with 1:100 dilution of a mouse monoclonal antibody raised against amino acids 1-403 representing full length PTEN of human origin (Catalog Number: sc- 133242, Concentrated form, Santa cruz biotechnology). Tissue sections were incubated with goat serum secondary antibody followed by streptavidin biotin for ten minutes, separated by washing in BPS for five minutes after each step. The reaction products were visualized by immersing the sections in diaminobenzidine (DAB) for 15 minutes at room temperature ( ScyTek, P.O. Box 3286- Logan, Utah 84323, USA). slight nuclear counterstaining was done by immersion in Harris’ Hematoxylin for few seconds followed by rapid washing in tap water to remove extra dye. Sections were dehydrated by ascending grades of alcohol. Clearance was done by xylene. Each staining run included positive and negative control sections to confirm that staining systems were working accurately and the positive signals were specific. The positive control tissue sections were prepared from human skin tissue as recommended in the data sheet. Negative control were obtained from endometrial tissue sections, but BPS was used instead of primary antibody.
Evaluation of PTEN immunostaining
The endometrial specimens were scored on the basis of a well-established immunoreactivity scoring system (IRS). The intensity of PTEN immune-expression was scored as follows: 0 (negative staining), 1 (weak), 2 (moderate) and 3 (strong). The percentage of positive cells was calculated as follows: 0 (0%), 1 (<10%), 2 (10-50%), 3 (51-80%) and 4 (>80%). The final score was calculated by multiplying the intensity score with the percentage of positive cells. The resulting IRS was categorized as negative (0), low (1-4), intermediate (6,8) and high staining (9,12) [18].
Statistical analysis
Data was analyzed using SPSS version 20 (Statistical Software package version 20). Quantitative data was represented as mean, standard deviation, median and range. Shapiro-Wilk test was used to determine if the data normally distributed or not. Data was analyzed using Kruskal- Wallis test for comparison of the means of three groups of non-parametric data. Qualitative data was represented as frequencies and percentages. When the data was categorical Chi-square (Χ2) test was used to compare between groups. P value was considered significant if it was < 0.05.
Results
Patients characteristics
The current study included 92 women, their ages ranged from 29-72 years old (mean= 50.4). The studied endometrial specimens were diagnosed as normal endometrium, EHs and ECs in 6, 54 and 32 patients respectively. As regards to the studied ECs, all cases of ECs were of endometrioid variant. The included EC specimens were graded into grades I, II and III in 14, 12 and 6 patients. On applying TNM and FIGO classifications of ECs; 10 cases were in T1 stage, T2 and T3 stages were reported in 10 and 12 patients respectively. Myometrium invasion by the neoplastic cells were reported as positive and negative in 22 and 10 patients respectively. Tables 1 and 2 summarize the pathological characteristics of the studied cases.
| Diagnosis of the studied cases | Frequency |
| Normal. | 6 |
| Hyperplastic endometrium. | 54 |
| Endometrial hyperplasia without atypia | 25 |
| Atypical endometrial hyperplasia | 29 |
| Endometrial adenocarcinoma. | 32 |
| Pathological characteristics of EC cases | Frequency |
| Tumor Grade | |
| G I | 14 |
| G II | 12 |
| GIII | 6 |
| Tumor Stage | |
| T 1 | 10 |
| T 2 | 10 |
| T3 | 12 |
| Myometrial invasion | |
| Positive | 22 |
| Negative | 10 |
Immunohistochemical detection of PTEN
On applying IRS system, 88 endometrial specimens were scored as positive, while 4 specimens were scored as negative for PTEN expression. The positive cases were categorized as mild, moderate and strong for PTEN expression in 25, 30 and 33 cases, respectively. PTEN immunostaining was detected in the endometrial glands, (Figure 1).
Figure 1. H&E Staining of Proliferative Endometrium (a), EH without Atypia (b), EC (c), Strong PTEN Expression in Proliferative Endometrium (d), Moderate PTEN Intensity in EH (e), Negative PTEN Expression in EC (f).
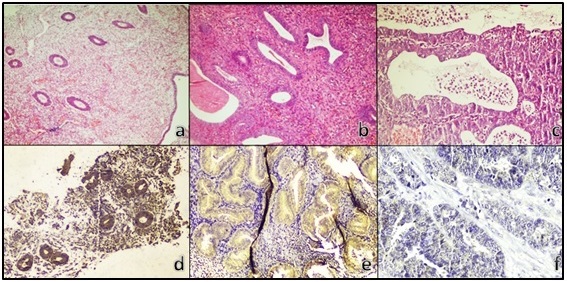
The statistical evaluation of PTEN immunostaining findings was compared with the studied clinical and pathological data. There was no statistically significant correlation between PTEN expression and the ages of the studied patients. On comparing the expression of PTEN in normal endometrium, EH without atypia and atypical EH; all cases of studied normal endometrial tissues and EH which lacked any evidence of cytological atypia showed strong cytoplasmic expression of PTEN, while most of atypical EH specimens showed decreased intensity of PTEN expression (p< 0.0001), (Table 3).
| Variable | Cases | PTEN Expression | P value | |||
| Negative | Mild | Moderate | Strong | < 0.0001** | ||
| Normal Endometrium. | 6 | 0 | 0 | 0 | 6 | |
| EH without atypia. | 25 | 0 | 0 | 0 | 25 | |
| Atypical EH. | 29 | 0 | 7 | 20 | 2 |
Chi-square test was used; **, highly significant.
PTEN immunostaining was evaluated in cases of EH without atypia versus atypical EH, all cases of EH without atypia retained strong PTEN immunostaining pattern, while the great majority of atypical EH specimens (27/29 cases) showed either mild or moderate PTEN expression (p< 0.0001), (Table 4).
| Variable | Cases | PTEN Expression | P value | |||
| Negative | Mild | Moderate | Strong | |||
| EH without atypia. | 25 | 0 | 0 | 0 | 25 | < 0.0001** |
| Atypical EH. | 29 | 0 | 7 | 20 | 2 |
Chi-square test was used; **, highly significant.
A highly significant relationship was noticed when the expression of PTEN in normal endometrial tissues was compared to its expression in EC specimens. All the included normal endometrial specimens showed strong PTEN immunostaining, while none of the studied EC specimens showed strong expression. In addition, all the encountered negative PTEN tissue sections were histologically diagnosed as ECs (p< 0.0001), (Table 5).
| Variable | Cases | PTEN Expression | P value | |||
| Negative | Mild | Moderate | Strong | |||
| Normal Endometrium. | 6 | 0 | 0 | 0 | 6 | |
| Endometrial Carcinoma | 32 | 4 | 18 | 10 | 0 | < 0.0001** |
Chi-square test was used; **, highly significant
Expression of PTEN in hyperplastic endometrial tissues (atypical and non-atypical EHs, separately) was compared to its expression in EC specimens, all cases of EH without atypia were strongly expressing PTEN (25/25 cases), while atypical endometrial hyperplastic specimens and EC specimens were either mildly or moderately expressing PTEN (p< 0.0001), (Table 6).
| Variable | Cases | PTEN Expression | P value | |||
| Negative | Mild | Moderate | Strong | < 0.0001** | ||
| EH without atypia. | 25 | 0 | 0 | 0 | 25 | |
| Atypical EH. | 29 | 0 | 7 | 20 | 2 | |
| Endometrial Carcinoma | 32 | 4 | 18 | 10 | 0 |
Chi-square test was used; **, highly significant.
immunohistochemical expression of PTEN in ECs was compared to different tumor parameters. There was a statistically significant relationship between PTEN and tumor grade. All the studied ECs that showed negative PTEN expression were included in tumor grade I, the intensity of PTEN immunostaining was noticed to be increased with higher tumor grades (p= 0.006). A similar observation was noticed when a comparison between PTEN expression in ECs and tumor stage was performed. Negative and mild PTEN expressions were noticed in stages 1 and 2, while stage 3 showed an increase in intensity of PTEN immunostaining (p< 0.0001). Finally, as regards to the relationship between PTEN expression and the status of myometrium invasion; immunohistochemical expression of PTEN in ECs is directly related to invasion of the myometrium by the neoplastic cells (p= 0.001), (Table 7).
| Endometrial Carcinoma | Cases= 32 | PTEN Expression | p- value | ||
| Negative= 4 | Mild= 18 | Moderate= 10 | |||
| Tumor Grade | |||||
| G I | 14 | 4 | 10 | 0 | 0.006* |
| G II | 12 | 0 | 6 | 6 | |
| G III | 6 | 0 | 2 | 4 | |
| Tumor Stage | |||||
| T 1 | 10 | 4 | 6 | 0 | < 0.0001** |
| T 2 | 10 | 0 | 10 | 0 | |
| T 3 | 12 | 0 | 2 | 10 | |
| Muscle Invasion | |||||
| Positive | 22 | 0 | 12 | 10 | 0.001* |
| Negative | 10 | 4 | 6 | 0 |
Chi-square test was used. *, significant; **, highly significant.
Discussion
Endometrial carcinoma (EC) is the most common gynecological malignant tumor in western world [1]. In more than 80% of cases, EC is preceded by endometrial hyperplasia (EH), which is defined as an irregular proliferation of the endometrial glands [7]. According to WHO classification of EH in 2014; EH was sub classified according to the cytological atypical features into EH without atypia and atypical EH. The 20-years risk of progression of EH without atypia to EC is less than 5%. On the contrary, atypical EH may progress to EC in 29% of cases [19].
The discrimination between different types of EHs, based only on the histological evaluation, seems to be of low accuracy. Endometrial samples frequently show ambiguous features, even within the same sample. Moreover, small sized samples and technical artifacts may result in over or under diagnosis of the endometrial hyperplastic lesions. So, depending only on the histological evaluation of the endometrial hyperplastic sample seems to be unable to accurately determine the malignant potential of different types of endometrial hyperplasia [20].
In t he current study, we evaluated the immunohistochemical expression of PTEN in normal and abnormal proliferative endometrial samples; both proliferative and neoplastic specimens. The study included 92 women. The histopathological examination of their submitted endometrial samples were normal, hyperplastic and endometroid type of endometrial carcinoma in 6, 54 and 32 patients, respectively.
We evaluated the expression of PTEN in the submitted endometrial samples. We found that the intensity of PTEN immunostaining was down-regulated with increasing the cytological atypical features. All the included normal endometrium and EH without atypia showed a strong PTEN expression, compared to only 6.89% of the examined atypical EH. None of the examined endometrioid ECs showed strong PTEN expression (p< 0.0001).
When PTEN immunoreactivity was evaluated in the enrolled endometrial hyperplastic tissues, we found that 100% of the examined EH without atypia were strongly expressed PTEN, compared to 6.89% of EH with atypical cytological features (p< 0.0001).
These findings were keeping with Sarmadi, et al. They noted that all the examined normal proliferative and simple hyperplastic endometrial samples were positive for PTEN, while 75% and 48% of atypical EH and endometrioid ECs, respectively, were positive for PTEN. Furthermore, they noted that PTEN immunostaining in atypical EH and endometrioid ECs was heterogeneous either between different endometrial glands or within the same gland [21]. In addition, Shanmugapriya, et al, postulated that PTEN immunoreactivity is down-regulated in endometrial pathological conditions (atypical EH and endometrioid ECs). They highlighted the usefulness of PTEN immunohistochemical evaluation as a screening method in cases of EH to detect precancerous lesions and early stages of endometrial carcinogenesis [22].
The current study included 32 cases of ECs, all were of endometrioid type. On correlating PTEN immunostaining with tumor grade, stage and myometrium invasion of the enrolled ECs, we found that PTEN immunostaining was accentuated in higher tumor grades. 28.6% of grade I were negative for PTEN, while 100% of grades II and III showed mild to moderate staining intensity (p=0.006). similarly, PTEN expression was positively correlated with advanced tumor stage (p< 0.0001). The latter finding was supported when PTEN expression was compared with myometrium invasion. We detected that 40% of ECs that didn’t show myometrium invasion were negative for PTEN expression. On the other hand, 100% of the myo- invasive ECs showed positive PTEN expression.
This was against to what was observed by Ray, et al. They found that PTEN expression in ECs was 93.7% in grades I and II, compared to only 8.6% in grade III. However, it’s worth to mention that they included in their study different variants of ECs; endometrioid, serous, clear and malignant mixed mullerian type of carcinoma [23].
Gene mutations may be missense or nonsense mutations. In missense mutation, there is a substitution of a different amino acid into amino acid sequence as a result of nucleotide change. So, missense mutation results in a different amino acid sequence. However, it doesn’t introduce a stop codon and it doesn’t prevent protein production. Nonsense mutation is a point mutation which introduce a premature stop codon, which in turn, results in shorter and unfinished protein product [24].
PTEN alterations were detected in 45% of ECs. In the vast majority of PTEN-altered ECs, gene mutations occur. Among PTEN gene mutations; missense substitution are reported in 43.84%, followed by frame shift deletion and nonsense substitutions in 28.47% and 22.95%, respectively [24]. We supposed that during EC progression and acquiring higher grades and advanced stages, a selection of specific tumor cells with a specific type of PTEN mutation may occur, and this may cause differences in PTEN expression in different tumor grades. However, this needs to be confirmed in future studies.
In conclusion, PTEN is strongly expressed in normal endometrium and EH without atypia, while it is down-regulated in atypical EH and ECs. So, PTEN immunostaining can be used as a screening tool in hyperplastic endometrial tissues to detect the potentially precancerous cases.
Acknowledgements
Competing interests
The authors declare that they have no competing interests.
Funding
No financial support was received for this study.
References
- Molecular carcinogenesis of endometrial cancer Liu Fu-Shing. Taiwanese Journal of Obstetrics & Gynecology.2007;46(1). CrossRef
- Endometrial adenocarcinoma associated with intrauterine pregnancy. A report of five cases and a review of the literature Schammel D. P., Mittal K. R., Kaplan K., Deligdisch L., Tavassoli F. A.. International Journal of Gynecological Pathology: Official Journal of the International Society of Gynecological Pathologists.1998;17(4). CrossRef
- Altered PTEN expression as a diagnostic marker for the earliest endometrial precancerous changes Sharda Bela, Malik Reeni, Jain Pramila. International Journal of Medical Research and Review.2017;5(5). CrossRef
- Factors associated with Type I and Type II endometrial cancer Felix Ashley S., Weissfeld Joel L., Stone Roslyn A., Bowser Robert, Chivukula Mamatha, Edwards Robert P., Linkov Faina. Cancer causes & control: CCC.2010;21(11). CrossRef
- Type II endometrial cancers: A case series Lobo Flora D., Thomas Eliz. Journal of Mid-Life Health.2016;7(2). CrossRef
- Prognostic significance of POLE proofreading mutations in endometrial cancer Church David N., Stelloo Ellen, Nout Remi A., Valtcheva Nadejda, Depreeuw Jeroen, Haar Natalja, Noske Aurelia, Amant Frederic, Tomlinson Ian P. M., Wild Peter J., Lambrechts Diether, Jürgenliemk-Schulz Ina M., Jobsen Jan J., Smit Vincent T. H. B. M., Creutzberg Carien L., Bosse Tjalling. Journal of the National Cancer Institute.2015;107(1). CrossRef
- Expression of miRNAs and PTEN in endometrial specimens ranging from histologically normal to hyperplasia and endometrial adenocarcinoma Lee Heejeong, Choi Hyun Joo, Kang Chang Suk, Lee Hee Jin, Lee Weon Sun, Park Chul Soo. Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc.2012;25(11). CrossRef
- WHO Classification of Tumors of the Female Reproductive Organs. 4th, editor. Lyon: IARC; 2014 Kurman RJ, Carcangiu ML, Herrington CS, Young RH. .
- Expression of p53 and PTEN in human primary endometrial carcinomas: Clinicopathological and immunohistochemical analysis and study of their concomitant expression Stavropoulos Aggelis, Varras Michail, Vasilakaki Thivi, Varra Viktoria-Konstantina, Tsavari Aikaterini, Varra Fani-Niki, Nonni Aphrodite, Kavantzas Nikolaos, Lazaris Andreas C.. Oncology Letters.2019;17(5). CrossRef
- Assessment of the quality and frequency of mutations occurrence in PTEN gene in endometrial carcinomas and hyperplasias Konopka Bozena, Paszko Zygmunt, Janiec-Jankowska Aneta, Goluda Marian. Cancer Letters.2002;178(1). CrossRef
- Concomitant depletion of PTEN and p27 and overexpression of cyclin D1 may predict a worse prognosis for patients with post-operative stage II and III colorectal cancer Li Jing, Yin Lin-Lin, Su Ke-Li, Zhang Gang-Feng, Wang Jing. Oncology Letters.2014;8(4). CrossRef
- PTEN protects p53 from Mdm2 and sensitizes cancer cells to chemotherapy Mayo Lindsey D., Dixon Jack E., Durden Donald L., Tonks Nickolas K., Donner David B.. The Journal of Biological Chemistry.2002;277(7). CrossRef
- PTEN gene mutations correlate to poor prognosis in glioma patients: a meta-analysis Han Feng, Hu Rong, Yang Hua, Liu Jian, Sui Jianmei, Xiang Xin, Wang Fan, Chu Liangzhao, Song Shibin. OncoTargets and Therapy.2016;9. CrossRef
- PTEN functions as a melanoma tumor suppressor by promoting host immune response Dong Y., Richards J.-Ae, Gupta R., Aung P. P., Emley A., Kluger Y., Dogra S. K., Mahalingam M., Wajapeyee N.. Oncogene.2014;33(38). CrossRef
- Clinical implications of PTEN loss in prostate cancer Jamaspishvili Tamara, Berman David M., Ross Ashley E., Scher Howard I., De Marzo Angelo M., Squire Jeremy A., Lotan Tamara L.. Nature Reviews. Urology.2018;15(4). CrossRef
- Tumor Suppressor PTEN in Breast Cancer: Heterozygosity, Mutations and Protein Expression Kechagioglou P, Papi RM, Provatopoulou X, Kalogera E, Papadimitriou E, Grigoropoulos P, Nonni A, et al . Anticancer Resesarch.2014;34(3):1387-1400.
- PTEN expression is a prognostic marker for patients with non-small cell lung cancer: a systematic review and meta-analysis of the literature Xiao Jian, Hu Cheng-Ping, He Bi-Xiu, Chen Xi, Lu Xiao-Xiao, Xie Ming-Xuan, Li Wei, He Shu-Ya, You Shao-Jin, Chen Qiong. Oncotarget.2016;7(36). CrossRef
- Immunoreactivity score using an anti-sst2A receptor monoclonal antibody strongly predicts the biochemical response to adjuvant treatment with somatostatin analogs in acromegaly Gatto Federico, Feelders Richard A., Pas Rob, Kros Johan M., Waaijers Marlijn, Sprij-Mooij Diana, Neggers Sebastian J. C. M. M., Lelij Aart-Jan, Minuto Francesco, Lamberts Steven W. J., Herder Wouter W., Ferone Diego, Hofland Leo J.. The Journal of Clinical Endocrinology and Metabolism.2013;98(1). CrossRef
- PTEN as a predictive marker of response to conservative treatment in endometrial hyperplasia and early endometrial cancer. A systematic review and meta-analysis Travaglino Antonio, Raffone Antonio, Saccone Gabriele, Insabato Luigi, Mollo Antonio, De Placido Giuseppe, Zullo Fulvio. European Journal of Obstetrics, Gynecology, and Reproductive Biology.2018;231. CrossRef
- PTEN immunohistochemistry in endometrial hyperplasia: which are the optimal criteria for the diagnosis of precancer? Travaglino Antonio, Raffone Antonio, Saccone Gabriele, Mascolo Massimo, Pignatiello Sara, Mollo Antonio, De Placido Giuseppe, Insabato Luigi, Zullo Fulvio. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica.2019;127(4). CrossRef
- Altered PTEN expression; a diagnostic marker for differentiating normal, hyperplastic and neoplastic endometrium Sarmadi Soheila, Izadi-Mood Narges, Sotoudeh Kambiz, Tavangar Seyed Mohammad. Diagnostic Pathology.2009;4. CrossRef
- A study of PTEN expression in endometrial hyperplasia and endometrioid type of endometrial carcinoma Shanmugapriya Dr M., Sudha Dr M., Prakash Dr Geetha. Tropical Journal of Pathology and Microbiology.2017;3(1). CrossRef
- Study of prognostic and diagnostic significance of P53 and PTEN mutation in proliferative lesions of endometrium. Ray Suchandra, Jha Ashish, Islam Ayesha Afreen, Sengupta Moumita. Journal of Current Medical Research and Opinion.2020;3(08). CrossRef
- PTEN and Gynecological Cancers Nero Camilla, Ciccarone Francesca, Pietragalla Antonella, Scambia Giovanni. Cancers.2019;11(10). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2022
Author Details