Moringa oleifera Lamarck (1785), Moringaceae and Cancer I: A Systematic and Comprehensive Review of 24 Years of Research
Download
Abstract
This review was aimed to assess the anti-cancer properties of Moringa oleifera Lamarck. (Moringaceae: MO) reported from 1998 till 10 November 2021. A total 71 PubMed relevant papers were discussed here. Among all parts of MO which used to assess antitumor activities, the leaves (52%), seeds (22%), pods (7%), and phytocompounds (7%; like moringin and its congeners) would be considered as a source of putative phyto-onco-lytics or phyto-onco-statics. The partitioning of secondary metabolites with pharmacological value in source (leaf) and sink (roots, flowers, pods, callus, and fruits) organs of MO dictates the best choice of the solvents for their extraction. The polar: water (29%) > ethanol (17%) > methanol (13%) > hydro-alcohol (11%); intermediate polar: dichloromethane (4%); and nonpolar: n-hexane = ethyl acetate (7%) > chloroform (3% of studies) solvents have been employed for extractions among studies. The human colorectal cancer, leukemia, non-small cell adenocarcinoma, and breast cancer consisted 22, 14,10, and 8% of screened studies, respectively and the rest of tumors consisted less than 5% of studies. The in vitro (51%), in vivo chemically induced model (21%), and tumor graft models (8%) were reported and there were no clinical trials among studies. Totally, from 118 cell lines used, healthy cell lines (control; n = 19), MCF-7 (n = 12), HepG2 (n = 12), and Hela (n = 6) consisted top list amongst studies. From 76 anti-cancer portals curated amongst studies, induction of apoptosis (n = 29), anti-proliferation (n = 17), anti-angiogenesis (n = 8), and DNA/RNA fragmentation (n = 6) were the main antitumor portals and the cytotoxicity and anti-inflammation may be considered as minor ones. To sum up, the rational extraction and purification of MO, phytochemistry, and computational and experimental pharmacology of various extracts of MO should be pursued to decipher phyto-onco-lytic and/or phyto-onco-static drug-like phytocompounds suitable to be employed in clinical trials.
Introduction
Economics of cancer and botanicals
Cancer stands amongst the top-ranking mortality causes across the world, although spectacular onco-diagnostic and onco-therapeutic advances have been occurred in previous decades. At the present time, the imprisonment of humankind in a heavily polluted environment replete with tumorigenic agents has posed a radical challenge to the human health recognized as “cancer boom”. This critical issue has especially placed a tremendous strain upon those countries confronted by inadequate medical infrastructure and limited access to modern onco-therapeutic arsenals such as chemo- and radio-therapies.
Numerous institutes and academia have put forth a great deal of effort towards developing effective oncotherapeutics, but, by contrast, stiff resistance has been gained by different tumors in response to the available chemo-therapeutics and radio-therapeutics.
There exists a long list consisting of a vast spectrum of modern botanical-based anti-cancer drugs which are also referred to as botanical onco-therapeutics [1]. It is considerably needed to labor this noteworthy point that a huge number of anti-cancer phytomedicine or formulations never reach to clinical trial setting due to many challenges and barriers such as the lack of systematic integration in preclinical studies and financial support [2]. Along with all is known and reported, miscellaneous undiscovered and must-be-explored aspects exist as to the potential biological targets which could be targeted and help us battle against cancer.
All considered, an array of pharmacodynamics has been summarized in Figure 1 proposed for anti-cancer botanicals in seminal reviews [3, 4].
Figure 1. The Major Anti-cancer Portals of Botanicals. ┴, Inhibition; ↑, Activation; CYPs, Cytochrome P Enzymes; GST, Glutathione-S-Transferase; NQO1, Quinone Oxidoreductase 1; NRF2, Nuclear Transcription Factor Erythroid 2p45 (NF-E2)-related Factor 2 Signaling Pathway; ARE, Antioxidant Responsive Elements; COX, Cyclooxygenase; NO, Nitrogen Oxidase; MAP, Microtubule-associated Protein; NF-κB, Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B cells; ROS, Reactive Oxygen Species.
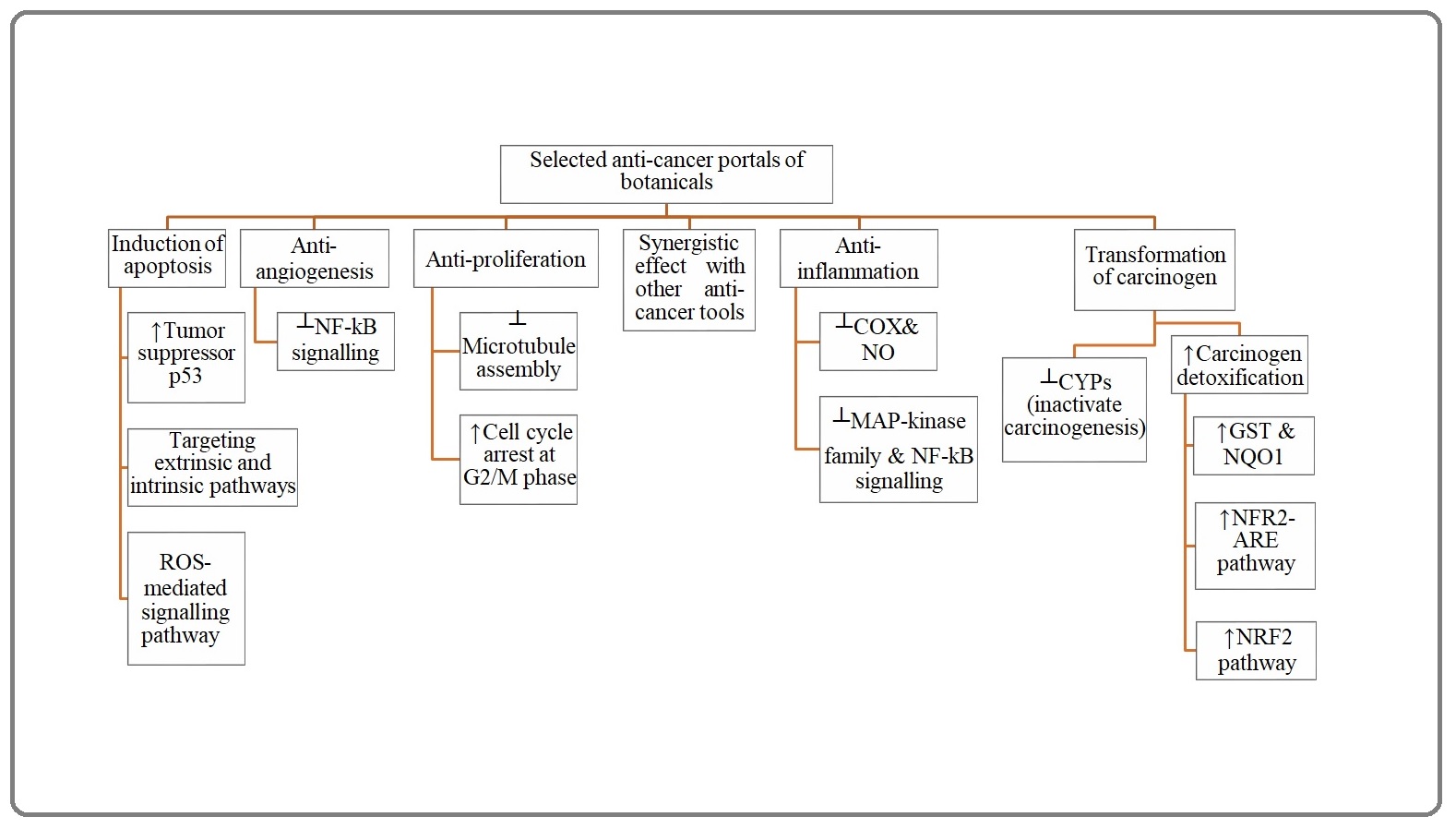
Nowadays, developing countries have risen to the challenge of low economic income as well as unstable market of anti-cancer drugs which have been posed by complex problems including undeveloped infrastructures for the development of antitumor drugs, restricted access to the knowledge should be replaced by technology urgently required to develop pharmaceutical products by means of advanced bioreactors, resilient political and economic import systems, and tight restrictions and hard sanctions imposed upon the import of required products. To sum up, tremendous efforts have been focused on seeking antitumor botanicals to come up with a solution in the face of onco-therapeutic challenges (Figure 1).
Moringa oleifera (Lamarck, 1785): An ultrashort overview
Moringa oleifera (Lamarck, 1785; MO; Figure 2) ) is a perennial deciduous tropical tree and medicinal plant which is taxonomically classified in the order Brassicales, family Moringaceae and genus Moringa native to West Africa and the Indian subcontinent.
Figure 2. Moringa oleifera Lam. Moringaceae cultivated in Laybid, Shahin Shahr, Isfahan, IRAN.

This immensely valuable tree is widely planted in India due to its profound significance in pharmaceutical industry. Likewise, this plant has been imported and planted in many countries such as Iran in which it is locally referred to as oil tamarix or castle tamarix. Globally, MO is known under various aliases Viz, Miracle Tree, Horse Radish Tree and Drumstick Tree in English, Ben Oil Tree, Benzoil Tree, the Murungai or Kelor in Malaysian, Zogale and Gergedi in Nigerian, Shigon in the Shushruta Sanhita of India, Sajiwan, Sajna, Soanjna and Munga across India. The plant is also known Shobhanjana (Sanskrit), Saragvo (Guja-rati), Sajna or Sajina (Bengali), Munanga (Telugu) and Murangai (Tamil) in various Indian linguistic variants.
Adopting a pharmacognostic perspective through looking askance at medicinal properties found in almost all parts of MO (for a review see [5]), it could serve a therapeutic role against various pathological circumstances including asthma, allergy, pain, fever, ulcers, neurological disorders, cardiovascular diseases, diabetes mellitus, hepatocellular disorders, inflammatory conditions, infertility, infectious diseases, and more specifically neoplastic conditions (for a review see [6-8]).
This plant contains a diverse array of functional bioactive components including vitamin A, vitamin C, proteins, alkaloids, quinines, saponins, flavonoids, tannins, steroids, glycosides, fixed oils and fats, and phytochemicals like niazinin A, niazinin B, niazimicin A, and niaziminin B (for a review see [6, 9, 10]). Adopting an approach in the light of orthodox medicine, this systematic review was designed to highlight anti-cancer properties of MO.
Methods
Search strategy
We extensively launched a search in PubMed database until November, 11, 2021 to find papers published with search string: “(Moringa) AND (cancer)” on the basis of PRISMA guidelines [11] as illustrated in Figure 3.
Figure 3. Flow ِDiagram of the Literature Search on the Antitumor Effect of Moringa oleifera Lam. in Preclinical Studies.
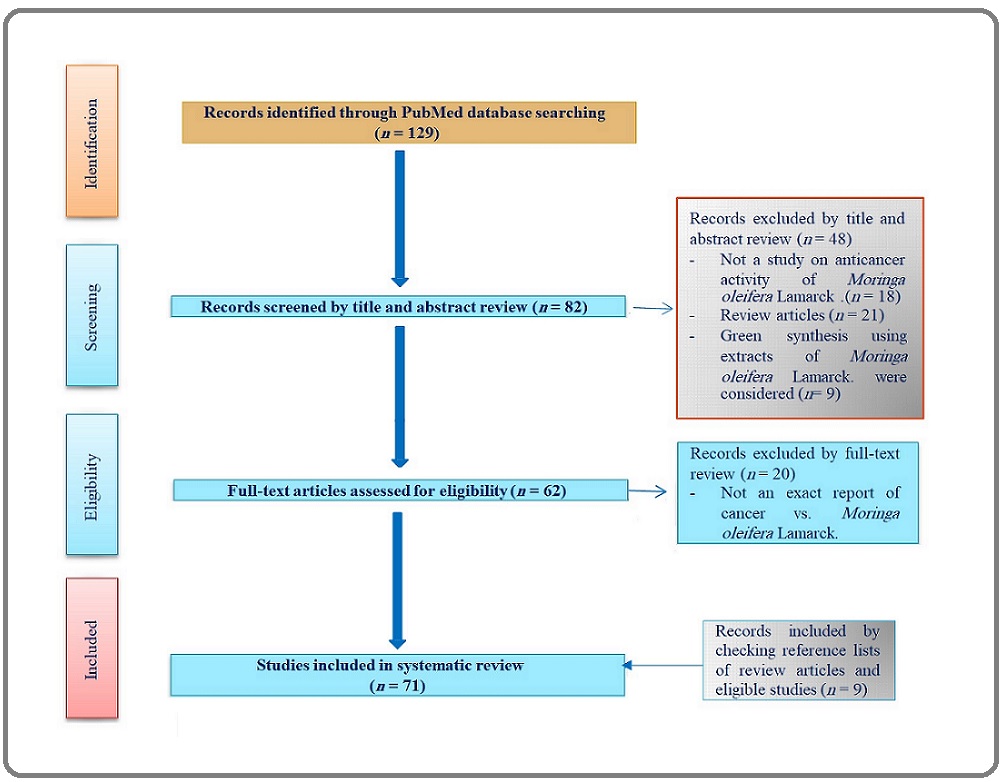
Studies were carefully screened and eligible ones were selected based upon the following inclusion criteria: (1) studies published in English; (2) studies aimed at reporting the anti-cancer effect (s) of MO; and (3) studies carried out at the level of human subjects or animal model. We excluded papers focused on the green synthesis of phyto-nanoparticles using MO and review articles; however, their references were scrutinized to seek relevant works.
Eligibility criteria
All included papers were subject to data extraction respecting the following information: (1) the part of MO used, (2) the employed formulation, (3) the animal or tumor model used and (4) the mechanism or targeted pathway.
Results
General view
I found 129 articles in accordance with the initial search script (vide supra). To begin with, based upon the close examination of titles and abstracts, 21 articles were excluded due to being review articles, not including MO, or lacking the evaluation of anti-cancer effects of MO.
In addition, after full text reading of the articles, 20 ineligible ones were excluded due to being focused on the green synthesis of nano-compounds using various types of MO extracts and-or not being exactly relevant to anti-cancer effects of MO. Finally, 71 eligible articles included for this first systematic review on MO and cancer plus 9 manually-curated records included by checking the reference lists of review articles and eligible studies were selected (Figure 3).
All selected studies are summarized in Tables 1.
| Reference | Formulation | Cell line/Animal model | Pathway | Outcome |
| Murakami et al., 1998 [26] | Leaf (niaziminin derived from methanol extract) | The activation of tumor-promoter- induced Epstein-Barr virus (EBV) in Raji cells as an EBV-containing Burkitt's lymphoma cell line | The inhibition of EBV activation | Promoting antitumor effect |
| Guevara et al., 1999 [12] | Seed (ethanolic extract) | Two-stage carcinogenesis in mouse skin initiated by 7,12-dimethylbenz (a) anthracene (DMBA) and promoted by 12-O-tetradecanoyl- phorbol-13-acetate | The inhibition of EBV-early antigen activation | Promoting antitumor effect and chemo-prevention of carcinogenesis |
| Bharali et al., 2003 [13] | Whole plant (ethanol extract) | DMBA-induced papillomagenesis in mouse skin | Increased activity of glutathione-S- transferase (GST), cytochrome p450 (CYP P450), cytochrome b5 (CYP b5), catalase, glutathione reductase (GR), acid soluble sulfhydryl content (-SH), glutathione peroxidase (GPx), and significantly reduced activity of hepatic malondialdehyde | Chemo-prevention against chemical carcinogenesis |
| Khalafalla et al., 2010 [20] | Leaf (cold water, hot water, and 80% (v/v) ethanol extracts) | Acute myeloblastic leukemia (AML), acute lymphoblastic leukemia (ALL) cells, and HpG2 human hepatocellular carcinoma (HCC) cells | Not access to data | Antitumor activity |
| Promkum et al., 2010 [27] | Pod (boiled; bMO) | Mitomycin C- and DMBA-induced clastogenicity in mouse | Altered number of micronucleated peripheral reticulocytes | Anti-clastogenic potential |
| Brunelli et al., 2010 [28] | Seed (glucomoringin-derived isothiocyanate) | Xenograft tumor model of RPMI8226 human myeloma cancer cells in severe combined immunodeficiency disease mice | Induced apoptosis through a caspase-dependent pathway, NF- κB inhibition | The reduced growth of myeloma tumor and antitumor activity |
| Budda et al., 2011 [21] | Pod (boiled; bMO) | Azoxymethane-initiated and dextran sodium sulfate-promoted colon carcinogenesis in mice | Significantly decreased PCNA index and expression of iNOS and COX-2 proteins | Chemo-prevention, anti-inflammation |
| Paliwal et al., 2011 [29] | Pod (hydro-ethanolic extract) | DMBA-induced renal carcinogenesis in Swiss albino mice | Normalization of renal oxidative stress markers including lipid peroxidation (LPO), superoxide dismutase (SOD) and catalase (CAT) | Anti-nephrotoxic effect, anti-oxidant |
| Sreelatha et al., 2011 [30] | Leaf (boiled/cooled aqueous extract) | Human KERATIN-forming tumor (KB) cell line | DNA Fragmentation, modulation of redox-sensitive mechanism, ROS production | Anti-proliferation, potent induction of apoptosis |
| Pamok et al., 2012 [22] | Leaf (hydro-ethanolic extract) | HCT15, SW48 and SW480 colon cancer cell lines | Not free access to fulltext | Anti-proliferative |
| Sharma et al., 2012 [23] | Pod (hydro-ethanolic extract) | DMBA-induced hepatic carcinogenesis in mice | Modulation cytochrome p450 (CYP P450) and cytochrome b5 (CYP b5), decreased level of glutathione (GSH), and glutathione-S-transferase (GST) | The induction of apoptosis, chemo-prevention |
| Waiyaput et al., 2012 [24] | Leaf (hydro-alcoholic extract) | HepG2 human liver cancer cells, COS-7 cells | Inhibited and significantly decreased hepatitis b virus (HB V) cccDNA in transiently transfected HepG2 cells | Cytotoxicity and anti-HBV activity |
| Gismondi et al., 2013 [14] | Leaf, root, cortex, flower, seeds (boiled aqueous extract) | B16-F10 melanoma cells | The scavenging of free radicals, the enhancement of p53, p21 WAF1/,Cip1 and p27 Kip1 protein levels | Anti-oxidant, anti-proliferative and differentiative effects |
| Berkovich et al., 2013 [25] | Leaf (boiled aqueous extract) | Panc-1 and COLO-357 human pancreatic cancer cell lines | The inhibition of growth and NF-κB signaling pathway, synergistic effect on chemotherapeutic efficacy of cisplatin | Cytotoxicity, chemotherapeutic effect on chemo-resistant tumors |
| Tiloke et al., 2013 [31] | Leaf (aqueous extract) | A549 lung cancer cells | Significantly increased expression of p53, increased activity of caspase-9, and caspase-3/7; enhanced expression of Smac/DIABLO, and induced cleavage and activation of PARP-1, reduced mRNA and protein expression of Nrf2, and reduced glutathione (GSH) | Anti-proliferation |
| Araújo et al., 2013 [32] | Seed (aqueous extract; lectins including cMoL and WSMoL) | NCI-H292, HT-29, HEp-2 / Male Balb/c mouse model of carrageenan-induced pleurisy | Exhibition of anti-inflammatory activity by means of reducing the production of nitric oxide, TNF-α, and IL-1β along with decreasing myeloperoxidase activity | Cytotoxicity, immuno-suppression, and anti-inflammatory activity |
| Ghosh, 2014 [33] | Leaf (methanol extract) | MCF 7 epithelial breast cancer cell line and MDA-MB-231 mesenchymal breast cancer cell line | Decreased adhesive capacity of MCF-7 and MDA-MB-231 cells | Anti-proliferation, cytotoxicity |
| Charoensin, 2014 [34] | Leaf (methanol and dichloromethane extracts) | HepG2 human hepatocellular carcinoma cells, Caco-2 human colorectal adenocarcinoma cells, MCF-7 human breast adenocarcinoma cells, and human dermal fibroblast | Quinone reductase induction assay, cytotoxicity | Antioxidative, a nti-proliferation, and chemo-prevention |
| Jafarain et al., 2014 [35] | Callus and Leaf (ethanol-water (70-30) extracts) | Hela human cervix carcinoma cell line | Decreased viability of Hela cells induced by the antioxidative activity | Induced cytotoxicity |
| Akanni et al., 2014 [36] | Leaf (ethanol extract) | Benzene-induced leukemia bearing rats | Potent antioxidative activity mediated through decreased malondialdehyde and enhanced glutathione (GSH) | Antioxidative, chemopreventive and anti-leukemic effect |
| Jung et al., 2014 [15] | Leaf (soluble aqueous extract) | MCF-7 breast adenocarcinoma cell line, A431 human epidermoid carcinoma cell line, HT1080 human fibrosarcoma cell line, and COS-7 monkey fibroblast/ fibroblast-like cells | Upregulation of heat shock proteins including HSPA8, HSPA1B, HSP90AB1, and HSPD1; downregulation of Akt, p-IkB, NF-kB, p-Erk, β-catenin, and cyclin D1 | Anti-proliferation, cytotoxicity |
| Leelawat and Leelawat, 2014 [37] | Leaf and seed extracts | RMCCA1 and KKU100 human hilar cholangiocarcinoma cell lines | Decreased mitochondrial membrane potential and increased pro- duction of ROS | Induced cytotoxicity |
| Elsayed et al., 2015 [38] | Seed (essential oil extracted by cold pressing) | HeLa human cervix carcinoma cells, HepG2 human hepatocellular carcinoma cells, MCF-7 human breast adenocarcinoma cells, CACO-2 human colorectal adenocarcinoma cells and L929 mouse fibroblast cell line | Cytotoxicity | Potent cytotoxic activities |
| Al-Asmari [39] et al., 2015 | Leaf, bark, and seeds (soluble ethanol extracts) | MDA-MB-231 human epithelial breast cancer cell line and HCT-8 human ileocaecal adenocarcinoma cell line | Induced cell cycle arrest at G2/M phase | Anti-cancer properties and induction of apoptosis (leaf and bark extracts) |
| Diab et al., 2015 [40] | Leaf (50% ethanol extract) | A549 lung adenocarcinoma cell line, PC-3 prostate adenocarcinoma cell line, T47D and MCF-7 breast cancer cell lines, HCT-16 and Colo-205 colon cancer cell lines, and THP-1, HL-60 and K562 leukemic cell lines | Cell cycle arrest at G0/G1 phase, increased DNA fragmentation | Anti-proliferative activity |
| Jung et al., 2015 [41] | Leaf (cold soluble aqueous extract) | A549 human non-small cell lung cancer, HepG2 human hepatocellular carcinoma cells, and male immune-deficient nude mice | DNA strand breaks | Inhibitory effect on tumor progression and clonogenicity of cancer cells, apoptosis induction, and anti-proliferation |
| Krishnamurthy et al., 2015 [42] | Leaf (n-hexane, chloroform, ethyl acetate, methanol extracts and 15 fractions (F1 to F15) of ethyl acetate extract) | Hep-2 hepatocellular carcinoma cell line and Dalton's lymphoma ascites model in mice | Potential cytotoxic effect on Hep-2 cell lines, downregulation of ERK1/2 phosphorylation | Antitumor activity, cytotoxicity |
| Fernandes et al., 2016 [43] | Leaf and flower (aqueous extracts) | MCF-7 human breast adenocarcinoma cells | Angiogenesis via chorioallantoic membrane assay, cytotoxicity | Anti-proliferation, cytotoxicity, anti-inflammation; proangiogenesis in normal tissue |
| Maiyo et al., 2016 [44] | Seed and leaves (crude extract) | HepG2 hepatocellular carcinoma cell line, Caco-2 human colorectal adenocarcinoma cell line, and HEK293 non-cancer cell line | Viability, cytotoxicity | Cytotoxicity and pro- apoptotic effect upon both cancer and non-cancer cell lines, antitumor effect |
| Rajan et al., 2016 [45] | Seed (glycosylated isothiocyanate moringin) | Human astrocytoma: grade IV CCF-STTG1 cells | The activation of p53 and Bax, Bcl-2 inhibition, modulation of Nrf2 transcription factor and its upstream regulator CK2-alpha expressions along with DNA and RNA fragmentation | The induction of oxidative stress-mediated apoptosis, antitumor activity |
| Tiloke et al., 2016 [46] | Leaf (boiled aqueous soluble extract) | SNO esophageal cancer cell line | Enhanced lipid peroxidation and DNA fragmentation, increased externalization of phosphatidylserine (PS), enhanced activities of caspase-9 caspase-3/7; and increased expression of Smac/ DIABLO protein and cleavage of PARP-1 | Anti-proliferation, apoptosis induction |
| Michl et al., 2016 [47] | Seed (glycosylated isothiocyanate moringin) | HeLa cells human cervix cancer cell line, the interleukin-3 (IL-3)-dependent mouse pro-B cell line Ba/F3, the Ba/ F3tet-on-1*6 cell line | Inhibition of TNF-induced NF-κB and IFNα-induced STAT1 and STAT2 activities | Chemo-prevention |
| Förster et al., 2016 [48] | Leaf (methanolic extract (α-4-rhamnopyranosyloxy- benzyl glucosinolate (Rhamno-Benzyl-GS) and acetyl-α-4- rhamnopyranosyloxy- benzyl glucosinolate Isomer III (Ac-Isomer-GS III)) | HepG2 hepatocellular carcinoma cells and V79-MZ Chinese hamster lung cells | The activation of the Nrf2 pathway | Induction of hepatic detoxifying enzymes; antitumor activity |
| Madi et al., 2016 [49] | Leaf (soluble boiled aqueous extract) | A549 adenocarcinomic human alveolar basal epithelial cells, HepG2 human hepatocellular carcinoma cells, Caco2 human colorectal carcinoma cells, Hek293 human embryonic kidney, and Jurkat cells | Decreased mitochondrial membrane potential, and ATP levels, followed by increased ROS, caspase activation, expression of pro-apoptotic proteins including p53, SMAC/Diablo, AIF, and PARP-cleavage | Apoptosis induction, cytotoxicity, and antitumor activity |
| Cuellar-Nuñez et al., 2017 [50] | Leaf (powder) | Azoxymethane/dextran sodium sulfate-induced colorectal carcinogenesis in CD-1 (ICR) mice | Decreased activity of harmful fecal enzymes including β-glucosidase, β-glucuronidase, tryptophanase,and urease | Chemo-protective, diminished colonic lesions, and decreased activity of fecal harmful enzymes |
| Sadek et al., 2017 [51] | Leaf (ethanol extract) | Diethyl nitrosamine-induced HCC in rat | Decreased expression of Bcl-2,Bcl-xl and b-arrestin-2, upregulated expression of Bax and caspase-3 | Chemo-prophylactic efficacy (through implementation of antioxidant activity and actuation of apoptosis) |
| Tragupakseerojn et al., 2017 [52] | Leaf (ethanol extract) | HCT116 human colon cancer cell line | Downregulation of ERK1/2 phosphorylation | Anti-proliferation |
| Leelawat and Leelawat, 2017 [53] | Fruit pulp and seed (ethanol extract) | RMCCA1 human cholangiocarcinoma (CCA) cell line | The activation of protein kinase B (Akt), increased expression level of mitogen-activated protein kinase (MAPK) pathways (including ERK1/2 and p38 MAPK), increased activity of pro-apoptotic molecules including caspase-3, poly (ADP-ribose) polymerase, checkpoint kinase 2 and tumor protein 53 | Anti-proliferation |
| Adebayo et al., 2017 [54] | Seed (crude water extract, n-hexane and dichloromethane fractions) | MCF7 human breast cancer cell line, MCF, 10A normal human mammary epithelial cells | Not reported | Anti-proliferation |
| de Andrade Luz et al., 2017 [16] | Seed (coagulant lectins) | B16-F10 murine melanoma cells | Mitochondrial ROS production, activation of caspases 3, 8 and 9 | Cytotoxicity and induced apoptosis |
| Giacoppo et al., 2017 [55] | 4- (α-L rhamnopyranosyloxy) benzyl ITC (moringin) complexed with alpha-cyclodextrin from MO seeds | SH-SY5Y human neuroblastoma cells | Inhibition of PI3K/Akt/mTOR pathway, (NF-κB) p65 activation and phosphorylation; downregulated MAPK pathway; apoptotic death triggered by MAC pathway (based on expression level of cleaved-caspase 3, Bax/Bcl-2 balance, p53 and p21) | Anti-proliferative efficacy, apoptosis induction |
| Antonini et al., 2018 [56] | Seeds (moringin (MOR), a glycosyl-isothiocyanate obtained by myrosinase-catalyzed hydrolysis of the precursor 4-(α-l-rhamnosyloxy)- benzyl glucosinolate or glucomoringin) | Hep3B cancer cells | AVN 2f-mediated caspase-8 activation (Extrinsic apoptosis), MOR-mediated increased level of intracellular ROS, caspases 2 and 9 activation, and downregulation of pro-survival gene BIRC5 | Anti-proliferative and pro-apoptotic effects |
| Asaduzzaman et al., 2018 [57] | Seed (Purified lectin) | Mouse model injected by Ehrlich ascites carcinoma (EAC) cells | Inhibited cell cycle at G2/M phase and caspase-3, suppressed expression of Bcl-2 and NF-κB genes, the activation of Bak | Anti-proliferative effect, apoptosis induction |
| Jaafaru et al., 2018 [58] | Pod (GMG-ITC rich soluble extract) | Sprague-Dawley (SD) rats, murine fibroblast (3T3), and PC-3 human prostate adenocarcinoma cells | Induced apoptosis | Anti-proliferative effect |
| Hagoel et al., 2019 [59] | Leaf (aqueous extract) | PANC-1 human pancreatic epithelioid carcinoma cell line, Ectopic (subcutaneous) tumor model in immune deficient athymic CD-1 nude mice | Decreased expression of pro-apoptotic protein Bcl-2 in combination with downregulation of PARP-1 and NF-κB-related proteins IκB-α, p65-subunit, and COX-2 | Anti-proliferative activity, anti-angiogenic effect, and enhancing the radiotherapeutic efficacy against tumor radioresistance |
| Paul et al., 2019 [60] | Leaf (bis (Isothiocyanatomethyl) benzene) | HeLa human cervical cancer cells, MCF-7 human breast adenocarcinoma cells, and MDA-MB-231 cells | Caspase 3 apoptotic pathway | Antitumor activity through establishing cancer cell growth and inhibiting carcinogenesis |
| Kumar et al., 2019 [61] | Leaf (n-hexane extract) | A549 non-small-cell lung cancer cells | Cytotoxicity | Anti-oxidant and antitumor activity |
| Potestà et al., 2019 [62] | Seed and Leaf (boiled and frozen aqueous extracts) | Human Jurkat E6-1 lymphoid and THP1 monocytoid cell lines; peripheral blood mononuclear cells (PBMCs) from healthy donors | Anti-proliferative and pro-apoptotic effects | Aqueous extracts differentially regulated proliferation and apoptosis in healthy cells and cancer cells, and they can be used as adjuvant in cancer therapy |
| Tiloke et al., 2020 [63] | Leaf (crude aqueous extract) | Human liver hepatocellular carcinoma (HepG2) cells; PBMCs; HEK293 (human embryonic kidney) | Apoptosis was assessed by caspase-9, -3/7 activities and ATP levels. Cell cycle, γH2AX, and cleaved PARP-1 were determined. Protein expression of c-myc, Bax, p-Bcl2, Smac/DIABLO, Hsp70, SRp30a and cleaved PARP-1 was assessed | Cell-cycle arrest and apoptosis in cancerous HepG2 cells |
| Khan et al., 2020 [64] | Leaf (methanolic extract) | PC-3 prostate cancer cells | Inhibiting Hedgehog signaling, cell cycle arrest in G0/G1, and significant alteration in mRNA expression of apoptosis-related and Hedgehog signaling pathway genes | Inhibitory effects against cancer cells |
| Luetragoon et al., 2020 [19] | Leaf (ethyl acetate extracts and their fractions, 3-hydroxy-β-ionone) | Squamous cell carcinoma SCC15 cell line | Cell viability on SCC15. Cell cycle and apoptosis markers were evaluated by immunoblotting | Anti-Cancer Effect of 3-Hydroxy-β-Ionone; antiproliferative |
| Dilworth et al., 2020 [65] | Leaf extract | Human promyelocytic leukemia cells (HL60 cells) subjected to oxidative stress | Viability assays; Antioxidant indexes | Protection from oxidative stress within the first 24 h, as well as increasing cell viability at certain concentrations |
| Xie et al., 2020 [66] | Leaf (alkaloids) | PC3 human prostate cancer cell line | Suppressing COX-2-mediated Wnt/β-catenin signaling pathway | Anti-migration, anti-proliferation |
| Aldakheel et al., 2020 [67] | Seed (ethanol extract) | Human colorectal carcinoma cells (HCT-116) cells | DNA nuclear morphology | Inhibitory action |
| Phannasil et al., 2020 [68] | Pod (dietary boiled extract) | Azoxymethane and dextran sodium sulfate-induced mouse colon carcinogenesis | Histological assay and colon proteomic assay; network analysis | Both preventive and therapeutic antitumor effects |
| Patriota et al., 2020 [69] | Flower (Moringa oleifera (drumstick tree) flower trypsin inhibitor (MoFTI) derived from protein extract) | Sarcoma 180-bearing mice | Tumor weight and vasogenesis | Reduced tumor weight and a reduced number of secondary vessels and lower gauge of the primary vessels |
| Wisitpongpun et al., 2020 [70] | Leaf (n-hexane, ethyl acetate and 95% ethanol extract and their fractionation) | MDA-MB-231 Cell Line | Apoptosis and cell cycle analysis | A fraction derived crude ethyl acetate extract reduced cell viability andclonogenic growth and increased cells apoptosis. |
| Do et al., 2020 [17] | Leaf (aqueous extract) | A375 cells and A2058 melanoma cells | Apoptosis which was expressed via chromatin condensation and PS externalization; decreased mitochondrial membrane potential; increased Bax/Bcl-2 ratio, activated Caspase-3/7, Caspase-9, PARP and AIF translocation, leading to apoptotic cell death | Anti-proliferation, apoptosis induction |
| Lim et al., 2020 [71] | Leaf and seed (the mixture of MO leaves and seed residue (MOLSr) extract) | MCF-7 breast cancer cells, PMECs primary mammary epithelial cells, non-malignant Chang's liver cells, HepG2 human hepatocellular carcinoma, HeLa cervical adenocarcinoma cells, and MCF-7 human breast adenocarcinoma cells | Significantly reduced expression of calcineurin (CaN) and vascular endothelial cell growth factor (VEGF) proteins as well as secreted frizzled-related protein 1 (SFRP1) and solute carrier family 39 member 6 (SLC39A6) genes. | Chemopreventive activity |
| Pandey and Khan, 2020 [72] | Leaf (methanolic extract) | HeLa Human cervical cancer cells | Down-regulated Jab1 expression, significantly increased p27 expression, enhanced ROS production and caspase-3 activation | Cell cycle arrest, targeting signalosome (Jab1) in cancer cells |
| Barhoi., et al 2021 [73] | The aqueous extract of MO (AEMO) | EAC (Ehrlich acites carcinoma) solid tumor-bearing mice and HEp-2 (human laryngeal carcinoma cells) | Apoptosis induction through changing mitochondrial membrane potential | The inhibition of tumor progression |
| Fuel et al., 2021 [74] | Seeds (ethanolic extract) | Colorectal cancer (CRC) cell lines T84, HCT-15, SW480 and HT-29, as well as with cancer stem cells (CSCs) | Overexpression of caspases 9, 8 and 3 and increased production of reactive oxygen species | Extracts presented the lowest IC50 in T-84 and HCT-15 (resistant) cells, highest level of inhibition of proliferation in multicellular tumor spheroids of HCT-15 cells. Synergistic activity with 5-Fu (5-Fluorouracil). cell death by autophagy |
| Siddiqui et al., 2021 [75] | Fruit (ethanolic extract) | HepG2 human hepatocellular carcinoma cells | ROS-mediated apoptosis induction and activation of caspase-3 | Anti-proliferative, apoptosis induction |
| Zunica et al., 2021 [76] | Seed (ethanol extract dried concentrate) | Immunocompromised female mice with diet-induced obesity bearing MDA-MB-231-derived xenograft tumors | Cytotoxicity assay | Anti-angiogenesis |
| Cuellar-Núñez et al., 2021 [77] | Leaf (lyophilized powder supplement) | Azoxymethane-induced colitis-associated colon carcinoma model in CD-1 mice | Down-regulated expression of pro-inflammatory mediators including IL-2, IL-6, TNF, IL-1ß, and INF-γ; as well as reduced pro-inflammatory cytokines (MCP-1, IL-6, TNF-α) in serum | Chemo-preventive, anti-inflammatory |
| Do et al., 2021 [18] | Leaf (methanolic extract, n-hexane, chloroform, ethyl acetate, and water-soluble | A375 human melanoma cells, A2058 human melanoma cells, and WS1 normal human skin fibroblasts | WST-1 assay, The caspase-dependent and caspase-independent pathways involved in apoptosis | Anti-proliferation, apoptosis induction |
| Xie et al., 2021 [78] | Leaf (extraction of alkaloids from leaf powder via using various ethanol and ethyl acetate solvents) | A549, HCT116, A375, MDA-MB-231, Hep-G2, NCI-H1975, NCI-H1781, NCI-H441, and GES-1 cells A549 lung cancer cells, GES-1 cells (human gastric mucosal epithelial cells). | The proliferation, apoptosis, cell cycle, and migration of cells; expression of caspase-3 and caspase-9, cyclin D1 and cyclin E | Strongest growth inhibitory effect against A549 cells but had low toxicity to GES-1 cells; induced cell cycle arrest; anti-migration, anti-proliferation, and apoptosis induction |
| Mohd Fisall et al., 2021 [79] | Leaf (methanolic extract) | MCF-7 Human breast adenocarcinoma cells | Increased expression of pro-apoptotic proteins including Bax, caspase 8 and p53; cytotoxicity assay | Anti-proliferation, apoptosis induction |
| Caicedo-Lopez et al., 2021 [80] | Leaf (fermented non-digestible | HT-29 colorectal cancer cells | Induced apoptosis, autophagy, and necrosis | Anti-proliferation |
| Lu et al., 2021 [81] | Seed (moringin isolated and purified from defatted moringa seed powders and its degraded products were prepared) | Three cancer cell lines (MCF-7, HepG2 and HCT-116) | Cytotoxicity | Moringin showed good cytotoxicity to cancer cells, however its degraded products showed very weak or no activity |
| Bhadresha et al., 2021 [82] | Leaf (digested and non-digested extracts) | Human prostate bone metastasis cancer cell line PC3 | The blockage of cell cycle in S phase; Cell proliferation assay; apoptosis assay via acridine orange and propidium iodide double staining | Cytotoxicity, cell cycle arrest, and apoptosis induction |
To conduct a comprehensive review, here, we have categorized the articles according to the following criteria (1) the type of MO-derived formulation, (2) the cell lines or animal models used to investigate anti-cancer effect of MO, (3) the targeted pathway or endpoints reported in studies. Meta-analysis could not be carried out owing to overt heterogeneity in tumor models and outcomes in the selected studies, however descriptive statistics has been pursued to discover the climate of papers have been selected (vide infra).
Dermatological cancers
Tender pod or the fruits of MO has a special place in Thai cuisine. Previous studies have demonstrated that MO pod extract can serve a bifunctional role in both displaying antioxidative properties and inhibiting skin papillomagenesis in mice as well [12]. In this respect, niazimicin has been isolated from ethanol extract of MO seeds and it exerted significant antitumor effect upon the two-stage carcinogenesis in mouse skin using 7,12-dimethylbenz (a) anthracene (DMBA) as the initiator and 12-O-tetradecanoyl-phorbol-13-acetate as the tumor promoter [12].
A hydro-alcoholic extract of whole MO brought about modulatory effects upon phase I (cytochrome b5 and cytochrome P450) and phase II (glutathione-S-transferase) drug metabolizing enzymes, anti-oxidant enzymes, glutathione content and lipid peroxidation within the liver tissues of female Swiss albino mice [13]. Additionally, it possessed chemopreventive efficacy in a two stage model of DMBA-induced skin papillomagenesis [13].
The anti-neoplastic properties of the leaves, root, cortex, and flowers of MO on murine B16F10 melanoma cells, brought about a significantly decreased growth and proliferation rate of tumor cells, cell cycle arrest, the increased expression of p21WAF1/Cip1, p53, and p27Kip1 proteins, and induction of differentiation [14]. The soluble cold distilled water extract (4°C; 300 µg/mL) from MO, greatly induced apoptosis, inhibited tumor cell growth, and lowered the level of internal reactive oxygen species (ROS) in A431 human epidermoid carcinoma [15]. In this regard, the seeds of MO contain carbohydrate- binding proteins, namely lectins, being able to exert strong cytotoxic effects against tumor cells. The cytotoxic effect of coagulant MO lectin (cMoL) upon B16-F10 murine melanoma cells has been previously reported [16].
In another study, the extract of MO leaves was fractionated using different solvents to figure out which fraction is the most effective one having anti-proliferative effect on melanoma cells [17]. In this line, they concluded that the phenolic-rich fraction exerted significant in vitro antitumor effects, mediated by mitochondrial ROS, on melanoma cells through both caspase-dependent (activation of caspase-3/7 and caspase-9) and caspase-independent (activation, and translocation of apoptosis-inducing factor into the nucleus) apoptosis pathways.
With regards to dermatological cancers, whole MO plant, its various parts, its cMoL, and even its phytochemical, namely niazimicin, happened to establish chemotherapeutic and chemopreventive impacts on murine B16F10 melanoma cells and skin papillomagenesis [12-14, 16].
Do and colleagues (2020) reported the anti-proliferation mediated by apoptosis of an aqueous extract of MO on human melanoma cell lines through a battery of tests focused on apoptosis [17]. Additionally, in a mechanistic study, Do and colleagues (2021) investigated the anti-proliferative activity activities of various extracts of MO against melanoma cells and normal cells and they decipher caspase-dependent and caspase-independent pathways which took part inter alia [18]. Finally, authors concluded that among all extracts and fractions, the phenolic-rich fraction exerted noteworthy anticancer effects on melanoma cells in vitro.
Luetragoon et al. (2021) investigated the anti-cancer effect of leaf extracts and their fractions, 3-hydroxy-β- ionone of MO on squamous cell carcinoma, SCC15 cell line. They found that MO extracts and 3-hydroxy-β-ionone significantly exhibited antiproliferative effects on SCC15. Moreover, they showed the inhibitory effects of aforementioned formulation on colony formation and cell migration of SCC15 [19].
To sum up, eight papers focused on the useful effects of various MO extract on four cancer cell lines including human melanoma, human epidermoid carcinoma, human keratin-forming tumor, and squamous cell carcinoma.
Gastrointestinal cancers
Three cold, hot, and ethanol extracts were prepared from the leaves of MO which caused death in the majority of hepatocellular carcinoma cells [20]. In this regard, hot water and ethanol extracts were more effective active against aforementioned cancer cells. The chemopreventive effect of boiled freeze-dried MO on azoxymethane (AOM)- initiated and dextran sodium sulfate (DSS)-promoted colon carcinogenesis in mice was demonstrated by suppressive effects induced in a colitis-associated colon carcinogenesis model [21]. The consumption of MO with the aim of providing an alternative/complementary therapy for cancer treatment, is on the rise in Thailand [22]. The inhibitory effect of the aqueous and ethanol extracts from MO leaves on the proliferation of colon cancer cells has been reported [22]. In line with this, two extracts were checked for their non-mutagenicity through safety test, Ames test, and antiproliferative effect on 3 types of colon cancer cell lines including HCT15, SW48 and SW480 showed both a dose-dependent and exposure time-dependent manner. To be more specific, the antiproliferative effect produced by the ethanol extract of MO appeared to be more powerful in comparison with that of the aqueous extract on all tested cell lines. In addition, SW48 was found to be the most sensitive cell line [22].
Hepatoprotective and antioxidative effects of MO against DMBA-induced hepatocellular damage has been reported in mice [23]. In this context, single oral administration of DMBA to mice resulted in significantly plummeted levels of xenobiotic enzymes such as cytochrome P450 and b5 which mediate hepatic carcinogenesis [23]. An extensive use of edible plants such as MO can be traced back to a long time ago in Thai culture so as to heal ulcers and the symptoms of hepatic disease. In this regard, buffered extracts from the fruits of MO showed to have anti-hepatitis B virus activity and also a mild cytotoxic effect on the hepatocellular carcinoma (HepG2) cells [24].
The effect of MO leaf extract alone and in combination with cisplatin on the survival of cultured human pancreatic cancer cells inhibited the growth of pancreatic cell lines including Panc-1, p34, and COLO 357 through deregulation of NF-κB signaling pathway [25]. Moreover, MO leaf extract synergistically enhanced the cytotoxic effect of cisplatin on Panc-1 cells [25].
Two coagulant lectins including cMol and water soluble lectin from MO seeds (WSMoL), extracted from the seeds of MO, have been used worldwide to refine drinking water [32]. The aqueous seed extract and cMol were potentially cytotoxic to human peripheral blood mononuclear cells, whereas WSMoL and diluted seed extract did not show any cytotoxic activity [32]. In addition, the MO aqueous seed extract and the lectins cMol and WSMoL were weakly/moderately cytotoxic to HT-29 human colorectal adenocarcinoma and HEp-2 human epithelial type 2 cancer cell lines [32]. Furthermore, the aqueous seed extract, cMol and WSMoL and diluted seed extract exhibited anti-inflammatory activity [32].
Another study has been focused on RMCCA1 and KKU100 human hilar cholangiocarcinoma cell lines treated with MO extracts which examined cell viability and apoptosis [37], showed that MO extracts inhibited cellular proliferation and induced cell apoptosis. Additionally, the leaf and seed extracts of MO incited cytotoxicity through a marked decrease in mitochondrial membrane potential and an increase in ROS production in cholangiocarcinoma cells.
The antiproliferative assay conducted on MO leaf demonstrated that dichloromethane extract have a higher antiproliferative activity compared to methanol extract on HepG2 and colorectal adenocarcinoma (Caco-2) cell lines [34]. In addition, the assessment of in vitro chemopreventive activity against cancer cells was implemented through quinone reductase (QR) induction assay as a useful strategy on hepatoma (Hepa-1c1c7) which revealed that the dichloromethane extract had the capacity to induce QR activity significantly, whereas the methanol extract appeared to have no inductive effect [34]. In a nutshell, this study provides evidence that MO leaves possess anti-oxidantive, cytotoxic and chemopreventive properties [34].
The in vitro antiproliferative potential of MO extracts against HCT-16 colorectal carcinoma cells and Colo-205 Dukes’ type D, colorectal adenocarcinoma cells; has been confirmed using SRB and MTT tetrazolium reduction assays [40]. The antitumor activity of MO leaf extracts against HepG2 cells has been reported [41]. In a labor-intensive work, n-hexane, chloroform, ethyl acetate, methanol extracts of the MO leaves and 15 fractions (F1 to F15) of ethyl acetate extract were evaluated in vitro and in vivo for their antitumor activity using Hep-2 cell lines and Dalton’s lymphoma ascites model in mice, respectively [42]. Authors stated the potential cytotoxic impact of F1 fraction on Hep-2 cell lines, a significantly reduced body weight, and increased mean survival time in the animal model of Dalton’s lymphoma ascites.
The different cell lines including HepG2, HeLa, and Caco-2 were treated with essential oil derived from MO seeds with a range of concentrations from 0.15 to 1 mg/mL for 24h; additionally, the cytotoxicity was assessed using MTT assay. Findings showed a significant reduction in cell viability in response to the ascending dose of essential oil in all treated cell lines [38].
The viability of Caco-2 and HepG2 cells were reduced by treating with aqueous leaf extract of MO [49]. In a pioneering study, the cytotoxicity caused by the major aromatic multi-glycosylated glucosinolates of MO leaf extracts, namely 4-α-rhamnopyranosyloxy- benzyl glucosinolate (Rhamno-Benzyl-GS) and acetyl- 4-α-rhamnopyranosyloxy-benzyl glucosinolate Isomer III (Ac-Isomer-GS III), to HepG2 cells and two Salmonella typhimurium strains (Ames test), was confirmed. As per the findings of this study, no genotoxicity caused by glucosinolates was reported through Ames test; nevertheless, stimulating effects produced by these glucosinolates have been corroborated both at the transcriptional and translational levels of antioxidative systems such as NRF2 (nuclear transcription factor erythroid 2p45 (NF-E2)-related factor 2) pathway [48]. In addition, the MO crude aqueous leaf extract showed antiproliferative effects on esophageal cancer cell line (SNO) through the augmentation of DNA fragmentation, lipid peroxidation, and the induction of apoptosis [46].
Quercetin-3-O-glucoside and 4-(β-D-glucopyranosyl- 1→4-α-L-rhamnopyranosyloxy)-benzyl isothiocyanate, isolated from the leaves and seeds of the MO from Kenya, showed antioxidative and cytotoxic activities against HepG2 and Caco-2 cell lines [44]. In this respect, one study reported that MO leaves which contain dietary fiber and phenolic compounds exerted a suppressive effect on an in vivo model of AOM/DSS-induced colorectal carcinogenesis [50].
The antiproliferative activity against RMCCA1 cholangiocarcinoma cells promoted by MO seeds extract has been confirmed with the cell proliferation assay [53]. The activation of protein kinase B (Akt) has been demonstrated in RMCCA1 cells pre-treated with the ethanolic extracts of MO seed [53]. Moringa oleifera leaf ethanol extract showed hepato-protective effects upon diethyl nitrosamine (DEN)-induced hepatocellular carcinoma may have been mediated by its antioxidative and apoptosis-promoting activities [51].
Reportedly, a fermented MO extract exerted an ameliorative effect upon glucose intolerance and mitigated the hepatic adiposity in the high-fat diet-induced non- alcoholic fatty liver disease, by means of decreasing endoplasmic reticulum stress, oxidative stress, and inflammation in C57BL/6 mice [83].
In another study, the in vitro antiproliferative effect and the mechanism of action by which successive fractions from the methanol extract of MO leaves exerts, have been investigated on HCT116 human colon carcinoma cell line [52]. Results demonstrated the presence of astragalin and isoquercetin in some fractions and significantly suppressed cell proliferation through the downregulation of ERK1/2 phosphorylation caused by them.
In a noteworthy study, the acute toxic and apoptosis- promoting effects of MO soluble extracts (GMG-ITC- RSE) replete with glucomoringin-isothiocyanate were investigated both in vivo and in vitro, respectively [84]. In this continuum, GMG-ITC-RSE did not appear to prompt adverse effects to rats even at high doses (2000 mg/kg body weight). Non-toxic GMG-ITC-RSE, triggered significant morphological aberrations indicative of apoptosis which confirmed its antiproliferative effect on PC-3 human prostate adenocarcinoma cell line.
Moringin (MOR), a glycosyl-isothiocyanate obtained through the myrosinase-catalyzed hydrolysis of the precursor 4-(α-l-rhamnosyloxy)-benzyl glucosinolate (glucomoringin), found predominantly in the seeds of MO, showed antiproliferative and pro-apoptotic effects upon Hep3B cancer cells resistant to anti-neoplastic agents. In this regard, the intrinsic apoptotic pathway was triggered by MOR-induced intracellular increase in ROS, the MOR-mediated activation of caspases 2 and 9, and the MOR-mediated downregulation of the pro-survival gene BIRC5 [56].
Another study has been focused upon the effect of MO in combination with radiation both on survival and metastasibility of pancreatic cancer cells, and on tumor growth [59]. The authors concluded that the combination of MO and radiation significantly inhibited PANC-1 cell survival in a dose-dependent manner, reduced metastasibility of these cells, and inhibited the growth of subcutaneous tumors engendered by PANC-1 cells in nude mice [59].
Tiloke and colleagues (2019) reported MO crude aqueous extracts displayed minimal cytotoxicity in peripheral blood mononuclear cell and human embryonic kidney (Hek293) cells while it led to increased lipid peroxidation, DNA damage and γH2AX levels with a decrease in G1, S and G2-M phase for 24 h in human liver HepG2 cells. Finally, they concluded that MO extracts induced cell-cycle arrest and apoptosis in cancerous HepG2 cells [63].
Another impressive study has put its concentration upon the potential antitumor effect of the aqueous extract of MO in mice bearing both EAC (Ehrlich ascites carcinoma)-induced solid tumor and tumor composed of HEp-2 human laryngeal carcinoma cells, and antitumor activity was reported. A vast range of phytochemicals including quinic acid, octadecanoic acid, hexadecanoic acid (palmitic acid), α-tocopherol (vitamin E), and ɣ-sitosterol, as the predominant bioactive components of the aqueous extract of MO, was reported [73].
Aldakheel and colleagues (2020) showed inhibitory action of seed ethanol extract of MO against human colorectal carcinoma cells (HCT-116) cells beside its biosafety for HEK-293 cells (non-cancerous cells) [67]. Phannasil et al. (2020) dogged deeper to decipher the antitumor activity of boiled MO pod-supplemented diets on azoxymethane/dextran sodium sulfate induced mouse colon carcinogenesis [68]. They reported the protein expression profiles responsible for the suppressive effect of this supplementation including 125 proteins that were differentially expressed in mouse colon tissues potentially involved in regulating apoptotic and inflammatory signaling networks in colorectal cancer prevention and therapy.
2 2 Caicedo-Lopez and colleagues (2021) reported antitumor activities of the impact of the colonic metabolites obtained from the fermented non-digestible fraction MO leaves on cell death mechanisms from human colorectal adenocarcinoma cell line (HT-29) cells [80]. In this scenario, this novel fermented formula exerted antiproliferative effect against HT-29 colorectal cancer cells via induction of increased H2O2 production, apoptosis, autophagy, and necrosis.
Fuel et al. (2021) analyzed chromatographically ethanolic extracts of the seeds of MO and declared that its extracts had excellent capacity to enhance activities of antioxidative enzymes such as glutathione S-transferase and QR [74]. they showed antitumor activity of this MO extracts against a bunch of colorectal cell lines and more surprisingly the showed its antitumor activity against cancer stem cells (CSCs) which are self-renewable cell types that are identified in most types of liquid and solid cancers and contributed to tumor onset, expansion, resistance, recurrence, and metastasis after therapy. This seminal study will open a research avenue to make MO-derived formulations as a candidate for clinical studies for human or veterinary mono- or poly-oncotherapy since this extract showed synergism with 5 fluorouracil.
Siddiqui et al. (2021) reported the antiproliferative activity of MO fruit extract on human liver cancer HepG2 cells and computational validation of cell death through ROS-mediated apoptosis and activation of caspase-3 enzyme [75]. They introduced benzyl glucosinolate as the best binders of caspase-3 enzyme while we cannot discriminate among activators or inhibitors in molecular docking and further experimental studies will (dis)prove this impressive finding.Cuellar-Núñez et al. (2021) experimentally showed that intake of a supplement contained 10% MO leaf powder induced differential expression of 65 genes in colon tissue such as IL-2, IL-6, TNF, IL-1ß, and INF-γ [77]. In this context, MO downregulated proinflammatory mediators showing chemopreventive properties against inflammatory response and colon carcinogenesis.
Respiratory cancers
A weak/moderate cytotoxicity to the NCI-H292, human mucoepidermoid pulmonary carcinoma cancer cell lines, was reported to be induced by both aqueous seed extract (ASE) of MO and the lectins cMol and WSMoL [32]. Both leukocyte migration and the number of leukocytes present in the lungs were diminished by ASE [32]. The cytotoxicity of ASE and cMol to immune cells may be the case and this supports the hypothesis of extract having immunosuppressive activity [32]. The antiproliferative effect of MO leaf extract in A549 lung cancer cells has been confirmed to be associated with the enhancement of oxidative stress, DNA fragmentation and apoptosis [31]. The MO aqueous leaf extract exerts its cytotoxic effect on A549 adenocarcinomic human alveolar basal epithelial cells by means of affecting the mitochondrial viability and inducing apoptosis through a ROS–dependent manner [49]. The MO water extract greatly promoted apoptosis, inhibited tumor cell growth, and reduced the level of internal ROS in human lung cancer cells and other types of cancer cells including A549 adenocarcinomic human alveolar basal epithelial cells, H23 human adenocarcinoma (non-small cell lung carcinoma), and H358 human bronchoalveolar carcinoma (non-small cell lung carcinoma) cell lines [15]. The in vitro antiproliferative activity of MO extracts against a diverse range of cancer cell lines such as A549 adenocarcinomic human alveolar basal epithelial cells, has been confirmed using sulphorhodamine-B (SRB) and MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) tetrazolium reduction assays [40]. In another ground-breaking study, two phytocompounds derived from MO leaf extracts, namely 4-α-rhamnopyranosyloxy-benzyl glucosinolate (Rhamno- Benzyl-GS) and acetyl-4-α-rhamnopyranosyloxy-benzyl glucosinolate Isomer III (Ac-Isomer-GS III), were reported to have cytotoxic effects upon V79-MZ Chinese hamster lung cells [48]. In the other study, antioxidative activity and moderate antitumor effect of various MO extracts have been reported in A549 adenocarcinomic human alveolar basal epithelial cells [61]. Xie et al. (2021) reported antitumor activity of an alkaloid extract of leaves of MO on a bunch of cancer cell lines and they concluded that this extract exerted the strongest growth inhibitory effect through inducing apoptosis and increasing the expression of the apoptosis- related proteins caspase-3 and caspase-9 against A549 cells but had low toxicity to GES-1 cells (human gastric mucosal epithelial cells), therefore this extract has potential for therapy and prophylaxis of lung cancer [66].
Neurological cancers
Neuro-protective effect of glucomoringin- isothiocyanate (GMG-ITC) also known as moringin suggests that GMG-ITC has the potential to protect the neuronal integrity in response to cytotoxicity associated with increased oxidative stress which is recognized as one of the hallmarks of neurodegenerative diseases [58]. Polar extracts prepared out of dried and milled leaves of MO grown in Jamaica showed significantly higher antioxidative activity through the assay using 2,2-diphenyl-1-picrylhydrazyl (DPPH) than those of other phytocompounds [85].
The in vivo and in vitro antiproliferative activity of an MO seed lectin (MOSL) was investigated to examine whether it exerts any activity against the proliferation of Ehrlich ascites carcinoma (EAC) cells, and the results showed dramatic inhibitory effects [57]. In particular, isothiocyanates (ITCs), which exert antitumor effects; and a newly prepared MO-derived 4-(α-L-rhamnopyranosyloxy) benzyl ITC (moringin) combined with alpha-cyclodextrin (moringin + α-CD; MAC) decreased the growth of SH-SY5Y human neuroblastoma cells in a concentration-dependent manner specifically through inhibiting the PI3K/Akt/mTOR pathway, whose aberrant activation was found to be associated with numerous types of tumors [55].
The in vitro antitumor effect caused by glycosylated isothiocyanate moringin (4-(α-l-rhamnopyranosyloxy) benzyl isothiocyanate), produced through the quantitative myrosinase-induced hydrolysis of glucomoringin (GMG) under a neutral pH value, upon human astrocytoma cells has been reported [45].
Genitourinary cancers
In another study, the antitumor effects of Saudi Arabian MO leaves, bark and seed extracts have been investigated against MDA-MB-231, a “basal” type and triple negative (ER, PR and HER2 negative) breast cancer cell line [39]. The authors found that in spite of neutral effect of seed extracts, the extracts of leaves and bark exhibited remarkable antitumor properties through decreasing cell survival, dramatic decrease (about 70–90%) in colony formation, diminished cell motility, increased apoptosis, and arrested progression of cell cycle at the G2/M phase. Coupled with aforementioned descriptive findings, the authors proposed that the anti-neoplastic effects of MO extracts are associated with a vast spectrum of compounds chemically detected in MO extracts and reportedly showing antitumor activity including eugenol, isopropyl isothiocynate, D-allose, and hexadeconoic acid ethyl ester [39].
The leaf extract of MO showed both dose- and time- dependent antiproliferative effect in MCF-7 human breast adenocarcinoma cell line and MDA-MB-231; a highly aggressive, invasive and poorly differentiated triple-negative breast cancer; comparable to Cis-Platinum [33]. The MO soluble cold aqueous extract significantly induced apoptosis, inhibited tumor cell growth, and lowered the level of internal ROS in MCF-7 human breast adenocarcinoma cell line [15]. The in vitro antiproliferative effect of MO extracts upon a wide spectrum of cancer cell lines including PC-3 prostate cancer cells, T47D human ductal carcinoma cells, and MCF-7 human breast adenocarcinoma cells, have been confirmed using SRB and MTT tetrazolium reduction assays [40]. The chemopreventive effect incited by the hydro-ethanolic extract of MO pods on DMBA-induced renal carcinogenicity may be mediated through its antioxidative properties [29].
Adopting a mechanistic perspective, the antiproliferative and apoptotic effect of benzyl isothiocyanate (BITC) upon ovarian cancer (OC) have been investigated. In line with this, BITC inhibited the proliferation of OC cells and made them undergo apoptosis as well [86]. Reportedly, the dichloromethane extract of MO leaf showed higher antiproliferative effect on MCF-7 (human breast adenocarcinoma cell line) of MO leaf was assessed and it was revealed that dichloromethane extract has higher activity in comparison with that of methanol extract on MCF-7 breast adenocarcinoma cell lines [34].
The MCF-7 human breast adenocarcinoma was treated with the essential oil extracted from MO seeds at a range of concentrations from 0.15 to 1 mg/mL for 24h, and the cytotoxicity was assessed through the MTT assay. Findings revealed that cell viability was significantly reduced in response to the ascending dose of essential oil in the treated cell line [38].
The aqueous leaf extract of MO decreased the viability of HEK293 human embryonic kidney 293 cells as well [49]. The aqueous extracts of MO leaf and flower produced different effects depending upon which cells they are being treated with [43]. The aqueous leaf extract brought about a moderate increment in the proliferation of mesenchymal stem cells (MSCs), angiogenesis, and a substantially enhanced hepato-protectivity, whereas it showed prominent cytotoxicity to cancer cell line (MCF-7). The aqueous extract of MO flower showed to a stimulatory effect on cellular proliferation; however, the same effect on cancer cell lines was not exerted. In addition, the leaf extract, had a prominent antitumor and hepato-protective effects [43].
The hydro-alcoholic extract of MO seeds and its fractions with four solvents (n-hexane, dichloromethane, chloroform, and n-butanol), significantly inhibited the cellular growth which has been tested on MCF 10A (human fibrocystic disease) cell line. Crude water extract, n-hexane and dichloromethane fractions of the seeds inhibited the proliferation of MCF-7 cells with half-maximal inhibitory concentration (IC50) of 280 μg/ml, 130 μg/ml and 26 μg/ml respectively; however, amongst the 3 samples, n-hexane fraction had the minimal cytotoxic effect on MCF 10A (IC50 > 400μg/ml) [54].
The bis (isothiocyanatomethyl) benzene, an anti- neoplastic phytocompound, has been purified from MO leaf extract which suppresses cancer cell growth and imposes inhibitory effect upon carcinogenesis within HeLa, MCF-7, and MDA-MB-231 cell lines by means of caspase 3 apoptotic pathway. Although not being quite similar, it bears much resemblance to drug Cis-Platinum, an anti-neoplastic agent available at the market [60].
Khan and colleagues (2020) proposed the inhibitory potential of MO methanolic extract of leaves against PC-3 prostate cancer cells via inhibiting hedgehog signaling pathway. They also observed a dose-dependent G0/G1 cell cycle arrest and a significant alteration in mRNA expression of genes related to apoptosis and hedgehog signaling pathway following the treatment with MO extract [64].
In a seminal work, Wisitpongpun and colleagues (2020) performed bioassay-guided fractionation and documentation of phytocompounds derived from MO leaf have been assessed against MDA-MB-231 breast cancer cells. The authors reported that the best anticancer activities were related to a fraction derived from crude ethyl acetate extract [70]. This fraction considerably reduced cell viability and clonogenic growth and increased apoptosis in aforementioned cell line and it possessed a plethora of phytocompounds among them 7-octenoic acid, oleamide, and 1-phenyl-2-pentanol showed anticancer activities by inducing cell cycle arrest and triggering apoptosis through suppressed Bcl-2 expression which subsequently promoted activation of caspase 3, an indicator for the apoptosis pathway.
Pandey and Khan (2020) reported the inhibitory role of methanolic extract of MO leaves against Jab1 signalosome in cervical cancer by analyzing several in vitro battery tests which reflected decreased cell growth throughout inducing apoptosis in a dose and time dependent manner [72].
Xie et al. (2020) evaluated mechanistically the inhibitory effect of MO alkaloids (MOA) on proliferation and migration of PC3 human prostate cancer cells in vitro and in vivo [66]. The results showed that MOA inhibited proliferation of PC3 cells and induced apoptosis and cell cycle arrest beside suppression of PC3 cell migration, inhibition of the expression of matrix metalloproteinases (MMP)-9, downregulation of the expression of cyclooxygenase 2 (COX-2), β-catenin, phosphorylated glycogen synthase 3β2, and vascular endothelial growth factor, and suppression of production of prostaglandin E2 (PGE2 ) and reversion of PGE2-induced PC3 cell proliferation and migration. Furthermore, in vivo experiments showed that MOA significantly inhibited growth of xenograft tumors in mice, and significantly reduced the protein expression levels of COX-2 and β-catenin in tumor tissues.
Lim et al. (2020) investigated chemopreventive values of the combined mixture of MO leaves and seed residues on the primary mammary epithelial cells (PMECs), non-malignant Chang’s liver cells and various human cancer cell lines (including breast, cervical, colon and liver cancer cell lines) [71]. They also tried this mixture in xenograft mice injected with MDA-MB-231 cells for in vivo. This mixture showed chemopreventive or cytotoxic effects on in MCF-7 breast cancer cells and PMECs with alteration of the levels of candidate genes involving in tumorigenesis.
Zunica et al. (2021) focused on the antitumor effects of dietary MO seed supplementation in immunocompromised female mice with diet-induced obesity bearing MDA-MB- 231-derived xenograft tumors [76]. More surprisingly, moringa supplementation could not disturb tumor progression and authors concluded that consumption of moringa seed extracts while receiving chemotherapy for breast cancer treatment should be cautiously assessed. The striking feature of this study was using triple negative breast cancer which is an aggressive and highly metastatic breast cancer subtype with limited treatment options.
Mohd Fisall et al. (2021) prepared various leaf extracts and fractions of MO and assessed their cytotoxic and apoptotic effects on breast cancer (MCF7) cells [79]. They finally reported that the dichloromethane extract was selectively cytotoxic to MCF7 cells (5 μg/mL) without significantly inhibiting the non-cancerous breast (MCF 10A) cells.
Bhadresha et al. (2021) investigated digestion effects on the bio-efficacy of leaves of MO on a PC3 cell line and finally they concluded that although in vitro digestion will decrease antioxidative potency of digesta but potentiates its antitumor effects against prostate cancer bone metastasis. This study will open a research aperture to the pharmacology of oral consumption of MO leaves as functional table vegetable in daily food menu.
Hematological cancers
Cold, hot, and ethanol extracts prepared from MO leaves could kill the majority (70 - 86%) of the cells among primary cells harvested from the clinical samples belonging to patients suffering from acute lymphoblastic leukemia and acute myeloid leukemia [20]. In this regard, hot water and ethanol extracts were more effective against abovementioned cancer cells.
The clastogenic and anti-clastogenic effects of freeze-dried boiled MO pod (bMO) were assessed in the presence of direct-acting clastogens (mitomycin C, MMC) and an indirect-acting agent (DMBA) using the in vivo erythrocyte micronucleus assay in mice [27]. Authors reported that freeze-dried bMO not only showed no clastogenicity but also produced an anti-clastogenic effect, particularly on the direct-acting clastogen in mouse [27]. The chemopreventive and anti-leukemic effects induced by the ethanolic extracts of MO leaves on benzene-induced leukemia, have been reported in rats [36]. Reportedly, the aqueous leaf extract of MO decreased the viability of Jurkat cells, the immortalized line of human T lymphocytes, which are used to study acute T-cell leukemia [49]. The in vitro antiproliferative potential of MO extracts against leukemia, namely THP-1 human acute monocytic leukemia, HL-60 human acute promyelocytic leukemia, and K562 human chronic myelogenous leukemia, has been confirmed through SRB and MTT tetrazolium reduction assays [40].
Potestà et al. (2019) reported the antiproliferative and pro-apoptotic effects of various aqueous extracts derived from leaves and seeds of MO on lymphoid and monocytoid cells not on peripheral blood mononuclear cells from healthy donors [62]. They concluded that this ability could be associated with the microRNA present in the extracts which highlighted their possible use as an adjuvant in traditional cancer therapy.
Dilworth et al. (2020) emphasized the antioxidative properties of MO leaf extract on markers of oxidative stress in HL60 cells, a human leukemia cell line, exposed to oxidative stress [65]. They showed that supplementation with the MO leaf extract at all concentrations resulted in significant reductions in lipid peroxidation in cells and increased cell viability with unknown mechanism.
Miscellaneous tumors
The antiproliferative effect of different fractions and the crude methanol extract of MO leaf were tested on the HeLa cell line. MO leaf extracts inhibited the proliferation of KB cells (a subline of the ubiquitous keratin-forming tumor cell line HeLa) through induction of apoptosis in a dose-dependent manner [30]. The ethanol-water [70-30] extracts of MO callus and leaf, significantly decreased the viability of Hela cells in a dose-dependent manner. However, the leaf extract of MO appeared to be more effective compared to callus extract since the content of phenolic compounds present in leaf extract was higher than that of callus extract. Authors proposed that phenolic compounds are involved in the cytotoxicity triggered by MO [35]. Different cell lines including HeLa, were treated with the essential oil extracted from MO seeds at a range of concentrations from 0.15 to 1 mg/mL for 24h, and the cytotoxicity was assessed using MTT assay. Findings showed a significant reduction in cell viability in response to the ascending dose of essential oil in all cell lines [38]. The MO soluble cold distilled water extract, greatly induced apoptosis, inhibited tumor cell growth, and lowered the level of internal ROS in HT1080 human fibrosarcoma cell line [15]. The L929 cell line (subcutaneous connective tissue, areolar and adipose) was treated with the essential oil from MO seeds and a significantly reduced cell viability was reported in response to the ascending dose of essential oil in all treated cell line [38]. Recently, bis (isothiocyanatomethyl) benzene, an anti-neoplastic phytocompound, has been purified from MO leaf extract which posed a suppression on cancer cell growth and inhibited carcinogenesis on HeLa cell line by caspase 3 apoptotic pathway in comparison to Cis-Platin [60].
Patriota et al. (2020) reported the antitumor activity of the MO flower trypsin inhibitor in sarcoma 180 inoculated mice [69]. They reported profound tumor regression and anti-angiogenesis without histological damages in other organs.
Lu et al. (2021) investigated the degradation of moringin (4-((α-l-rhamnosyloxy) benzyl)-isothiocyanate), a major bioactive isothiocyanate (ITC) found in MO seeds, at various food processing conditions [81]. They concluded that processing may lead to inactivation or perturbation of cytotoxicity of moringin against cancer cells.
Discussion
The MO is an omnipotent plant and almost all its parts have been used in various schools of traditional medicine and in culinary menus of south America, India, and Asia. This plant can be considered as an efficient tool for green synthesis of anticancer phyto-nanoparticles (for a review see Tiloke et al., 2018 [87]), nutraceutical source contains general (vitamin A, vitamin C, milk protein, and active phyto-constituents like alkaloids, protein, quinine, saponins, flavonoids, tannin, steroids, glycosides, fixed oil and fats) and special compounds like niazinin A, niazinin B and niazimicin A, niaziminin B [5] with a panaceas therapeutic power including anti-inflammatory, antioxidative, anti-cancerous, hepatoprotective, neuroprotective, hypoglycemic, and blood lipid-reducing functions [8], pharmaceutical source [6], agricultural resourse for its cultivations and preparation of germplasm bank for assessing the reasons of its diversities in both morphology and compositions and even for biodiesel production [10] and more specifically oncotherapeutic and targeting chemopreventive properties of MO (for review see Karim et al., 2016; Khor et al., 2018 [4] [88]). In this regard, Khor and colleagues (2018) reported anti-cancer potentials of MO in a seminal review till 2018, however their review has not been covered all relevant studies and selection biases were obvious in presenting data [88]. It is worthy to mention, the numbers of studies focusing on anti-cancer properties of MO showed a surge after 2018 and to the best of my knowledge this is the first systematic review inter alia. More recently, Ercan et al. (2021) has reviewed antioxidative potential of MO and proposed it as an antiproliferative source [89]. Both anecdotal narratives belonging to various schools of traditional medicine and mainstream scientific evidence propose that MO-derived phytocompounds and botanical formulations have antitumor potential [48, 90]. The chemopreventive characteristics of MO have been extensively reported through a seminal review [4]; however, the mentioned review adopted a disintegrated approach and haphazardly examined general chemopreventive mechanisms, phytochemistry, and pharmacognosy and cannot offer us a conclusion regarding oncotherapeutic or onco-prophylactic properties of MO although give us a good cue for focusing on this miracle tree. Accordingly, current systematic review, from 1998 to 2021, was implemented so as to obtain more scrutinized information (≠ knowledge) and gain further insight in order to put a conclusive end on ~ 24 years of research concerning the antitumor properties of MO. This area of research is very active and anybody looks over Table 1 will find that it is a great research of interest especially among Asian and Indian researchers, however these researchers just playing at the temporary field of in vitro (51% of studies) or in vivo chemically induced model (21% of studies) or tumor graft models (8% of studies; Figure 4) and there is no clinical studies in both medicine and veterinary medicine and it seems that there is courage to incite scientists to go from traditional medicine to bench but there is no encourage to leave bench to the destination of bed or human clinical studies. The reason of this stagnation in the in vitro or preclinical phase in this research avenue should be discussed in the cancer societies like Asian Clinical Oncology Society (asia-acos. org) and Indian Cancer Society (indiancancersociety.org) or their allies.
Figure 4. The Typology of Studies focused on Anti-cancer Effects of Maringa oleifera Lam. CITMs: Chemically Induced Tumor Model.
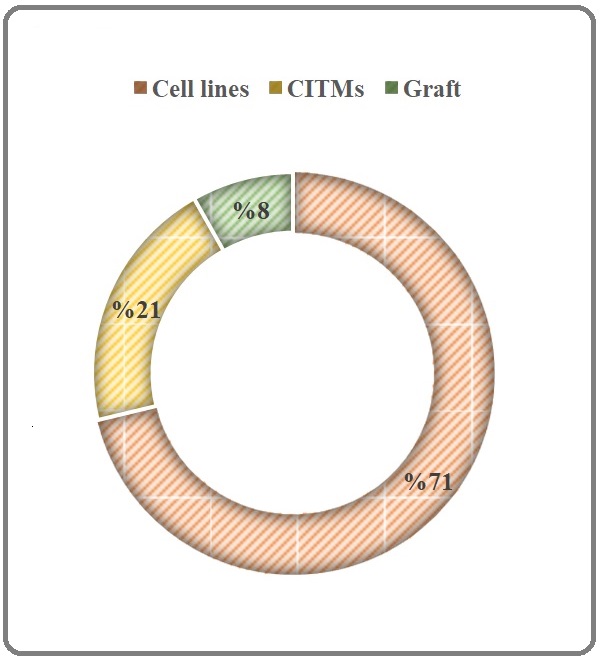
In this line, the higher proportion of works only reported therapeutic or preventive effects of MO extract and rarely specified phytochemicals mediating the antitumor effects except more recently investigations which have mechanistic insight to anti-tumor effects of MO in both gene and protein levels [75, 77]. To be more precise, Rhamno-Benzyl-GS and Ac-Isomer-GS III, quercetin-3-O-glucoside, 4-(β-D-glucopyranosyl-1→4- α-L-rhamnopyranosyloxy)-benzyl isothiocyanate, and moringin, are remarkable MO phytochemicals having been reported to serve strong antitumor effects. Further scrutiny is urgently required to shed light upon the sphere of the unknown on which phytochemicals are the major bioactive compounds found in MO extract possessing potential antitumor effect against cancers. To the best of my knowledge, there is not any report in terms of antitumor activity of MO against metastatic cancers. Interestingly, a few studies have put their steps beyond the descriptive reports of antitumor effect of MO on colorectal cancers and introduced those phytochemicals promoting antitumor activities. Reportedly, both dietary fiber and phenolic compounds of MO leaves showed to impose suppressive effect on chemically-induced colorectal cancer. A fermented formulation of MO, established substantial anti-inflammatory effects and possibly may be involved in producing antitumor as well as antioxidative effects. A long way still exists to determine the profile of MO prebiotics found in various parts in both fermented and non-fermented formulations and this can be considered as a gap of knowledge in terms of MO pharmacognosy. In another seminal work, the inhibitory effect of MO leaf extract on human pancreatic cancer has been confirmed both alone and in combination with cisplatin. The number of studies that focused on synergistic effects of MO on the other canonical anti-neoplastic drugs and radiotherapeutics is very scarce.
All 21 review articles which targeted in this systematic review have been deeply analyzed technically to distillate their contents for discussing here. I should declare that the framework and backbone of them were heterogeneous and amalgam of traditional approaches mixed by orthodox medicine approaches covered ethnobotanical, pharmacognostic, agricultural and pharmacological investigations. Although this broad vision may reflect the miracle panacean properties of MO but cannot give us a cue to highlight the present and the future of this pipeline of research based on previous achievements. If I drew an analogy, reviewers just hit in outer ring of dartboard distant to high score rings or even bullseye. In nutshell, good samples of interest to understand general phytochemistry and therapeutic benefits of MO have been recently published (for a review see Arora and Arora, 2021; Hassan et al., 2021 [91, 92]).
As shown in Figure 5, leaves of MO as major botanical part (52%) have been used for its anticancer activities and since its decoction and boiled formulations are reported (Table 1) and there are some profound documented traditions [34] which convey prior-experiences of humankind regarding drinking of MO, it can be concluded that future market of functional tea of this plant would be at the top list of functional anticancer soft- or even hard-drinks.
Figure 5. The Classification of Selected Studies Based on the Part Used for Evaluation of Anti-cancer Potential of Moringa oleifera Lam.
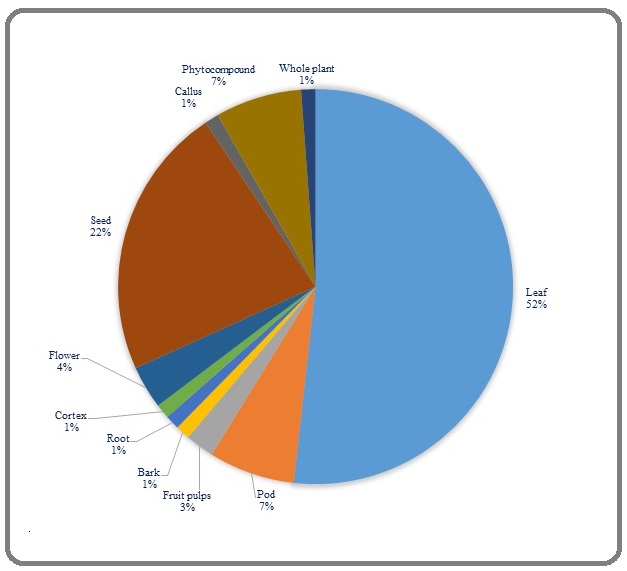
A simple and fast snapshot of “moringa tea” in Google image will show us the current market of functional products of MO, however based on my personal experience, fresh leaves would be more fragrant and aromatic and drying will diminish significantly the quality of hot and cold teas. It seems that employing of some conservation methods like cryopreservation would be strategic to hold natural flavor of moringa tea (for a review see Arora and Arora, 2021). After leaves, seeds of MO have been used to prepare various anti-cancer formulations including fresh or frozen aqueous extracts, coagulant or purified lectins, essential oil derived via cold pressing, and organic, enzyme-treated or defatted moringin-rich extract (Table 1). The MO seed would be considered as an oil seed source with high content of anticancer phytocompounds, however the more phytochemical and mechanistic investigations are requested to decipher its major phytocompound. Based on studies which analyzed in this systematic review (Table 1) moringin and its analogs would be considered as putative phyto-onco-lytics or phyto-onco-statics that I coined here arrogantly. In this line, approximately 7% of studies reported some anticancer phytocompounds derived from various parts of MO. In this context, more phytochemical and computational studies are requested to delve chemical spaces of phyto-onco- lytics, phyto-onco-statics or tumorigenic, proto-oncogenic, and proangiogenic compounds of MO. For instance, Fernandes et al. (2016) warned us to survey the effect of MO extracts or its phytocompounds on angiogenesis before considering them as prospective candidates for antitumor drug [43].
Pods would be considered as an initial guard of seeds and their nutritional values are very different among cultivars and ecotypes (for a review see Arora and Arora, 2021). Many tribes cook pods and use them as food,however 7% of reported studies in this systematic review mentioned to their anticancer activities (Table 1) and moringin has been introduced as their main functional components. The phytochemistry and deciphering functional components of the powders of mature and immature pods may be an unknown features of MO which requests more scientific investigations. The contributions of other parts of MO including callus, bark, root, fruit pulp and flowers were less than 5 percentage of studies and would be investigated in the future (Table 1). In addition, it is worthy to note the partitioning of secondary metabolites with pharmacological values in source (leaf) and sink (roots, stems, pods, callus, and fruits) organs of MO for selecting the best solvents for their extractions. The secondary metabolites usually produced in plants exposed to a plethora of (a) biotic stresses therefore it is possible to manipulate MO to decipher enriching ecotypes or to concentrate high amount of phyto-oncolytics or phyto-oncostatics or engineer new cultivars by using biopharming and transgenic tools.
In this continuum, various techniques of extractions including maceration, decoction, percolation, cold pressing, etc. have been reported in some selected studies and it seems that rational extractions have not been pursued in all studies. The various physicochemical attributes of extracts and the phytocompound of interest dictate us to select the best solvents for extraction or fractionation of a part of MO to reach the most potent formulations or even phytocompounds (for a review see Abubakar et al., 2020). As shown in Figure 6, solvents commonly used in extraction of MO are polar solvent (e.g., water, ethanol, hydro-alcohol, methanol), intermediate polar (e.g., acetone for fractionation, dichloromethane), and nonpolar (e.g., n-hexane, ethyl acetate, chloroform).
Figure 6. The Classification of Selected Studies Based on the Solvent Used for Evaluation of Anti-cancer Potential of Moringa oleifera Lam.
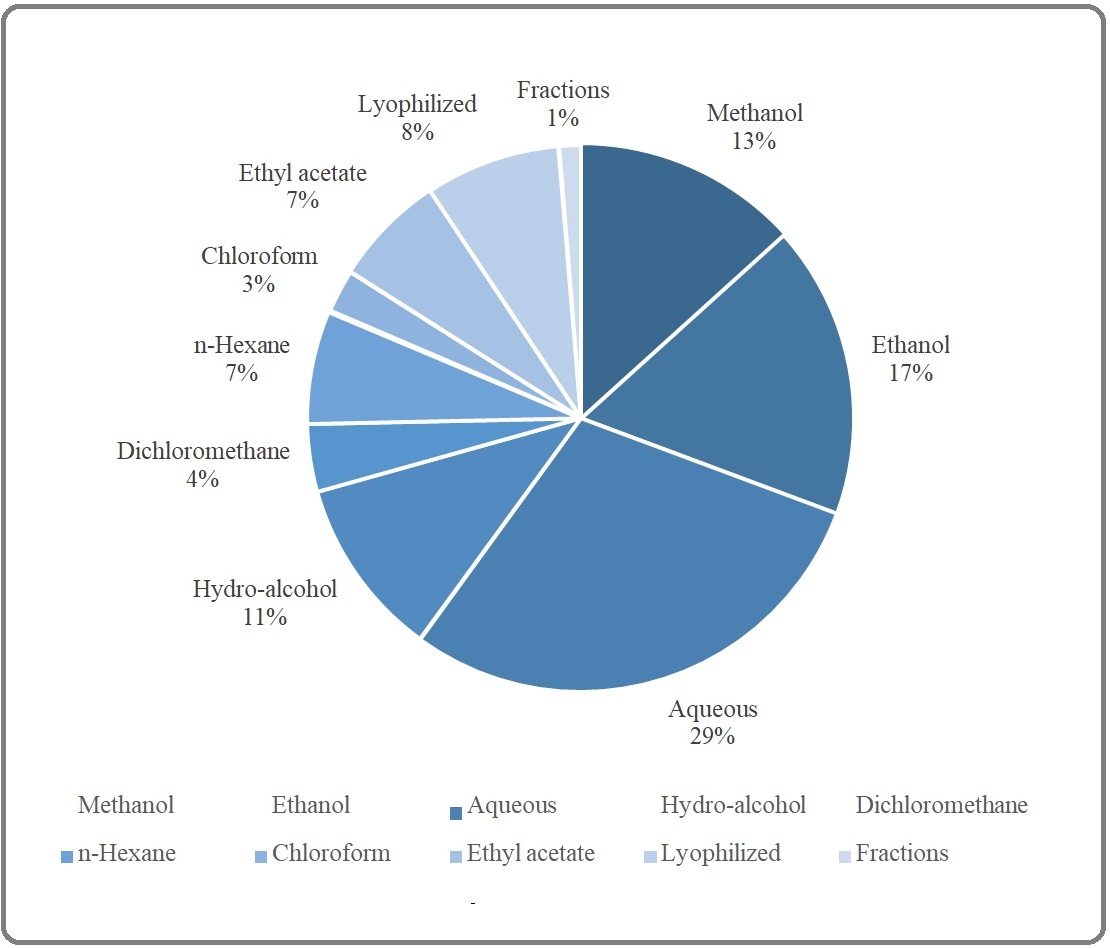
Some studies (8%) have been employed lyophilization as a new method to break cell walls and to extract its content by using appropriate solvents. Among the solvents have been utilized to extract or to fractionate (1% of studies) resulting extracts of MO, polar solvents had contributions in this order: water (29%) > ethanol (17%) > methanol (13%) > hydro-alcohol (11%) and the rest of intermediate polar (dichloromethane 4%) and nonpolar (n-hexane (7%), ethyl acetate (7%), chloroform (3%)) solvents. To sum up, future studies must be focused on the rational extraction and purification of MO with special thanks to nonpolar solvents or phytochemical and pharmacological features of menstruum and marc.
As shown in Figure 7, human colorectal cancer was the main tumor phenotype which was studied among other phenotypes and consisted 22% of screened studies. Leukemia, non-small cell adenocarcinoma, and breast cancer consisted 14, 10, and 8% of screened studies, respectively and the rest of tumors consisted less than 5% of studies. To sum up, various extracts of MO have been showed phyto-onco-lytic and/or phyto-onco-static activities against a wide ranges of tumor phenotypes including benign, malignant, and metastatic ones. Figure 7 gives us a research cue to test antitumor activity of MO in other tumors, to select specific phenotype for in vivo animal model or even to try clinical trial for some aforementioned phenotypes in medicine or veterinary medicine.
Figure 7. The Classification of Selected Studies Based on the Cancer Phenotype Used for Evaluation of Anti-cancer Potential of Moringa oleifera Lam.
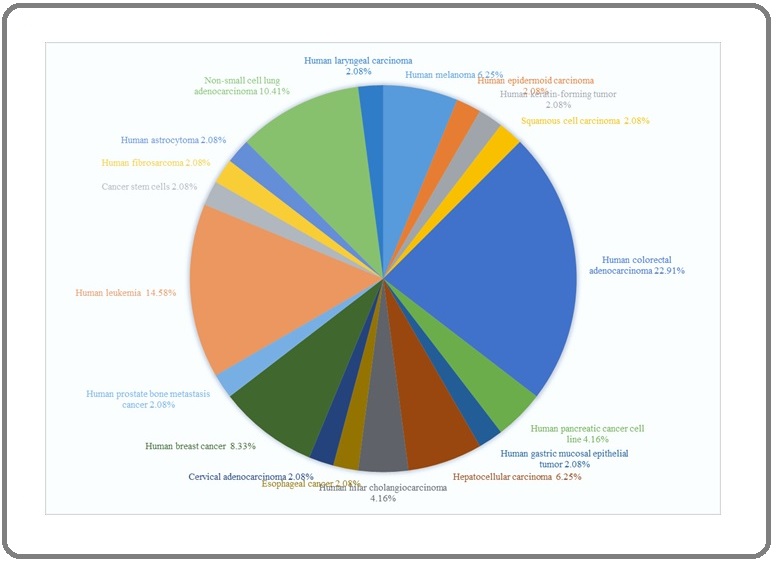
As depicted in Figure 8 in a radar format, totally 118 cell lines have been used to detect antitumor activity of various extracts of MO and in this context, healthy cell lines (control; n = 19), MCF-7 (n = 12), HepG2 (n = 12), and Hela (n = 6) consisted top list cell lines employed in various studies. This radar format gives us an opportunity to select new cell lines for novel works to understand broad spectrum of antitumor activity of MO and it also can give us the highly responsive cell lines to dig mechanisms of MO antitumor activities. Young researchers can curate more information regarding disease, disease subtype, lineages, source, gender, and primary/metastasis of all mentioned cell lines in depmap (https://depmap.org/ portal/depmap/).
Figure 8. The Classification of Selected Studies Based on the Cell Lines Used for Evaluation of Anti-cancer Potential of Moringa oleifera Lam.
The major anti-cancer portals of MO which have been classified in Figure 9 may be considered redundant however they are distilled from selected studies of this systematic review via both text mining and semantics.
Figure 9. The Major Anti-cancer Portals of Moringa oleifera Lam.
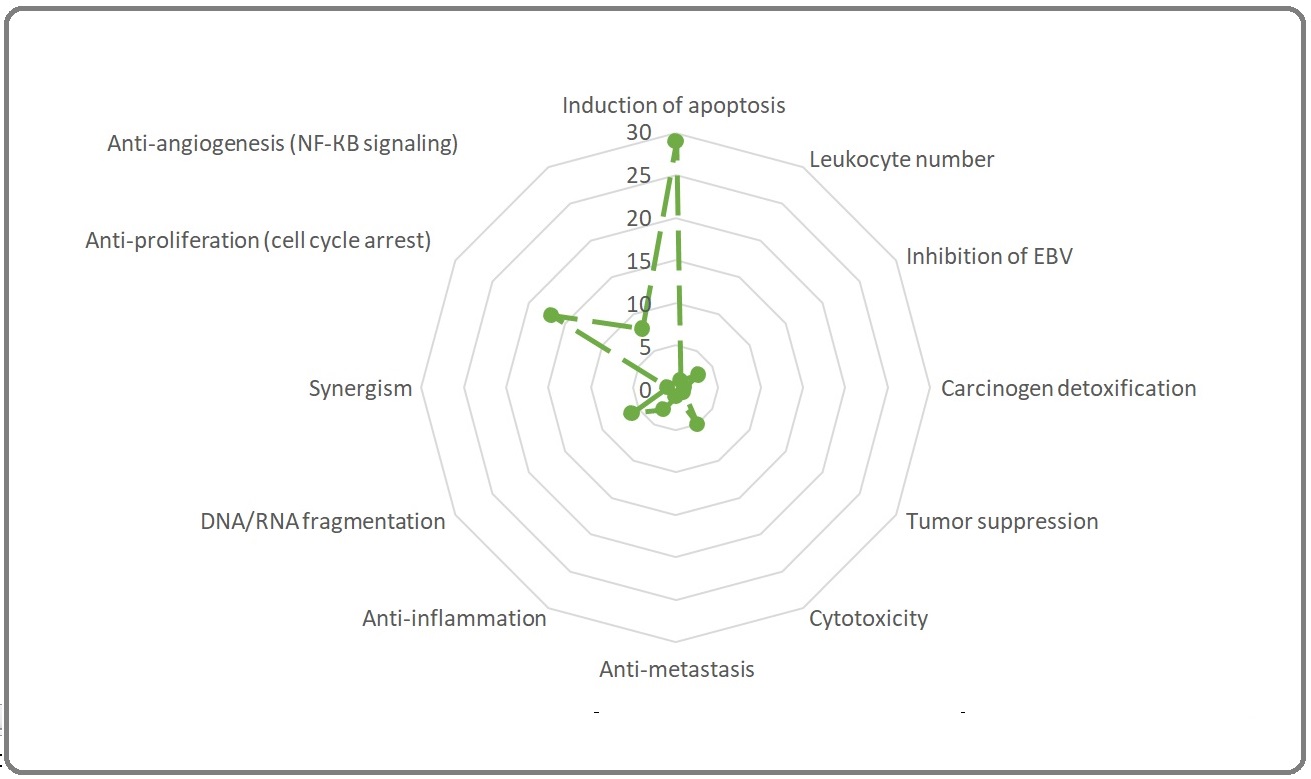
In this scenario, the summation of all anti-cancer portals was 76 for all selected papers. The induction of apoptosis (n = 29), anti-proliferation (n = 17), anti-angiogenesis (n = 8), and DNA/RNA fragmentation (n = 6) were the main portals mediated antitumor effects of MO. The cytotoxicity and anti-inflammation may be considered as minor antitumor portals. The difference between the number of selected studies of this systematic review (n = 71) and total number of reported antitumor portals (n = 76) is due to report of more than one determinant outcomes per each study.
In conclusion, future studies must be focused on the rational extraction and purification of MO, phytochemistry, and computational and experimental pharmacology of various extracts of MO to decipher phyto-onco-lytic and/or phyto-onco-static drug-like phytocompounds and the necessity of starting clinical trials should be discussed and pursued especially for tumors which have sufficient preclinical evidence. In this line, I am focusing on a hybrid study (computational plus experimental) to decipher pharmacological mechanism of ani-breast cancer activities of MO.
Funding
This study was supported by intramural fund of Razi University.
Acknowledgments
The author thanks Dr. Naser Karimi for introducing him this plant; Nima Yakhchalian who gave some comments technically and grammatically in the preparation of manuscript.
References
- Weber D. Botanical Oncology; isolates. Sydney: Panaxea Publishing 2015 .
- Challenges Conducting Clinical Trials with Herbal Products in Oncology. In: Alaoui-Jamali M. (eds) Alternative and Complementary Therapies for Cancer Sood A, Prasad K. Springer, Boston, MA.2010.
- Plants vs. cancer: a review on natural phytochemicals in preventing and treating cancers and their druggability Wang Hu, Khor Tin Oo, Shu Limin, Su Zheng-Yuan, Fuentes Francisco, Lee Jong-Hun, Kong Ah-Ng Tony. Anti-Cancer Agents in Medicinal Chemistry.2012;12(10). CrossRef
- Moringa oleifera Lam: targeting chemoprevention Karim NA, Ibrahim MD, Kntayya SB, Rukayadi Y, Hamid HA, Razis AF. Asian Pac J Cancer Prev.2016;17(8):3675-3686.
- Health benefits of Moringa oleifera Abdull Razis Ahmad Faizal, Ibrahim Muhammad Din, Kntayya Saie Brindha. Asian Pacific journal of cancer prevention: APJCP.2014;15(20). CrossRef
- Phytochemistry and Pharmacology of Moringa oleifera Lam Paikra Birendra Kumar, Dhongade Hemant kumar J., Gidwani Bina. Journal of Pharmacopuncture.2017;20(3). CrossRef
- Bioactive Components in Moringa Oleifera Leaves Protect against Chronic Disease Vergara-Jimenez Marcela, Almatrafi Manal Mused, Fernandez Maria Luz. Antioxidants (Basel, Switzerland).2017;6(4). CrossRef
- Nutraceutical or Pharmacological Potential of Moringa oleifera Lam Kou Xianjuan, Li Biao, Olayanju Julia B., Drake Justin M., Chen Ning. Nutrients.2018;10(3). CrossRef
- Review: an exposition of medicinal preponderance of Moringa oleifera (Lank.) Hussain S, Malik F, Mahmood S. Pak J Pharm Sci.2014;27(2).
- Cultivation, Genetic, Ethnopharmacology, Phytochemistry and Pharmacology of Moringa oleifera Leaves: An Overview Leone Alessandro, Spada Alberto, Battezzati Alberto, Schiraldi Alberto, Aristil Junior, Bertoli Simona. International Journal of Molecular Sciences.2015;16(6). CrossRef
- The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration Liberati Alessandro, Altman Douglas G., Tetzlaff Jennifer, Mulrow Cynthia, Gøtzsche Peter C., Ioannidis John P. A., Clarke Mike, Devereaux P. J., Kleijnen Jos, Moher David. BMJ.2009;339. CrossRef
- An antitumor promoter from Moringa oleifera Lam Guevara A. P., Vargas C., Sakurai H., Fujiwara Y., Hashimoto K., Maoka T., Kozuka M., Ito Y., Tokuda H., Nishino H.. Mutation Research.1999;440(2). CrossRef
- Chemomodulatory effect of Moringa oleifera, Lam, on hepatic carcinogen metabolising enzymes, antioxidant parameters and skin papillomagenesis in mice Bharali R, Tabassum J, Azad MR. Asian Pac J Cancer Prev.2003;4(2):131-139.
- Antioxidant extracts of African medicinal plants induce cell cycle arrest and differentiation in B16F10 melanoma cells Gismondi Angelo, Canuti Lorena, Impei Stefania, Di Marco Gabriele, Kenzo Maurice, Colizzi Vittorio, Canini Antonella. International Journal of Oncology.2013;43(3). CrossRef
- Soluble extract from Moringa oleifera leaves with a new anticancer activity Jung Il Lae. PloS One.2014;9(4). CrossRef
- Cytotoxicity of the coagulant Moringa oleifera lectin (cMoL) to B16-F10 melanoma cells Andrade Luz Luciana, Rossato Franco Aparecido, Costa Rute Alves Pereira E., Napoleão Thiago Henrique, Paiva Patrícia Maria Guedes, Coelho Luana Cassandra Breitenbach Barroso. Toxicology in vitro: an international journal published in association with BIBRA.2017;44. CrossRef
- Phenolic Extraction of Moringa Oleifera Leaves Induces Caspase-Dependent and Caspase-Independent Apoptosis through the Generation of Reactive Oxygen Species and the Activation of Intrinsic Mitochondrial Pathway in Human Melanoma Cells Do Bich Hang, Hoang Nghia Son, Nguyen Thi Phuong Thao, Ho Nguyen Quynh Chi, Le Thanh Long, Doan Chinh Chung. Nutrition and Cancer.2021;73(5). CrossRef
- Phenolic extraction of Moringa Oleifera leaves induces caspase-dependent and caspase-independent apoptosis through the generation of reactive oxygen species and the activation of intrinsic mitochondrial pathway in human melanoma cells Do BH, Hoang NS, Nguyen TPT, Ho NQC, Le TL, Doan CC. Nutr Cancer.2021;73(5):869-888. CrossRef
- Anti-Cancer Effect of 3-Hydroxy-β-Ionone Identified from Moringa oleifera Lam. Leaf on Human Squamous Cell Carcinoma 15 Cell Line Luetragoon Thitiya, Pankla Sranujit Rungnapa, Noysang Chanai, Thongsri Yordhathai, Potup Pachuen, Suphrom Nungruthai, Nuengchamnong Nitra, Usuwanthim Kanchana. Molecules (Basel, Switzerland).2020;25(16). CrossRef
- Active principle from Moringa oleifera Lam leaves effective against two leukemias and a hepatocarcinoma Khalafalla MM, Abdellatef E, Dafalla HM, Nassrallah AA, Aboul-Enein KM, Lightfoot DA, et al . African Journal of Biotechnology.2010;9:8467-8471.
- Suppressive effects of Moringa oleifera Lam pod against mouse colon carcinogenesis induced by azoxymethane and dextran sodium sulfate Budda S, Butryee C, Tuntipopipat S, Rungsipipat A, Wangnaithum S, ,Lee LS, et al . Asian Pac J Cancer Prev.2011;12(12):3221-3228.
- Antiproliferative effect of Moringa oleifera Lam. and Pseuderanthemum palatiferum (Nees) Radlk extracts on the colon cancer cells Pamok Supaporn, Saenphet Supap, Saenphet Kanokporn. Journal of medicinal plant research.2012;6. CrossRef
- Chemopreventive efficacy of Moringa oleifera pods against 7, 12-dimethylbenz[a]anthracene induced hepatic carcinogenesis in mice Sharma Veena, Paliwal Ritu, Janmeda Pracheta, Sharma Shatruhan. Asian Pacific journal of cancer prevention: APJCP.2012;13(6). CrossRef
- Inhibitory effects of crude extracts from some edible Thai plants against replication of hepatitis B virus and human liver cancer cells Waiyaput Wanwisa, Payungporn Sunchai, Issara-Amphorn Jiraphorn, Panjaworayan Nattanan T.-Thienprasert. BMC complementary and alternative medicine.2012;12. CrossRef
- Moringa Oleifera aqueous leaf extract down-regulates nuclear factor-kappaB and increases cytotoxic effect of chemotherapy in pancreatic cancer cells Berkovich Liron, Earon Gideon, Ron Ilan, Rimmon Adam, Vexler Akiva, Lev-Ari Shahar. BMC complementary and alternative medicine.2013;13. CrossRef
- Niaziminin, a thiocarbamate from the leaves of Moringa oleifera, holds a strict structural requirement for inhibition of tumor-promoter-induced Epstein-Barr virus activation Murakami A., Kitazono Y., Jiwajinda S., Koshimizu K., Ohigashi H.. Planta Medica.1998;64(4). CrossRef
- Nutritive evaluation and effect of Moringa oleifera pod on clastogenic potential in the mouse Promkum C, Kupradinun P, Tuntipopipat S, Butryee C. Asian Pac J Cancer Prev.2010;11(3):627-632.
- The isothiocyanate produced from glucomoringin inhibits NF-kB and reduces myeloma growth in nude mice in vivo Brunelli Dario, Tavecchio Michele, Falcioni Cristiano, Frapolli Roberta, Erba Eugenio, Iori Renato, Rollin Patrick, Barillari Jessica, Manzotti Carla, Morazzoni Paolo, D'Incalci Maurizio. Biochemical Pharmacology.2010;79(8). CrossRef
- Anti-nephrotoxic effect of administration of Moringa oleifera Lam in amelioration of DMBA-induced renal carcinogenesis in Swiss albino mice Paliwal R, Sharma V, Pracheta A, Sharma S, Yadav S, Sharma S. Biol Med Special .2011;3:27-35.
- Antiproliferation and induction of apoptosis by Moringa oleifera leaf extract on human cancer cells Sreelatha S., Jeyachitra A., Padma P. R.. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association.2011;49(6). CrossRef
- The antiproliferative effect of Moringa oleifera crude aqueous leaf extract on cancerous human alveolar epithelial cells Tiloke Charlette, Phulukdaree Alisa, Chuturgoon Anil A.. BMC complementary and alternative medicine.2013;13. CrossRef
- Evaluation of Cytotoxic and Anti-Inflammatory Activities of Extracts and Lectins from Moringa oleifera Seeds Araújo Larissa Cardoso Corrêa, Aguiar Jaciana Santos, Napoleão Thiago Henrique, Mota Fernanda Virgínia Barreto, Barros André Luiz Souza, Moura Maiara Celine, Coriolano Marília Cavalcanti, Coelho Luana Cassandra Breitenbach Barroso, Silva Teresinha Gonçalves, Paiva Patrícia Maria Guedes. PLoS ONE.2013;8(12). CrossRef
- Anticancer effect of Moringa oleifera leaf extract on human breast cancer cell. Thesis submitted to Jadavpur University Faculty of Engineering and Technology School of Bioscience & Engineering Ghosh N. Kolkata – 700 032, India.2014.
- Antioxidant and anticancer activities of Moringa oleifera leaves Charoensin Suphachai. Journal of Medicinal Plants Research.2014;8. CrossRef
- Evaluation of cytotoxicity of Moringa oleifera Lam. callus and leaf extracts on Hela cells Jafarain Abbas, Asghari Gholamreza, Ghassami Erfaneh. Advanced Biomedical Research.2014;3. CrossRef
- Chemopreventive and anti-leukemic effects of ethanol extracts of Moringa oleifera leaves on wistar rats bearing benzene induced leukemia Akanni E. O., Adedeji A. L., Adedosu O. T., Olaniran O. I., Oloke J. K.. Current Pharmaceutical Biotechnology.2014;15(6). CrossRef
- Moringa oleifera extracts induce cholangiocarcinoma cell apoptosis by induction of reactive oxygen species production Leelawat S, Leelawat K. Int J Pharmacog Phytochem Res.2014;6:183-189.
- In vitro Evaluation of Cytotoxic Activities of Essential Oil from Moringa oleifera Seeds on HeLa, HepG2, MCF-7, CACO-2 and L929 Cell Lines Elsayed Elsayed Ahmed, Sharaf-Eldin Mahmoud A., Wadaan Mohammad. Asian Pacific journal of cancer prevention: APJCP.2015;16(11). CrossRef
- Moringa oleifera as an Anti-Cancer Agent against Breast and Colorectal Cancer Cell Lines Al-Asmari Abdulrahman Khazim, Albalawi Sulaiman Mansour, Athar Md Tanwir, Khan Abdul Quaiyoom, Al-Shahrani Hamoud, Islam Mozaffarul. PloS One.2015;10(8). CrossRef
- In Vitro Anticancer Activities of Anogeissus latifolia, Terminalia bellerica, Acacia catechu and Moringa oleiferna Indian Plants Diab Kawthar A. E., Guru Santosh Kumar, Bhushan Shashi, Saxena Ajit K.. Asian Pacific journal of cancer prevention: APJCP.2015;16(15). CrossRef
- A potential oral anticancer drug candidate, Moringa oleifera leaf extract, induces the apoptosis of human hepatocellular carcinoma cells Jung Il Lae, Lee Ju Hye, Kang Se Chan. Oncology Letters.2015;10(3). CrossRef
- Identification and characterization of a potent anticancer fraction from the leaf extracts of Moringa oleifera L. Krishnamurthy PT, Vardarajalu A, Wadhwani A, Patel V. Indian J Exp Biol.2015;53(2):98-103.
- Probing Regenerative Potential of Moringa oleifera Aqueous Extracts Using In vitro Cellular Assays Fernandes Evangeline E., Pulwale Anubha V., Patil Gauri A., Moghe Alpana S.. Pharmacognosy Research.2016;8(4). CrossRef
- Cytotoxicity, Antioxidant and Apoptosis Studies of Quercetin-3-O Glucoside and 4-(β-D-Glucopyranosyl-1→4-α-L-Rhamnopyranosyloxy)-Benzyl Isothiocyanate from Moringa oleifera Maiyo Fiona C., Moodley Roshila, Singh Moganavelli. Anti-Cancer Agents in Medicinal Chemistry.2016;16(5). CrossRef
- Anticancer activity of glucomoringin isothiocyanate in human malignant astrocytoma cells Rajan Thangavelu Soundara, De Nicola Gina Rosalinda, Iori Renato, Rollin Patrick, Bramanti Placido, Mazzon Emanuela. Fitoterapia.2016;110. CrossRef
- The Antiproliferative Effect of Moringa oleifera Crude Aqueous Leaf Extract on Human Esophageal Cancer Cells Tiloke Charlette, Phulukdaree Alisa, Chuturgoon Anil A.. Journal of Medicinal Food.2016;19(4). CrossRef
- The chemopreventive phytochemical moringin isolated from Moringa oleifera seeds inhibits JAK/STAT signaling Michl C, Vivarelli F, Weigl J, De Nicola GR, Canistro D, Paolini M, et al . PLoS One.2016;11(6:e0157430). CrossRef
- Characteristic single glucosinolates from Moringa oleifera: Induction of detoxifying enzymes and lack of genotoxic activity in various model systems Förster Nadja, Mewis Inga, Glatt Hansruedi, Haack Michael, Brigelius-Flohé Regina, Schreiner Monika, Ulrichs Christian. Food & Function.2016;7(11). CrossRef
- Moringa oleifera's Nutritious Aqueous Leaf Extract Has Anticancerous Effects by Compromising Mitochondrial Viability in an ROS-Dependent Manner Madi Niveen, Dany Mohammed, Abdoun Salah, Usta Julnar. Journal of the American College of Nutrition.2016;35(7). CrossRef
- Physicochemical and nutraceutical properties of moringa (Moringa oleifera) leaves and their effects in an in vivo AOM/DSS-induced colorectal carcinogenesis model Cuellar-Nuñez M. L., Luzardo-Ocampo I., Campos-Vega R., Gallegos-Corona M. A., González de Mejía E., Loarca-Piña G.. Food Research International.2018;105. CrossRef
- The chemo-prophylactic efficacy of an ethanol Moringa oleifera leaf extract against hepatocellular carcinoma in rats Sadek Kadry M., Abouzed Tarek K., Abouelkhair Reham, Nasr Sherif. Pharmaceutical Biology.2017;55(1). CrossRef
- Anti-proliferative effect of Moringa oleifera Lam (Moringaceae) leaf extract on human colon cancer HCT116 cell line Tragulpakseerojn Jintana, Yamaguchi Naoto, Pamonsinlapatham Perayot, Wetwitayaklung Penpun, Yoneyama Tatsuro, Ishikawa Naoki, Ishibashi Masami, Apirakaramwong Auayporn. Tropical Journal of Pharmaceutical Research.2017;16(2). CrossRef
- Molecular mechanisms of cholangiocarcinoma cell inhibition by medicinal plants Leelawat Surang, Leelawat Kawin. Oncology Letters.2017;13(2). CrossRef
- ANTIPROLIFERATIVE EFFECT ON BREAST CANCER (MCF7) OF MORINGA OLEIFERA SEED EXTRACTS Adebayo Ismail Abiola, Arsad Hasni, Samian Mohd Razip. African journal of traditional, complementary, and alternative medicines: AJTCAM.2017;14(2). CrossRef
- Moringa isothiocyanate complexed with α-cyclodextrin: a new perspective in neuroblastoma treatment Giacoppo Sabrina, Iori Renato, Rollin Patrick, Bramanti Placido, Mazzon Emanuela. BMC complementary and alternative medicine.2017;17(1). CrossRef
- A Combination of Moringin and Avenanthramide 2f Inhibits the Proliferation of Hep3B Liver Cancer Cells Inducing Intrinsic and Extrinsic Apoptosis Antonini Elena, Iori Renato, Ninfali Paolino, Scarpa Emanuele Salvatore. Nutrition and Cancer.2018;70(7). CrossRef
- Moringa oleifera seed lectin inhibits Ehrlich ascites carcinoma cell growth by inducing apoptosis through the regulation of Bak and NF-κB gene expression Asaduzzaman A. K. M., Hasan Imtiaj, Chakrabortty Aninda, Zaman Sadnima, Islam Shaikh Shohidul, Ahmed Fazle Rabbi Shakil, Kabir K. M. Ahsanul, Nurujjaman Md, Uddin Md Belal, Alam Mohammad Taufiq, Shaha Ranajit Kumar, Kabir Syed Rashel. International Journal of Biological Macromolecules.2018;107(Pt B). CrossRef
- Isothiocyanate from Moringa oleifera seeds mitigates hydrogen peroxide-induced cytotoxicity and preserved morphological features of human neuronal cells Jaafaru Mohammed Sani, Nordin Norshariza, Shaari Khozirah, Rosli Rozita, Abdull Razis Ahmad Faizal. PloS One.2018;13(5). CrossRef
- Combined Effect of Moringa oleifera and Ionizing Radiation on Survival and Metastatic Activity of Pancreatic Cancer Cells Hagoel Lior, Vexler Akiva, Kalich-Philosoph Lital, Earon Gideon, Ron Ilan, Shtabsky Alex, Marmor Silvia, Lev-Ari Shahar. Integrative Cancer Therapies.2019;18. CrossRef
- Bis (Isothiocyanatomethyl) Benzene, A Plant Derived Anti-Neoplastic Compound: Purified from Moringa Oleifera Leaf Extract Paul Samrat, Basak Piyali, Maity Namrata, Guha Chayan, Jana Nandan Kumar. Anti-Cancer Agents in Medicinal Chemistry.2019;19(5). CrossRef
- Anticancer activity of plant leaves extract collected from a tribal region of India Kumar Gourav, Gupta Rashmi, Sharan Shruti, Roy Partha, Pandey Dev Mani. 3 Biotech.2019;9(11). CrossRef
- Cytotoxic and apoptotic effects of different extracts of Moringa oleifera Lam on lymphoid and monocytoid cells Potestà Marina, Minutolo Antonella, Gismondi Angelo, Canuti Lorena, Kenzo Maurice, Roglia Valentina, Macchi Federico, Grelli Sandro, Canini Antonella, Colizzi Vittorio, Montesano Carla. Experimental and Therapeutic Medicine.2019;18(1). CrossRef
- Moringa oleifera Aqueous Leaf Extract Induces Cell-Cycle Arrest and Apoptosis in Human Liver Hepatocellular Carcinoma Cells Tiloke Charlette, Phulukdaree Alisa, Gengan Robert M., Chuturgoon Anil A.. Nutrition and Cancer.2019;71(7). CrossRef
- Moringa oleifera methanolic leaves extract induces apoptosis and G0/G1 cell cycle arrest via downregulation of Hedgehog Signaling Pathway in human prostate PC-3 cancer cells Khan Fahad, Pandey Pratibha, Ahmad Varish, Upadhyay Tarun Kumar. Journal of Food Biochemistry.2020;44(8). CrossRef
- Effects of Moringa oleifera Leaf Extract on Human Promyelocytic Leukemia Cells Subjected to Oxidative Stress Dilworth Lowell L., Stennett Dewayne, Omoruyi Felix O.. Journal of Medicinal Food.2020;23(7). CrossRef
- Moringa oleifera Alkaloids Inhibited PC3 Cells Growth and Migration Through the COX-2 Mediated Wnt/β-Catenin Signaling Pathway Xie Jing, Luo Feng-xian, Shi Chong-ying, Jiang Wei-wei, Qian Ying-yan, Yang Ming-rong, Song Shuang, Dai Tian-yi, Peng Lei, Gao Xiao-yu, Tao Liang, Tian Yang, Sheng Jun. Frontiers in Pharmacology.2020;11. CrossRef
- Bactericidal and In Vitro Cytotoxicity of Moringa oleifera Seed Extract and Its Elemental Analysis Using Laser-Induced Breakdown Spectroscopy Aldakheel Reem K., Rehman Suriya, Almessiere Munirah A., Khan Firdos A., Gondal Mohammed A., Mostafa Ahmed, Baykal Abdulhadi. Pharmaceuticals (Basel, Switzerland).2020;13(8). CrossRef
- Protein expression profiles that underpin the preventive and therapeutic potential of Moringa oleifera Lam against azoxymethane and dextran sodium sulfate-induced mouse colon carcinogenesis Phannasil Phatchariya, Roytrakul Sittiruk, Phaonakrop Narumon, Kupradinun Piengchai, Budda Sirintip, Butryee Chaniphun, Akekawatchai Chareeporn, Tuntipopipat Siriporn. Oncology Letters.2020;20(2). CrossRef
- Antitumor activity of Moringa oleifera (drumstick tree) flower trypsin inhibitor (MoFTI) in sarcoma 180-bearing mice Patriota Leydianne Leite de Siqueira, Ramos Dalila de Brito Marques, Dos Santos Angela Caroline Lima Amorim, Silva Yasmym Araújo, Gama E Silva Mariana, Torres Diego José Lira, Procópio Thamara Figueiredo, Oliveira Alisson Macário, Coelho Luana Cassandra Breitenbach Barroso, Pontual Emmanuel Viana, Silva Diego César Nunes, Paiva Patrícia Maria Guedes, Lorena Vírginia Maria Barros, Mendes Rosemairy Luciane, Napoleão Thiago Henrique. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association.2020;145. CrossRef
- In Vitro Bioassay-Guided Identification of Anticancer Properties from Moringa oleifera Lam. Leaf against the MDA-MB-231 Cell Line Wisitpongpun Prapakorn, Suphrom Nungruthai, Potup Pachuen, Nuengchamnong Nitra, Calder Philip C., Usuwanthim Kanchana. Pharmaceuticals (Basel, Switzerland).2020;13(12). CrossRef
- Significant Decreased Expressions of CaN, VEGF, SLC39A6 and SFRP1 in MDA-MB-231 Xenograft Breast Tumor Mice Treated with Moringa oleifera Leaves and Seed Residue (MOLSr) Extracts Lim Wai Feng, Mohamad Yusof Mohd Izwan, Teh Lay Kek, Salleh Mohd Zaki. Nutrients.2020;12(10). CrossRef
- Jab1 Inhibition by Methanolic Extract of Moringa Oleifera Leaves in Cervical Cancer Cells: A Potent Targeted Therapeutic Approach Pandey Pratibha, Khan Fahad. Nutrition and Cancer.2021;73(11-12). CrossRef
- Aqueous Extract of Moringa oleifera Exhibit Potential Anticancer Activity and can be Used as a Possible Cancer Therapeutic Agent: A Study Involving In Vitro and In Vivo Approach Barhoi Dharmeswar, Upadhaya Puja, Barbhuiya Sweety Nath, Giri Anirudha, Giri Sarbani. Journal of the American College of Nutrition.2021;40(1). CrossRef
- Antioxidant and antiproliferative potential of ethanolic extracts from Moringa oleifera, Tropaeolum tuberosum and Annona cherimola in colorrectal cancer cells Fuel Marco, Mesas Cristina, Martínez Rosario, Ortiz Raul, Quiñonero Francisco, Prados José, Porres Jesús M., Melguizo Consolación. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie.2021;143. CrossRef
- Cytotoxicity of Moringa oleifera fruits on human liver cancer and molecular docking analysis of bioactive constituents against caspase-3 enzyme Siddiqui Sahabjada, Upadhyay Shivbrat, Ahmad Imran, Hussain Arshad, Ahamed Maqusood. Journal of Food Biochemistry.2021;45(5). CrossRef
- Moringa Oleifera Seed Extract Concomitantly Supplemented with Chemotherapy Worsens Tumor Progression in Mice with Triple Negative Breast Cancer and Obesity Zunica Elizabeth R. M., Yang Shengping, Coulter Ann, White Christy, Kirwan John P., Gilmore Linda A.. Nutrients.2021;13(9). CrossRef
- Moringa oleifera leaves alleviated inflammation through downregulation of IL-2, IL-6, and TNF-α in a colitis-associated colorectal cancer model Cuellar-Núñez M. L., Gonzalez de Mejia E., Loarca-Piña G.. Food Research International (Ottawa, Ont.).2021;144. CrossRef
- Alkaloid Extract of Moringa oleifera Lam. Exerts Antitumor Activity in Human Non-Small-Cell Lung Cancer via Modulation of the JAK2/STAT3 Signaling Pathway Xie Jing, Peng Lin-Jie, Yang Ming-Rong, Jiang Wei-Wei, Mao Jia-Ying, Shi Chong-Ying, Tian Yang, Sheng Jun. Evidence-Based Complementary and Alternative Medicine: eCAM.2021;2021. CrossRef
- Dichloromethane fraction of Moringa oleifera leaf methanolic extract selectively inhibits breast cancer cells (MCF7) by induction of apoptosis via upregulation of Bax, p53 and caspase 8 expressions Mohd Fisall Umiey Fahietah, Ismail Noor Zafirah, Adebayo Ismail Abiola, Arsad Hasni. Molecular Biology Reports.2021;48(5). CrossRef
- Colonic metabolites from digested Moringa oleifera leaves induced HT-29 cell death via apoptosis, necrosis, and autophagy Caicedo-Lopez Laura H., Cuellar-Nuñez M. Liceth, Luzardo-Ocampo Ivan, Campos-Vega Rocio, Lóarca-Piña Guadalupe. International Journal of Food Sciences and Nutrition.2021;72(4). CrossRef
- The degradation kinetics and mechanism of moringin in aqueous solution and the cytotoxicity of degraded products Lu Yuyun, Maria Vos Romy Dorothea, Zhang Yuyu, Zhang Molan, Liu Yunjiao, Fu Caili, Liu Shao Quan, Huang Dejian. Food Chemistry.2021;364. CrossRef
- Assessing the Protective Effect of Moringa oleifera Extract against Bone Metastasis: An In Vitro Simulated Digestion Approach Bhadresha Kinjal P., Jain Nayan K., Rawal Rakesh M.. Nutrition and Cancer.2021. CrossRef
- Fermented Moringa oleifera Decreases Hepatic Adiposity and Ameliorates Glucose Intolerance in High-Fat Diet-Induced Obese Mice Joung Hyunchae, Kim Bobae, Park Hyunjoon, Lee Kyuyeon, Kim Hee-Hoon, Sim Ho-Cheol, Do Hyun-Jin, Hyun Chang-Kee, Do Myoung-Sool. Journal of Medicinal Food.2017;20(5). CrossRef
- Nontoxic Glucomoringin-Isothiocyanate (GMG-ITC) Rich Soluble Extract Induces Apoptosis and Inhibits Proliferation of Human Prostate Adenocarcinoma Cells (PC-3) Jaafaru Mohammed Sani, Abd Karim Nurul Ashikin, Mohamed Eliaser Enas, Maitalata Waziri Peter, Ahmed Hamidu, Mustapha Barau Mohammed, Kong Liliya, Abdull Razis Ahmad Faizal. Nutrients.2018;10(9). CrossRef
- An Investigation of the Antioxidant Capacity in Extracts from Moringa oleifera Plants Grown in Jamaica Wright Racquel J., Lee Ken S., Hyacinth Hyacinth I., Hibbert Jacqueline M., Reid Marvin E., Wheatley Andrew O., Asemota Helen N.. Plants (Basel, Switzerland).2017;6(4). CrossRef
- Benzyl isothiocyanate (BITC) induces apoptosis in ovarian cancer cells in vitro Kalkunte S, Swamy N, Dizon DS, Brard L. J Exp Ther Oncol.2006;5(4):287-300.
- Moringa oleifera and their phytonanoparticles: Potential antiproliferative agents against cancer Tiloke Charlette, Anand Krishnan, Gengan Robert M., Chuturgoon Anil A.. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie.2018;108. CrossRef
- The In Vitro and In Vivo Anticancer Properties of Moringa oleifera Khor Kang Zi, Lim Vuanghao, Moses Emmanuel J., Abdul Samad Nozlena. Evidence-Based Complementary and Alternative Medicine: eCAM.2018;2018. CrossRef
- Moringa Oleifera: A Review of Its Occurrence, Pharmacological Importance and Oxidative Stress Ercan Kenan, Gecesefa Omer Faruk, Taysi Muhammed Enes, Ali Ali Omeed Akbar, Taysi Seyithan. Mini Reviews in Medicinal Chemistry.2021;21(3). CrossRef
- Local knowledge, use pattern and geographical distribution of Moringa oleifera Lam. (Moringaceae) in Nigeria Popoola Jacob O., Obembe Olawole O.. Journal of Ethnopharmacology.2013;150(2). CrossRef
- Nutritional significance and therapeutic potential of Moringa oleifera: The wonder plant Arora Shalini, Arora Saurabh. Journal of Food Biochemistry.2021;45(10). CrossRef
- Health benefits and phenolic compounds of Moringa oleifera leaves: A comprehensive review Hassan Mohamed Ahmed, Xu Tao, Tian Yang, Zhong Yongheng, Ali Fatma Abo Zakaib, Yang Xuan, Lu Baiyi. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology.2021;93. CrossRef
- Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes Abubakar Abdullahi R., Haque Mainul. Journal of Pharmacy & Bioallied Sciences.2020;12(1). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2022
Author Details