Influence of Autoantibodies to Estradiol and Progesterone on the Blood Serum Hormones Concentrations in Postmenopausal Healthy Women and Breast Cancer Patients
Download
Abstract
Background: It is known that antibodies to steroid hormones are associated with some human diseases (systemic lupus erythematosus, thrombosis, recurrent pregnancy loss, autoimmune dermatitis et al.). There were no any data about action of specific autoantibodies on the sex hormones in hormone-dependent cancer patients.
Objectives: The purpose of this study was to investigate the probable links between estradiol (Es) and progesterone (Pg) levels and autoantibodies of A-class specific to these hormones (IgA-Es and IgA-Pg) in the blood serum of healthy women and breast cancer patients.
Patients and Methods: There were examined 521 nearly diagnosed breast carcinoma patients from Regional Clinical Oncology Dispensary (Kemerovo, Russian Federation) and 143 healthy women from Kemerovo (Russian Federation). The serum concentration of Es and Pg were analyzed by competitive immunoassay using specific monoclonal antibodies. The serum IgA-Pg and IgA-Es were analyzed by solid-phase non-competitive immunoassay using Pg and Es conjugated with bovine serum albumin as adsorbed antigens. The data analysis was performed using software STATISTICA 8.0 (Stat Soft Inc., USA).
Results: We discovered that low ratios Pg/Es<4.3 were associated with breast cancer. Personal IgA-Pg/IgA-Es ratios negatively correlated with Es levels and positively correlated with Pg levels and Pg/Es ratios in compared groups. The influence of IgA-Pg/IgA-Es ratio on the hormones levels and their ratio in heathy women was more strong than in breast cancer patients.
Conclusions: Our clinical results correspond to the known experimental data about influence of specific antibodies on the levels of steroid hormones in immunized animals. Human autoantibodies to Es and Pg could stimulate or inhibit promotion of carcinogenesis by influence on Pg/Es ratio depending on individual IgA-Pg/IgA-Es ratio.
Introduction
It is well known that estradiol (Es) and progesterone (Pg) play an important role in the promotion of the breast carcinogenesis by influence on the proliferation of mammary cells. In addition, Es acts as initiator of carcinogenesis by the formation of mutagenic adducts with DNA [1-3]. On the other hand immunization of animals against Es and Pg resulted in elevated blood serum hormones levels and modulation of their biological actions [4-10]. Caldwell et al. (1971) [11] revealed that immunization of rats by Es conjugated with bovine serum albumin (Es-BSA) induced regression of Es-sensitive mammary adenocarcinoma. They were confident that the neutralization of the circulating Es by antibodies (Abs) was the primary cause of extended survival time and longer interval between implantation and tumor growth. The low-weight Es adducted with macromolecular carrier could induce the synthesis of specific antibodies as a hapten. There were found the associations of anti-Es Abs with some human diseases: systemic lupus erythematosus [12] and thrombosis in women on oral contraceptives [13]. Anti-Pg Abs was associated with recurrent pregnancy loss [14] and autoimmune dermatitis [15]. The specific immune reaction on both Es and Pg were revealed in women with premenstrual syndrome [16, 17] and recurrent pregnancy loss [18]. Autoantibodies to Es-receptor were markers of systemic lupus erythematosus [19] and systemic sclerosis [20]. They acted as Es-agonists in mammary carcinoma cell culture [21,22] and their serum levels correlated with breast cancer cell proliferation [23].
There were no any data about action of specific autoantibodies on the sex hormones in hormone-dependent cancer patients.
Previously we have proposed that Abs to Es and Pg could stimulate or inhibit hormone-sensitive carcinogenesis according to individual features of their formation [24-26]. In the present study, we explored the influence of autoantibodies to Es and Pg on the blood serum hormones levels in postmenopausal healthy women (HW) and breast cancer patients (BCP).
Materials and Methods
Patients
The study was performed in 664 non-smoking postmenopausal women, of which 521 women were primarily diagnosed with invasive breast cancer (BC) in the Regional Clinical Oncology Dispensary (Kemerovo, Russian Federation). Each case diagnosis was confirmed morphologically in the oncology dispensary. Data related to tumor size, grade, and stage were collected from surgical pathology reports. All cases were unilateral - 267 (51.2%) right breast, 254 (48.8%) left breast. The histopathological subtype of the all cases was ductal carcinoma. Most cases belonged to differentiation grade II (247, 47.4%), followed by grade I (185, 35.5%), grade III (86, 16.5%) and grade
IV (3, 0.6%). 143 HW without breast pathology were included for comparison. The median ages of participants were 63 (ranging from 42 to 85) for BCP and 58 (ranging from 43 to 80) for HW.
The study protocol conforms to the ethical guidelines of the 2013 Declaration of Helsinki and was approved by ethics committee of Institute of Human Ecology of Siberian Branch of Russian Academy of Sciences (protocol №46/1). All women provided informed consents.
Inclusion and exclusion criteria
Inclusion criteria: for BCP group – patients with primarily diagnosed invasive breast cancer, non-smoking postmenopausal women, patients were aged over 40 years old; for control group - healthy postmenopausal women without breast pathology, non-smoking, women were aged above 40 years old.
Exclusion criteria: patients with other types of tumor, patients without any treatment (chemotherapy, X-ray therapy, hormone replacement therapy, surgery); smoking women, young women under 40 years old.
Immunoassay of Antibodies to Estradiol and Progesterone
Abs to Es and Pg were detected by solid phase non-competitive enzyme-linked immunosorbent assays. Microtiter wells were coated with 2 μg/ml Es or Pg conjugated with bovine serum albumin BSA (Amresco, USA) in 100 μl phosphate-buffered saline (PBS) pH 7.4 (Amresco, USA) at room temperature overnight. The Es-BSA conjugate was received from Sigma-Aldrich (Germany). Pg-BSA conjugate was obtained by conjugation of hemi-glutarate of 21-hydroxyprogesterone (Sigma-Aldrich, Germany) and BSA by the carbodiimide method. Coated wells were blocked for 30 min in blocking buffer saline (0.5% BSA in PBS, 0.05% Tween 20). The serum samples were diluted at 1/20 in blocking buffer saline and were incubated with coated antigens (100 μl/well) for 1 h at 37◦C. Bound Abs were detected by goat anti-human IgAantibody labeled with horseradish peroxidase (1/10000 dilution, Novex, USA). After each assay steps wells were washed 4-5 times with 250 μl/ well of washing buffer saline (PBS, 0.05% Tween 20). The amounts of bound Abs were determined through enzymatic reaction with the chromogenic substrate TMB (Vector Corp., USA). The reaction was then terminated by addition of 2 N HCl and absorbance was measured at 450 nm. All measurements were conducted in duplicate. The levels of Abs to Es or Pg were expressed in arbitrary units and calculated based on the following
formula:
IgA-Х = (OD Х-BSA −OD BSA )/OD BSA
where X were Es or Pg; OD Х-BSA was the absorbance of the binding to hapten–BSA conjugate, OD BSA was the absorbance of the binding to BSA.
Steroid Hormones Determination
The concentrations of Es and Pg were determined using commercial kits “ImmunoFA-Estradiol”, “ImmunoFA- PG” (“Immunotech”, Russia) according to the instructions for use.
Statistical Analysis
All statistical analyses were conducted using STATISTICA version 8.0 (StatSoft Inc., USA). Normality was evaluated by Shapiro-Wilk’s W-test. The Mann– Whitney U-test and χ2 with Yates’ correction were used for comparison of non-parametric data. The results are expressed as medians (Me), interquartile range (IQR). The relationship between the antibodies levels and the steroids hormones was assessed using the Spearman’s rank correlation analysis. All statistical analyses were two-sided, p<0.05 were considered statistically significant. To determine the threshold values of Abs and steroid hormones (cut-off) ROC analysis was performed [27]. Odds ratio (OR) was determined with 95% confidence interval (95% CI).
Results
Associations of the serum Es and Pg levels with the breast cancer in postmenopausal women
Breast cancer is typically Es- and Pg-dependent women malignant tumor. Interactions of Es and Pg with mammary cellular receptors are complicated. Incidence rates and risk factors for breast cancer differ according to Es- and Pg- receptors status [28]. It was revealed that Pg inhibited Es-mediated growth of Es-receptor α cell line xenografts and primary breast tumor explants, and had increased anti-proliferative affects when coupled with Es antagonist [29].
Here we studied the blood serum Pg and Es levels and calculated the individual Pg/Es ratios in 143 HW and 521 BCP. The results are shown in Figure 1.
Figure 1. Medians of Estradiol and Progesterone Concentrations (A) and Pg/Es Ratios (B) in Breast Cancer Patients (BCP) and Healthy Women (HW).
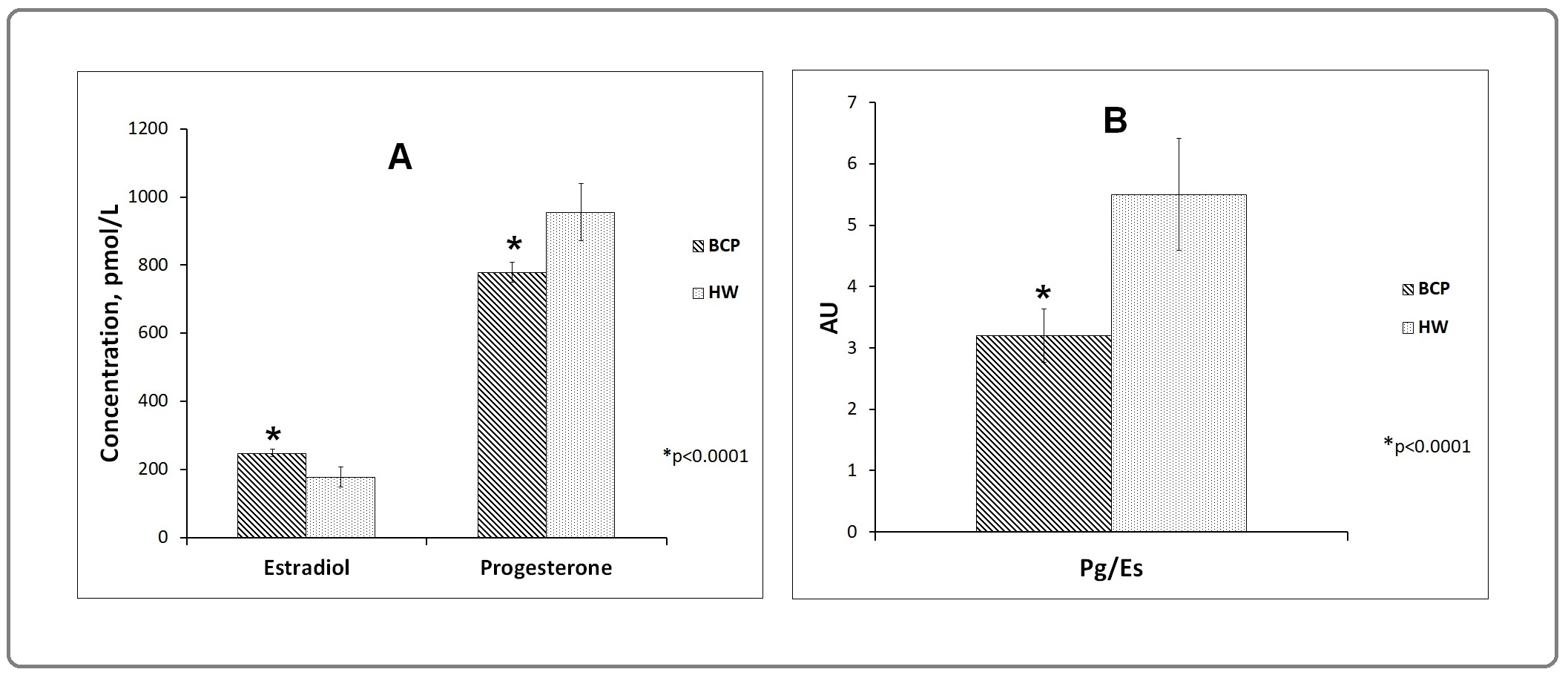
The Pg levels were significantly less but Es levels were high in BCP that in HW (p<0.0001). The Pg/Es ratio in BCP was less than in HW (p<0.0001). There were no any differences on these parameters between BCP according to Pg- and Es-receptors presence (data not shown).
The cut-off between compared groups on these parameters was calculated using ROC-analysis. The frequencies of cases with the low (<) and high (≥) hormones levels and ratios in HW and BCP were determinated. The high levels of Es (>200 pmol/L) were revealed in BCP more frequently than in HW (66.8% vs 44.1%; χ2=23.6; p<0.0001). The high levels of Pg (≥900 pmol/L) were found more rarely in BCP than in HW (30.9% vs 58.7%; χ2=36.2; p<0.0001). The high Pg/ Es ratios (≥4) were revealed in BCP more rarely than in HW (31.5% vs 55.9%; χ2=27.8; p<0.0001). It means that high levels of Es (>200 pmol/L) were associated with high BC risk (p<0.0001; OR=2.6; 95% CI=1.8-3.7) and high Pg levels (≥900 pmol/L) as well as high Pg/Es ratios (≥4.3) were not associated with BC risk (p<0.0001; OR=0.3; 95% CI=0.2-0.5 and OR=0.4; 95% CI=0.2-0.5 correspondingly). But low levels of Pg (<900pmol/L) and low Pg/Es ratios (<4.3) were associated with BC risk (p<0.0001; OR=3.2; 95% CI=2.2-4.7 and OR=2.8; 95% CI=1.9-4.0 correspondingly).
Influence of Abs to Es and Pg on the levels of these hormones in the blood serum of HW and BCP
Taken into attention the possibility of model antibodies against steroids to elevate the levels of sex hormones in immunized animals we studies the influence of autoantibodies to Es and Pg on their levels in the blood serum of HW and BCP. There were searched the specific antibodies of A class based on their known possibility to bind and transport the corresponding hormones into epithelial target cells. In the Table 1 the correlations between IgA-levels to Pg and Es (IgA-Pg and IgA-Es) and IgA-Pg/IgA-Es, on the one hand, and Pg and Es levels, and Pg/Es ratio, on the other hand, in compared groups are shown.
| (x) | (y) | Breast cancer patients | Healthy women | ||
| Abs levels, | Hormone | ||||
| Abs ratios | levels, ratios | r s (p) | y=a * xx+b | r s (p) | y=a * x+b |
| 1. IgA-Es | Es | -0.01 (0.91) | ---- | 0.21 (0.01) | y=0.02х+0.2 |
| Pg | 0.04 (0.33) | ---- | -0.01 (0.95) | ---- | |
| Pg/Es | 0.03 (0.47) | ---- | -0.17 (0.06) | ---- | |
| 2. IgA-Pg | Es | -0.13 (0.004) | y=-0.01х+0.3 | -0.05 (0.55) | ---- |
| Pg | 0.19 (<0.0001) | y=0.05х+0.8 | 0.31 (0.0001) | y=0.1х+0.9 | |
| Pg/Es | 0.20 (<0.0001) | y=0.5х+3.9 | 0.15 (0.07) | ---- | |
| 3. IgA-Pg/IgA-Es | Es | -0.15 (0.0007) | y=-0.04х+0.3 | -0.35 (<0.0001) | y=-0.2х+0.4 |
| Pg | 0.18 (<0.0001) | y=0.2х+0.8 | 0.50 (<0.0001) | y=0.6х+0.6 | |
| Pg/Es | 0.21 (<0.0001) | y=1.9х+3.6 | 0.45 (<0.0001) | y=6.1х+4.1 |
rs - Spearman's rank correlation, p<0.05 was statistically significant
In HW the Es levels, but not Pg levels and Pg/Es ratio positively correlated with the IgA-Es levels. The Pg levels but not Es levels and Pg/Es ratio positively correlated with the IgA-Pg. The levels of Es (negatively) and Pg (positively) and Pg/Es ratio (positively) correlated with the IgA-Pg/IgA-Es ratio.
In BCP correlations of Es, Pg and Pg/Es with the IgA-Es were not revealed. The levels of Es negatively correlated with IgA-Pg and with IgA-Pg/IgA-Es. The levels of Pg and Pg/Es ratio correlated positively with IgA-Pg levels and IgA-Pg/IgA-Es ratio.
The study of Es and Pg concentrations and Pg/Es ratios in HW and BCP with the low (<1) and high (≥1) levels of IgA-Es and IgA-Pg and IgA-Pg/IgA-Es ratio are shown in Table 2.
| Antibodies ratios | Es | Pg | Pg/Es |
| Me (IQR) | Me (IQR) | Me (IQR) | |
| Healthy women | |||
| 1.1. IgA-Pg/IgA-Es<1 | 213(121-355) | 862 (711-1119) | 3.6 (1.9-10.6) |
| 1.2. IgA-Pg/IgA-Es≥1 | 138 (91-187) | 1414(1110-1797) | 12.5 (7.9-17.9) |
| p-value | 0.001 | <0.0001 | <0.0001 |
| Breast cancer patients | |||
| 2.1. IgA-Pg/IgA-Es<1 | 249 (185-411) | 760 (649-920) | 3.0 (1.8-4.6) |
| 2.2. IgA-Pg/IgA-Es≥1 | 236 (146-291) | 861 (706-1345) | 3.7 (2.7-9.02) |
| p-value | 0.006 | 0.0002 | 0.0001 |
p<0.05 was statistically significant
The average Es-concentration decreased from 213 pmol/L in HW with low IgA-Pg/IgA-Es ratio to 138 pmol/L with high IgA-Pg/IgA-Es ratio (i.e. on 35.2%). The respective decline in BCP was only 5.2%. The average Pg- concentration increased from 862 to 1414 pmol/L in HW (i.e. on 64%), but only on 13.3% in BCP. The average Pg/Es ratio increased from 3.6 in HW with low IgA-Pg/ IgA-Es ratio to 12.5 with high IgA-Pg/IgA-Es ratio (i.e. on 247.2%). The respective raise in BCP was only on 23.3%.
Discussion
In the present study the influence of specific auto Abs in human on the blood serum Es and Pg concentrations was shown for the first time.
It was found that the average Es-levels in BCP was higher than in HW and the average Pg-levels was lower in the corresponding groups. These data confirm that Es could stimulate but Pg could inhibit the promotion of carcinogenesis in mammary gland.
The positive correlations between Es and IgA-Es levels and between Pg and IgA-Pg were revealed in HW. IgA-Es correlated neither with Es nor with Pg in BCP. IgA-Pg positively correlated with Pg and negatively with Es in BCP. It means that specific autoantibodies could influence on the Es and Pg levels as well as in model experiments.
The personal Pg/Es ratio was calculated in compared group. ROC-analysis has conducted to determine the odds ratio (OR) for these parameters. The frequency of cases with high Pg/Es ratio (≥4.3) was higher in HW that in BCP (OR=0.4; 95%CI=0.2-0.5). Such hormonal state could be marked as “hormonal balance”. The frequency of cases with low Pg/Es ratio (<4.3) was corresponding higher in BCP than in HW (OR=2.8; 95%CI=1.9-4.0) and such hormonal state could be marked as “hormonal disbalance”.
We have proposed that hormonal balance/disbalance depended on individual IgA-Pg/IgA-Es ratio. It was marked as “immunological disbalance”.
There were positive correlation between IgA-Pg/IgA- Es ratio and Pg/Es ratio in HW and in BCP. It means that immunological and hormonal balance (disbalance) are interconnected and interdependent.
The average value of Pg/Es ratio was low (hormonal disbalance) in HW with IgA-Pg/IgA-Es ratio<1 (immunological disbalance). And average value of Pg/Es ratio was high (hormonal balance) in HW with IgA-Pg/ IgA-Es ratio ≥1 (immunological balance). But in BCP with IgA-Pg/IgA-Es ratio ≥1 (immunological balance) the state of hormonal disbalance was remained (average value of Pg/Es ratio was low). It means that immune- hormonal relationships in HW were strong but in BCP were weak. These differences could be explained by the strong affinity of Abs to Es and Pg in HW and weak affinity of corresponding Abs in BCP.
The proposal mechanism of immunomodulation of steroid-dependent carcinogenesis by specific auto-Abs needs the further investigations. First, the study of anti- idiotypic Abs to hormones has to be explored because they take part in hormone-antibody interaction and could influence on the cell behavior through the membrane steroid receptors [22, 23, 30-34].
Secondly, it’s need to study Abs to the chemical carcinogens because their metabolites interact with Es-receptors [35-38] Abs to carcinogens were found in the blood serum of healthy donors and cancer patients [39-42] the levels of Abs to steroid hormones correlated with levels of Abs to chemical carcinogens in human [24].
In conclusions, the levels of Es and Pg in the blood serum of postmenopausal women depend on the levels of specific autoantibodies. The individual IgA-Pg/IgA-Es ratios influence on the Pg/Es ratios in HW more strong than in BCP. The comprehensive analysis of auto-Abs to endogenous steroids and environmental carcinogens together with corresponding anti-idiotypic Abs will be helpful for the further study of immune-hormonal disbalance in human carcinogenesis.
Acknowledgements
We thank the many people who participated in these studies as researches, collaborators and donors.
Funding/Support
This study was funded by the Ministry of Science and Higher Education of Russian Federation (state assignment № 0286-2022-0008), project № VI.59.1.1. of Siberian Branch of Russian Academy of Sciences.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Evaluation of serum estrogen-DNA adducts as potential biomarkers for breast cancer risk Pruthi Sandhya, Yang Li, Sandhu Nicole P., Ingle James N., Beseler Cheryl L., Suman Vera J., Cavalieri Ercole L., Rogan Eleanor G.. The Journal of steroid biochemistry and molecular biology.2012;132(1-2). CrossRef
- Mechanisms of estrogen carcinogenesis: The role of E2/E1-quinone metabolites suggests new approaches to preventive intervention--A review Yager James D.. Steroids.2015;99(Pt A). CrossRef
- Critical depurinating DNA adducts: Estrogen adducts in the etiology and prevention of cancer and dopamine adducts in the etiology and prevention of Parkinson's disease Cavalieri Ercole L., Rogan Eleanor G., Zahid Muhammad. International Journal of Cancer.2017;141(6). CrossRef
- Effect of Immunization with Estrone-Protein Conjugate in Rhesus Monkeys1 Sundaram K., Tsong Y. Y., Hood W., Brinson A.. Endocrinology.1973;93(4). CrossRef
- The biological effects of injected antibodies to estradiol-17 beta and to progesterone in pregnant rats Csapo A., Dray F., Erdos T.. Endocrinology.1975;97(3). CrossRef
- Effects of active immunisation against steroids upon circulating hormone concentrations Hillier S. G., Groom G. V., Boyns A. R., Cameron E. H.. Journal of Steroid Biochemistry.1975;6(3-4). CrossRef
- A comparison of the effects of active immunization of female rhesus monkeys to estradiol-17 or progesterone-20-protein conjugates Schwartz U., Dyrenfurth I., Khalaf S., Wiele R. L. Vande, Ferin M.. Journal of Steroid Biochemistry.1975;6(3). CrossRef
- Endocrine and reproductive repercussions of immunization against progesterone and oestradiol in female rats Kaushansky A., Bauminger S., Koch Y., Lindner H. R.. Acta Endocrinologica.1977;84(4). CrossRef
- Effects of active immunization against oestradiol-17 beta, testosterone or progesterone on receptivity in the female rabbit and evaluation of specificity Elsaesser F.. Journal of Reproduction and Fertility.1980;58(1). CrossRef
- Increased Luteinizing Hormone Secretion and Ovarian Function in Heifers Actively Immunized against Estrogen and Progesterone2 Chang Ching-Fong, Roberts A. J., Reeves Jerry J.. Journal of Animal Science.1987;65(3). CrossRef
- Survival of Tumours after Immunization against Oestrogens Caldwell Burton V., Tillson Stephen A., Esber Henry, Thorneycroft Ian H.. Nature.1971;231(5298). CrossRef
- Anti-estrogen antibodies in systemic lupus erythematosus: a quantitative evaluation of serum levels Counihan K. A., Vertosick F. T., Kelly R. H.. Immunological Investigations.1991;20(3). CrossRef
- Beaumont V, Malinow MR, Sexton G, et al (1992). Hyperhomocyst(e)inemia, anti-estrogen antibodies and other risk factors for thrombosis in women on oral contraceptives. Atherosclerosis, 94, 147-52. .
- Menzhinskaya IV, Gladkova KA, Sidelnikova VM, Sukhikh GT (2008). Antiprogesterone antibodies in clinic of habitualloss of pregnancy. Immunology, 29, 34-7. (in Russian) .
- Progesterone autoimmune dermatitis with positive autologous serum skin test result García-Ortega Pilar, Scorza Enrique. Obstetrics and Gynecology.2011;117(2 Pt 2). CrossRef
- Hormone allergy Roby Russell R., Richardson Richard H., Vojdani Aristo. American Journal of Reproductive Immunology (New York, N.Y.: 1989).2006;55(4). CrossRef
- Steroid hormone hypersensitivity: clinical presentation and management Itsekson Alek M., Seidman Daniel S., Zolti Matityahu, Alesker Michael, Carp Howard J. A.. Fertility and Sterility.2011;95(8). CrossRef
- Recurrent pregnancy loss and inappropriate local immune response to sex hormones Itsekson Alek M., Seidman Daniel S., Zolti Matityahu, Lazarov Anneta, Carp Howard J. A.. American Journal of Reproductive Immunology (New York, N.Y.: 1989).2007;57(2). CrossRef
- Autoantibodies to estrogen receptor α interfere with T lymphocyte homeostasis and are associated with disease activity in systemic lupus erythematosus Colasanti Tania, Maselli Angela, Conti Fabrizio, Sanchez Massimo, Alessandri Cristiano, Barbati Cristiana, Vacirca Davide, Tinari Antonella, Chiarotti Flavia, Giovannetti Antonello, Franconi Flavia, Valesini Guido, Malorni Walter, Pierdominici Marina, Ortona Elena. Arthritis and Rheumatism.2012;64(3). CrossRef
- Autoantibodies to estrogen receptor α in systemic sclerosis (SSc) as pathogenetic determinants and markers of progression Giovannetti Antonello, Maselli Angela, Colasanti Tania, Rosato Edoardo, Salsano Felice, Pisarri Simonetta, Mezzaroma Ivano, Malorni Walter, Ortona Elena, Pierdominici Marina. PloS One.2013;8(9). CrossRef
- Natural antiestrogen receptor autoantibodies in man with estrogenic activity in mammary carcinoma cell culture: study of their mechanism of action; evidence for involvement of estrogen-like epitopes Tassignon J., Haeseleer F., Borkowski A.. The Journal of Clinical Endocrinology and Metabolism.1997;82(10). CrossRef
- Autoantibodies to estrogen receptors and their involvement in autoimmune diseases and cancer Ortona Elena, Pierdominici Marina, Berstein Lev. The Journal of Steroid Biochemistry and Molecular Biology.2014;144 Pt B. CrossRef
- Autoantibodies specific to estrogen receptor alpha act as estrogen agonists and their levels correlate with breast cancer cell proliferation Maselli Angela, Capoccia Sara, Pugliese Patrizia, Raggi Carla, Cirulli Francesca, Fabi Alessandra, Malorni Walter, Pierdominici Marina, Ortona Elena. Oncoimmunology.2016;5(2). CrossRef
- Immunological disbalance in carcinogenesis Glushkov Andrey N.. Medical Hypotheses.2014;83(2). CrossRef
- Immunomodulation of Human Carcinogenesis by the Blood Serum Antibodies against Benzo[a]pyrene, Estradiol and Progesterone Glushkov Andrey N., Polenok Elena G., Ustinov Valentin A.. Open Journal of Immunology.2016;6(3). CrossRef
- Immunomodulation of carcinogens-induced steroids-dependent human diseases Glushkov Andrew N., Polenok Elena G.. Saudi Journal of Biological Sciences.2019;26(2). CrossRef
- Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation Hajian-Tilaki Karimollah. Caspian Journal of Internal Medicine.2013;4(2).
- Risk factors for breast cancer according to estrogen and progesterone receptor status Colditz Graham A., Rosner Bernard A., Chen Wendy Y., Holmes Michelle D., Hankinson Susan E.. Journal of the National Cancer Institute.2004;96(3). CrossRef
- Progesterone receptor modulates estrogen receptor-α action in breast cancer Mohammed Hisham, Russell I. Alasdair, Stark Rory, Rueda Oscar M., Hickey Theresa E., Tarulli Gerard A., Serandour Aurelien A. A., Birrell Stephen N., Bruna Alejandra, Saadi Amel, Menon Suraj, Hadfield James, Pugh Michelle, Raj Ganesh V., Brown Gordon D., D’Santos Clive, Robinson Jessica L. L., Silva Grace, Launchbury Rosalind, Perou Charles M., Stingl John, Caldas Carlos, Tilley Wayne D., Carroll Jason S.. Nature.2015;523(7560). CrossRef
- Nongenomic effects of an anti-idiotypic antibody as an estrogen mimetic in female human and rat osteoblasts Sömjen D., Kohen F., Lieberherr M.. Journal of Cellular Biochemistry.1997;65(1). CrossRef
- Antibodies to the estrogen receptor-alpha modulate rapid prolactin release from rat pituitary tumor cells through plasma membrane estrogen receptors Norfleet A. M., Clarke C. H., Gametchu B., Watson C. S.. FASEB journal: official publication of the Federation of American Societies for Experimental Biology.2000;14(1). CrossRef
- Human spermatozoa as a model for studying membrane receptors mediating rapid nongenomic effects of progesterone and estrogens Luconi M., Francavilla F., Porazzi I., Macerola B., Forti G., Baldi E.. Steroids.2004;69(8-9). CrossRef
- Non-genomic membrane progesterone receptors on human spermatozoa Modi D. N., Shah C., Puri C. P.. Society of Reproduction and Fertility Supplement.2007;63.
- Role of ERα36 in membrane-associated signaling by estrogen Chaudhri Reyhaan A., Schwartz Nofrat, Elbaradie Khairat, Schwartz Zvi, Boyan Barbara D.. Steroids.2014;81. CrossRef
- Estrogenic/Antiestrogenic Activities of Benzo[a]pyrene Monohydroxy Derivatives Hirose Toshiharu, Morito Keiko, Kizu Ryoichi, Toriba Akira, Hayakawa Kazuichi, Ogawa Sumito, Inoue Satoshi, Muramatsu Masami, Masamune Yukito. Journal of Health Science.2001;47(6). CrossRef
- Effect of estrogen receptor (ER) on benzo[a]pyrene-DNA adduct formation in human breast cancer cells Kang Se Chan, Lee Byung Mu. Journal of Toxicology and Environmental Health. Part A.2005;68(21). CrossRef
- Estrogen promotes benzo[a]pyrene-induced lung carcinogenesis through oxidative stress damage and cytochrome c-mediated caspase-3 activation pathways in female mice Chen Zhaoli, Zhang Yunxiao, Yang Jie, Jin Min, Wang Xin-Wei, Shen Zhi-Qiang, Qiu Zhigang, Zhao Guofan, Wang Jingfeng, Li Jun-Wen. Cancer Letters.2011;308(1). CrossRef
- ERα phenotype, estrogen level, and benzo[a]pyrene exposure modulate tumor growth and metabolism of lung adenocarcinoma cells Lin Susana, Lin Chun-Ju, Hsieh Dennis P. H., Li Lih-Ann. Lung Cancer (Amsterdam, Netherlands).2012;75(3). CrossRef
- Detection of antibodies to the benzo(a)pyrene diol epoxide-DNA adducts in sera from individuals exposed to low doses of polycyclic aromatic hydrocarbons Galati R., Zijno A., Crebelli R., Falasca G., Tomei F., Iecher F., Carta P., Verdina A.. Journal of experimental & clinical cancer research: CR.2001;20(3).
- Circulating antibodies directed against "polycyclic aromatic hydrocarbon-like" structures in the sera of cancer patients Pouns Olivier, Mangas Arturo, Coveñas Rafael, Geffard Michel. Cancer Epidemiology.2009;33(1). CrossRef
- The relevance of monitoring of antibodies against the polycyclic aromatic hydrocarbon (PAH) and PAH-DNA adducts in serum in relation to lung cancer and chronic obstructive pulmonary disease (COPD) Pauk N., Klimesova S., Kara J., Topinka J., Labaj J.. Neoplasma.2013;60(2). CrossRef
- Serum level of antibody against benzo[a]pyrene-7,8-diol-9,10-epoxide-DNA adducts in people dermally exposed to PAHs Borska Lenka, Andrys Ctirad, Krejsek Jan, Palicka Vladimir, Chmelarova Marcela, Hamakova Kvetoslava, Kremlacek Jan, Borsky Pavel, Fiala Zdenek. Journal of Immunology Research.2014;2014. CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2022
Author Details