Squamous Cancer of the Esophagus in Africa: A Causal Pathway Established
Download
Abstract
Squamous cancer of the esophagus has been endemic in much of East, Central and Southern Africa since the 1940s. Much research has concentrated on attempts to identify major carcinogenic influences, and failure to do so has made it clear that the problem in high incidence areas (HIAs) is not primarily of potent environmental carcinogens, but of population susceptibility. In Africa the association with maize is constant and strong. Research in the last decade has helped to explain that association. Considered along with historical findings there is now enough evidence to establish causal associations of a nutritionally deficient maize diet and use of maize meal with squamous cancer of the esophagus. Evidence is available in a high incidence area of degenerating maize meal resulting in excess production of PGE2, gastric hypochlorhydria and a predominant pattern of non-acid gastroesophageal reflux. This pathway explains the existence of major population susceptibility: a poor maize-based diet provides specific nutritional deficiencies and n-6 fatty acid dominance which cause failure of homeostasis of the arachidonic acid cascade; with this background chemically degenerating maize meal then triggers duodenogastric reflux and non-acid gastro-esophageal reflux; non-acid reflux causes squamous cancer of the esophagus. Within a susceptible population, environmental carcinogens including tobacco increase individual risk. There is sufficient evidence that this is an active pathway, and the dominant pathway to SCCE in high incidence areas in Africa.There is sufficient evidence to justify appropriate preventative measures.
Introduction
Squamous cell carcinoma of the esophagus (SCCE) emerged with a sudden explosive rise in incidence in South Africa, spread to much of Southern, East and Central Africa in the 1940s to 1970s, and has remained highly prevalent within parts of the region since then [1-3]. Incidences remain high throughout East, Central and Southern Africa, peaking currently in Malawi. SCCE is a devastating disease in Africa, not amenable to cure or even satisfactory palliation in many of the affected regions.
The association of maize with SCCE in Africa is constant and strong, and has been the subject of speculation and hypothesis for many decades. Lack of certainty about the place of maize in the genesis of endemic SCCE led to a belief that the association may be spurious. The last decade has brought new evidence which clarifies that association. Key new findings have included demonstration of a causal role of non-acid reflux (NAR) in SCCE [4, 5], and demonstration of NAR in an affected community [6].
In this article I piece together strands of historical and recent research and put forward what I believe is no longer a hypothesis, but a satisfactorily evidenced pathway to SCCE, the dominant pathway to SCCE in high incidence areas (HIAs) in Africa, and an impetus for preventive action. The pathway is illustrated with evidence from HIAs.
Non-acid reflux
Two studies have shown evidence of an association between NAR and SCCE. Uno et al [4] in Japan found that NAR episodes were significantly higher in SCCE patients than in controls. The number of NAR episodes was significantly proportional to intragastric pH level. Kgomo et al [5] in South Africa found significantly more NAR in their SCCE group than in controls. Both these studies concluded that NAR is part of the causal pathway of SCCE (Figure 1).
Figure 1. A Causal Pathway to Squamous Cancer of the Oesophagus in Africa.
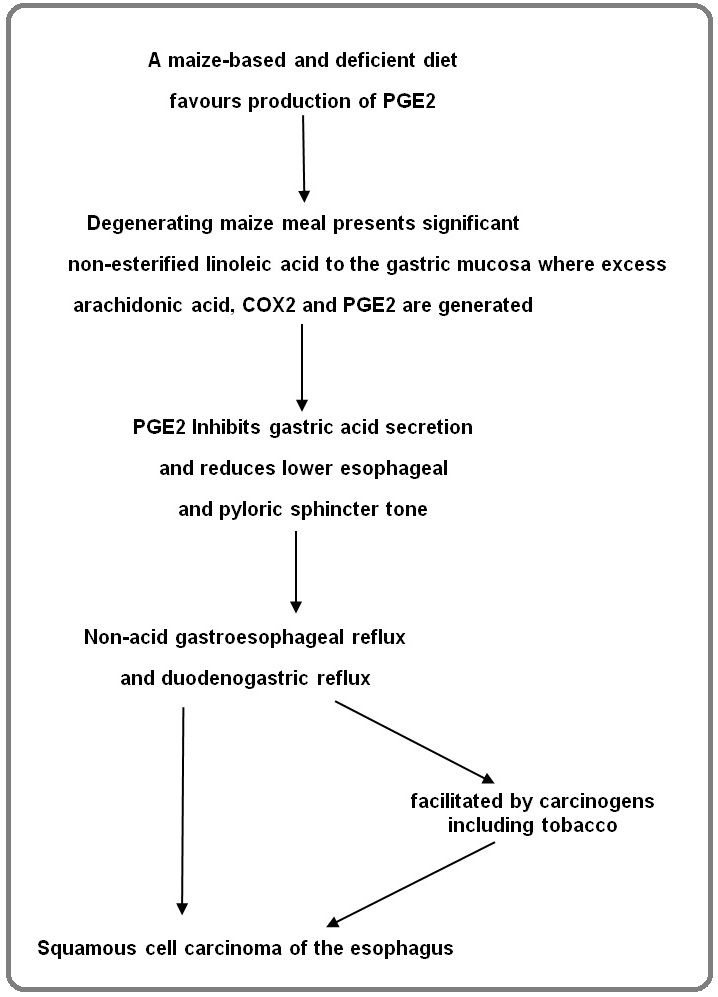
In related findings Iijima et al [7], also in Japan, found a strong association between SCCE patients and profound hypochlorhydria. They commented that ‘a lower level of gastric acid secretion, especially profound hypochlorhydria, was a strong risk factor for ESCC’ (SCCE). The association between gastric atrophy and SCCE is further diminished by a study showing no increased risk of SCCE with increasing histological changes of atrophy [8] supporting hypochlorhydria as the true risk factor for SCCE.
None of these studies examined the content of the refluxate. Evidence of the insufficiency of isolated NAR to cause SCCE is offered by the fact that proton pump inhibitors promote NAR but their widespread use has not resulted in an epidemic of SCCE in users. Animal studies have consistently shown that upper intestinal juice is strongly mitogenic to the oesophagus and that the combination of reflux of upper GI juices particularly pancreatic juice, in the absence of gastric acid, is a potent agent of squamous carcinogenesis [9, 10].
Maize
In very high incidence areas throughout the world people subsist on a nutritionally deficient monocereal diet of either wheat or maize [1]. In Africa there is a consistent and strong relationship between maize and high incidences [1]. It is of note that there was no significant incidence of SCCE in the nineteenth century in regions where maize was the staple diet [11]. Pellagra and kwashiorkor, diseases that accompany an otherwise deficient maize diet were common at that time [11, 12]. However the upsurge in incidence of SCCE did not occur until the fourth and fifth decades of the twentieth century [13, 14]. There is no evidence of the emergence of a powerful carcinogen accompanying that explosive rise, but there are remarkable historical parallels between emergence of small-scale rural milling technology, the change in dietary predominance from whole maize to maize meal [11], and the sudden growth in SCCE incidence.
Case-control studies within maize-based regions have not identified maize consumption as a risk factor. However several studies in Africa and elsewhere have identified the use of maize in the form of maize meal as conferring a very significant risk of SCCE [15-17]. A study in Malawi showed the strongest association of SCCE with the most highly refined maize meal [18].
Maize and n-6 fatty acid dominance
Linoleic acid (LA), bound in triglyceride form, constitutes most of the fat content of maize. People in HIAs of Africa obtain the majority of their calories from maize, and may not choose to or be able to supplement this with sources of n-3 fat with the result that n-6 fatty acids predominate.
Dietary n-6 dominance causes arachidonic acid cascade overreactions throughout the body, which lead to excess conversion of LA to arachidonic acid and arachidonic acid to PGE2 [19].
This shift of the arachidonic acid cascade towards production of PGE2 can be amplified by other dietary deficiencies in regions where the diet is almost exclusively based on maize, and consumed as maize meal products. Maize meal loses up to 60% of its fat content in the milling process. This accentuates the deficiency of n-3 fats. Milling also involves loss of vitamins and trace elements.
The consequences of deficiency of n-3 fatty acids may be augmented by deficiency of selenium [20] and of zinc [21], each of which upregulate COX-2, which in turn increases throughput of LA to PGE2. These deficiencies are common to HIAs [22].
Excess production of PGE2 has been found in a HIA in the former Transkei region of South Africa where salivary levels were investigated in a religious community who lived on maize and maize products, and chose not to eat any additional fat. Their salivary PGE2 was significantly higher than people in the same area who consumed a maize-based diet but used added fat regularly, and also significantly higher than the PGE2 level in UK controls eating a western diet [23].
Change of diet from whole maize to milled maize
In the late 1920s and early 1930s small hammer mills became commercially available, at first in the Eastern Cape of South Africa, and were distributed to rural general stores. Rural farmers could bring their maize for milling,
and take home finely ground maize meal. Significant quantities were milled, stored in homes and used over the course of weeks or even months [11]. Maize meal is chemically different from whole maize. The process of milling releases lipases which mix with and react with the fat content resulting in hydrolysis of esterified fatty acids to the free form. This occurs rapidly in maize and in wheat for the first few months after milling [24]. The progressive change to non-esterified fatty acids has been measured as up to 363 mg of free LA per 100g of prepared maize-meal based food [25]. Consumption of 1Kg per day of prepared maize meal or maize flour products provides a daily intake of up to 3.6 grams of non-esterified LA.
Non-esterified LA, when presented to the gastric mucosa is effective in small amounts, and rapid in its action: LA added to rat gastric mucosa cells increased the concentration of arachidonic acid, induced expression of COX-2 and augmented production of PGE2. These were time and dose-dependent increases. COX-2 mRNA was induced I hour after LA addition, and was maintained for 24 hours [26]. In a study with human volunteers 3g of LA was administered to healthy human volunteers. They showed a marked rise in both gastric PGE and 13,14-dihydro 15-keto prostaglandin E2 [27].
In a situation of n-6 dominance and arachidonic acid cascade overreaction, these responses to non-esterified linoleic acid may be greatly augmented and the consequences of excess production of PGE2 found.
Evidence from high incidence areas
Fatty acid imbalance is central to the failure of homeostasis of the arachidonic acid cascade. Supplement of the diet with other fats in a HIA has shown a significant protective effect against SCCE in case-control studies. Van Rensburg et al [15] found a reduced risk for those consuming margarine or butter daily. Sammon [28] found a protective effect of total bought fat against SCCE.
Alkali treatment of maize neutralises free fatty acids in maize meal and maize oil, and improves availability of trace elements and vitamins. In major contrast to maize- dependent HIAs of Africa, the incidence of SCCE is low or intermediate in Central and South America countries where an alkali process of nixtamalization is routinely used.
Two foodstuffs made from maize meal (amarewu, a fermented maize meal drink and umqa wethanga, a maize meal and pumpkin dish) caused heartburn in 60% of a sample of users in the former Transkei region. Of these who experienced heartburn 73 % regurgitated fluid into the mouth. This was prevented or reduced by aspirin taken 20 minutes before ingestion of the food. This is highly suggestive of a rapid prostaglandin effect [29].
PGE2 reduces acid secretion [30], relaxes the lower esophageal sphincter in a dose -dependent way [31] and relaxes the pyloric sphincter [32]. Inhibition of acid secretion and lower esophageal sphincter relaxation cause NAR. In a HIA gastric juice was aspirated from the gastric fundus of volunteers attending a rural clinic. Half of the samples had a pH over 4. High pH was significantly associated with frequency of maize consumption [33].
Recording of 24hr gastric pH was carried out in asymptomatic volunteers in the Eastern Cape of South Africa. The subjects had a raised median 24-hour pH of 2.84 and a night-time median pH of 3.7. There were abnormally long periods of night time alkalinisation [34].
NAR was predominant in an esophageal impedance study of 77 asymptomatic volunteers in a HIA in South Africa, and there was a higher frequency of gastroesophageal reflux compared with equivalent studies in Europe, China and USA [6 ].
The study by Kgomo et al [5] cited above was carried out in a high-risk community in South Africa. There was a significant association between NAR and SCCE.
Other factors
Tobacco has a proven association with SCCE in HIAs. The amount of tobacco used is low in some HIAs and there are significant percentages of non-smokers (up to 30% in South Africa) amongst SCCO victims [28, 35]. Alcohol does not have a consistent and clearly established role in HIAs [36]. Many other potential carcinogens have been suggested, including polycyclic aromatic hydrocarbons, and these substances may be active in HIAs, but there is none with consistently proven association [36].
While tobacco is significantly active as a carcinogen in HIAs, the number of non-users of tobacco who have no other known potent carcinogenic influence is high. This makes it probable that, as shown in animal studies [9, 10] NAR with upper gastrointestinal content exerts significant carcinogenic influence on the oesophageal mucosa even when there is no additional carcinogenic agent. A study of 24 hr gastric pH in a HIA found a high pH
/ gastric alkalinisation and unusually prolonged night-time alkalinisation. There were many rapid alkaline rises. The findings were suggestive of duodenogastric reflux [34]. A case-control study of diet and social factors in a HIA found no association between the cultural habit of self- induced vomiting and SCCO, but noted that the majority of those who used self-induced vomiting regurgitated bile when they vomited, evidence of the prevalence of duodenogastric reflux [34].
Discussion
Hypotheses to explain the very high levels of SCCE in regions of Africa have been put forward over the course of many years. Much research has concentrated on attempts to identify potential carcinogenic influences, and failure to do so has made it more obvious year by year that the problem in high incidence areas (HIAs) is not primarily of potent environmental carcinogens, but of population susceptibility.
The pathway outlined here is now well evidenced and established beyond reasonable doubt as fact, no longer as hypothesis. Evidence has been presented in a HIA of degenerating maize meal, excess production of PGE2, gastric acid suppression, and a predominant pattern of NAR. This pathway is sufficient to explain the existence of major population susceptibility: a maize-based diet provides the necessary nutritionally deficient background; chemically degenerating maize meal triggers NAR; NAR causes SCCE.
The evidence is sufficient to justify appropriate preventative measures.
The evidence presented applies widely to areas of East, Central and Southern Africa which depend heavily on maize. SCCE incidence is very high in monocereal cultures in some other parts of the world, and consideration could be given to whether similar mechanisms may be involved.
The importance of chronic micronutrient deficiencies in causing SCCE susceptibility in Africa as emphasized by van Rensburg and van Rensburg [22] needs assessed against inconsistent results from cohort interventions with diet supplements in HIAs in China [37].
In conclusion, there is sufficient evidence to establish causal associations of a nutritionally deficient maize diet and use of maize meal with NAR, and NAR with SCCE. There is sufficient evidence that this is an active pathway and the dominant pathway to SCCE in high incidence areas in Africa. It is no longer necessary that endemic SCCE should persist. The principal causal associations are known, are remediable, and should now be tackled.
References
- Epidemiologic and Dietary Evidence for a Specific Nutritional Predisposition to Esophageal Cancer Rensburg SJ . JNCI: Journal of the National Cancer Institute.1981;67(2). CrossRef
- The incidence of oesophageal cancer in Eastern Africa: identification of a new geographic hot spot? Cheng ML , Zhang L, Borok M, Chokunonga E, Dzamamala C, Korir A, Wabinga HR , Hiatt RA , Parkin DM , Van Loon K. Cancer Epidemiology.2015;39(2). CrossRef
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries Sung H, Ferlay J, Siegel RL , Laversanne M, Soerjomataram I, Jemal A, Bray F. CA: a cancer journal for clinicians.2021;71(3). CrossRef
- Direct measurement of gastroesophageal reflux episodes in patients with squamous cell carcinoma by 24-h pH-impedance monitoring Uno K, Iijima K, Hatta W, Koike T, Abe Y, Asano N, Kusaka G, Shimosegawa T. The American Journal of Gastroenterology.2011;106(11). CrossRef
- Non-acid gastro-oesophageal reflux is associated with squamous cell carcinoma of the oesophagus Kgomo M, Mokoena TR , Ker JA . BMJ Open Gastroenterology.2017;4(1). CrossRef
- Normal values of 24-hour ambulatory esophageal impedance-pH monitoring in a rural South African cohort of healthy participants Ndebia E. J., Sammon A. M., Umapathy E., Iputo J. E.. Diseases of the Esophagus: Official Journal of the International Society for Diseases of the Esophagus.2016;29(4). CrossRef
- Gastric hyposecretion in esophageal squamous-cell carcinomas Iijima K., Koike T., Abe Y., Yamagishi H., Ara N., Asanuma K., Uno K., Imatani A., Nakaya N., Ohara S., Shimosegawa T.. Digestive Diseases and Sciences.2010;55(5). CrossRef
- Increased risk of esophageal squamous cell carcinoma in patients with gastric atrophy: independent of the severity of atrophic changes Vries AC , Capelle LG , Looman CWN , Blankenstein M, Grieken NCT , Casparie MK , Meijer GA , Kuipers EJ . International Journal of Cancer.2009;124(9). CrossRef
- Histopathological findings of the lower esophagus after total gastrectomy in rat Aoyagi K., Kohfuji K., Yano S., Murakami N., Hori H., Terasaki Y., Takeda J., Shirouzu K.. The Kurume Medical Journal.1999;46(3-4). CrossRef
- The role of alkaline reflux in esophageal carcinogenesis induced by N-amyl-N-methylnitrosamine in rats Seto Y., Kobori O., Shimizu T., Morioka Y.. International Journal of Cancer.1991;49(5). CrossRef
- Squamous cancer of the oesophagus in Africa Sammon AM .. Retrieved from www.scoafrica.org 2009..
- Maize and grace. Harvard University Press. Cambridge McCann JC . Massachusetts. 2005..
- Epidemiology of oesophageal cancer in Southern Africa Rose EF . Adv Med Oncol Res Ed.1979;9:317-326.
- Cancer of the Oesophagus in Africa Cook P. British Journal of Cancer.1971;25(4).
- Oesophageal cancer in Zulu men, South Africa: a case-control study Van Rensburg S. J., Bradshaw E. S., Bradshaw D., Rose E. F.. British Journal of Cancer.1985;51(3). CrossRef
- 1982 Rilievi epidemiologici sul cancro esofageo nella Regione Veneto. [Epidemiological surveys on esophageal cancer in the Veneto region.] Rossi M, Ancona E, Mastrangelo G, Solimbergo D, Paruzzolo P, Azzarini G, et al . Minerva Medica.1982;73:1531-1540 (Italian).
- Maize and risk of cancers of the oral cavity, pharynx, and esophagus in northeastern Italy Franceschi S., Bidoli E., Barón A. E., La Vecchia C.. Journal of the National Cancer Institute.1990;82(17). CrossRef
- Environmental risk factors for oesophageal cancer in Malawi: A case-control study Mlombe Y. B., Rosenberg N. E., Wolf L. L., Dzamalala C. P., Chalulu K., Chisi J., Shaheen N. J., Hosseinipour M. C., Shores C. G.. Malawi Medical Journal: The Journal of Medical Association of Malawi.2015;27(3). CrossRef
- Omega-3 PUFAs Lower the Propensity for Arachidonic Acid Cascade Overreactions Lands B. BioMed Research International.2015;2015. CrossRef
- Selenium Deficiency-Induced Inflammation and Increased Expression of Regulating Inflammatory Cytokines in the Chicken Gastrointestinal Tract Gao X, Zhang Z, Xing H, Yu J, Zhang N, Xu S. Biological Trace Element Research.2016;173(1). CrossRef
- Dietary zinc modulation of COX-2 expression and lingual and esophageal carcinogenesis in rats Fong LYY , Zhang L, Jiang Y, Farber JL . Journal of the National Cancer Institute.2005;97(1). CrossRef
- Esophageal squamous cell cancer susceptibility: environmental and nutritional associations reveal a universally applicable pathogenesis scenario (Review) van Rensburg Schalk J, van Rensburg Susan J. World Acad Sci J .2019;1:219-228. CrossRef
- Dietary fat and salivary prostaglandin E2 Sammon AM , Morgan A. Prostaglandins & Other Lipid Mediators.2002;67(2). CrossRef
- Storage releases physiologically active content in milled maize and wheat Sammon AM , Whittington FM . African Journal of Food Science.2009;3(12). CrossRef
- Maize meal, non-esterified linoleic acid, and endemic cancer of the esophagus - preliminary findings Sammon AM. Prostaglandins and other lipid mediators .1999;57(2-3):167-171. CrossRef
- Helicobacter pylori alters n-6 fatty acid metabolism and prostaglandin E2 synthesis in rat gastric mucosal cells Nakaya A., Wakabayashi H., Imamura L., Fukuta K., Makimoto S., Naganuma K., Orihara T., Minemura M., Shimizu Y., Nagasawa T., Hamazaki T., Watanabe A.. Journal of Gastroenterology and Hepatology.2001;16(11). CrossRef
- Dietary linoleic acid, gastric acid, and prostaglandin secretion Grant H. W., Palmer K. R., Kelly R. W., Wilson N. H., Misiewicz J. J.. Gastroenterology.1988;94(4). CrossRef
- 1992 A case-control study of diet and social factors in cancer of the esophagus in Transkei Sammon, A. M. Cancer .1992;69:860-865. CrossRef
- Heartburn in Transkei – letter to the editor Sammon AM . Dis Oesophagus.1994;7:20.
- Oral PGE2 inhibits gastric acid secretion in man Befrits R., Johansson C.. Prostaglandins.1985;29(1). CrossRef
- Effect of prostaglandin E2 on esophageal motility in man Mukhopadhyay A., Rattan S., Goyal R. K.. Journal of Applied Physiology.1975;39(3). CrossRef
- Effects of aspirin and prostaglandin E2 on interdigestive motility complex and duodenogastric reflux in man Dooley C. P., Mello W. D., Valenzuela J. E.. Digestive Diseases and Sciences.1985;30(6). CrossRef
- Bimodal distribution of fasting gastric acidity in a rural African population Sammon A. M., Mguni M., Mapele L., Awotedu K. O., Iputo J. E.. South African Medical Journal = Suid-Afrikaanse Tydskrif Vir Geneeskunde.2003;93(10).
- 24-Hour Measurement of Gastric pH in Rural South Africa Sammon A, Ndebia E, Umapathy E, Iputo J. Gastroenterology Research and Practice.2015;2015. CrossRef
- Tobacco and alcohol as risk factors for oesophageal cancer in a high incidence area in South Africa Sewram V, Sitas F, O'Connell D, Myers J. Cancer Epidemiology.2016;41. CrossRef
- Aetiology of Oesophageal Cancer in Africa - A Review of Historical and Current Evidence Sammon A, Ndebia E. Global Journal of Health Science.2019;11. CrossRef
- Epidemiology of Esophageal Squamous Cell Carcinoma Abnet CC , Arnold M, Wei W. Gastroenterology.2018;154(2). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2022
Author Details