A Potentially Less Nephrotoxicity of Carboplatin Over Cisplatin as Radiosensitizer in Head-neck Cancer
Download
Abstract
Introduction: Head and neck cancers are the common cancers in developing countries, especially in Southeast Asia. Concurrent chemoradiation is the treatment of choice for locally advanced stage III to IVB squamous cell carcinoma of head and neck. Carboplatin can be used instead with reduced incidence of nephrotoxicity than Cisplatin.
Objectives: The study has been conducted to elucidate the reduced incidence of nephrotoxicity of Carboplatin as radiosensitizing agent instead of Cisplatin.
Materials and Methods: Total 60 patients were enrolled according to selection criteria among whom 30 patients received Cisplatin 40mg/m2 and Carboplatin AUC 2 along with 66Gy in 33 fractions of radiation. Every patient was evaluated routinely to see nephrotoxicity.
Results: Nephrotoxicity has been assessed at routine intervals in both arms. At first week of concurrent chemoradiotherapy 8.325% and 5.825% of patients developed nephrotoxicity in arm A and B respectively. On 2nd week follow-up 25% patients of arm A and 19.175% patients of arm B developed nephrotoxicity. The p-values over the whole period of chemoradiotherapy were not statistically significant (>0.05) but arm A patient who received Cisplatin showed more incidence of nephrotoxicity than arm B who received Carboplatin. In post chemoradiotherapy follow up, 10% patients of arm A and 1.65% patients of arm B retained some sorts of renal impairment but in 4th follow up 5% patients of arm A patient retained nephrotoxicity but on the other hand none of the arm B patients had nephrotoxicity at that time. During post chemoradiotherapy period p-values between both the arms were statistically significant (<0.05).
Conclusion: This study concluded that, due to low incidence of nephrotoxicity Carboplatin can be used as radiosensitizer instead of Cisplatin in locally advanced head-neck squamous cell carcinoma.
Introduction
Head and neck squamous cell carcinoma accounts for 90% of all malignant disease in the head and neck region of the body [1]. Nearly 60% of the population presents with locally advanced disease [2]. Head and neck cancers are mainly attributed to tobacco, areca nut, alcohol etc. [3].
Radiotherapy and concurrent chemotherapy represent the most commonly used strategy because some chemotherapeutic agents may both radio sensitize cells and provide additive cytotoxicity. Meta-Analysis of chemotherapy on Head and Neck Cancer demonstrated that the use of radiotherapy and concurrent chemotherapy resulted in a 19% reduction in the risk of death and an overall 6.5% improvement in 5-year survival compared to treatment with radiotherapy alone [2].
The platinum based (mainly Cisplatin and Carboplatin) concurrent chemoradiotherapy regimens can be used in head and neck cancers and cisplatin has priority over the other platinum-based drugs [1,4]. Concurrent chemoradiation with Cisplatin is the standard approach for resectable disease (where surgery followed by radiotherapy give same result) and for definitive management of unresectable locally advanced head and neck squamous cell carcinoma not only to increase loco-regional control but also decrease distal failure [5,6]. Carboplatin, though a platinum group of drugs, is generally well tolerated compared to Cisplatin [6].
The favorable toxicity profile and similar mechanism of action make it tempting to substitute Carboplatin for Cisplatin [6,7]. Significant Cisplatin induced toxicities include nausea and vomiting, nephrotoxicity, mucositis, dermatitis and potentially permanent ototoxicity [4,8]. Carboplatin is a second-generation platinum-based drug, has been frequently used to replace Cisplatin because of its similar mode of action, but lower rates of ototoxicity, nephrotoxicity and emesis [4,9]. The intricate anatomy and the critical functional and social roles of the head and neck region have no doubt also motivated significant efforts to identify alternatives to oncologic resection of malignant tumors in head and neck region [10].
This study was conducted to find out the incidence of nephrotoxicity in patients with locally advanced head and neck carcinoma by comparing concomitant use of weekly Cisplatin versus weekly Carboplatin with radiotherapy.
Materials and Methods
Patient population
With the institutional ethical committee permission, the study was conducted in the department of Radiation Oncology, NICRH, Dhaka, Bangladesh for a period of one year from 30th March 2018 to 29th March 2019 over 60 patients. We only included patients with an age range of more than 30 to less than 70 years having KPS of more than 70 with histologically proven squamous cell carcinoma of head and neck belonging to AJCC prognostic stage group of III to IVB. We excluded patients with primary tumors of nasopharynx, salivary glands, nasal cavity, paranasal sinuses, and unknown primaries or patients with non-squamous cell carcinomas. We also excluded patients who were treated previously with chemotherapy and/or radiotherapy, having multiple synchronous malignancies, recurrent disease, kidney and liver diseases.
Pretreatment evaluation
All of the patients underwent complete history, physical examination, necessary laboratory investigations and fitness evaluation with Karnofsky performance score.
Chemotherapy
We administered weekly Cisplatin at 40mg/m2 intravenously in 30 patients of Arm A as an outpatient basis along with adequate pre- and post-hydration, mannitol support. Rest of the 30 patients who belong to Arm B received weekly Carboplatin at AUC 2 intravenously.
Radiotherapy schedule
All patients received external beam radiotherapy of 66Gy in 33 fractions, 5 days in a week in two-dimensional treatment planning with parallel opposed fields using Linear Accelerator. The target treatment volume included primary tumor with adequate margins and regional cervical lymph nodes. Level IV cervical lymph node was treated with a separate low anterior neck field. The spinal cord was spared after 44Gy and if there was level V lymph node metastasis, it received electron therapy with appropriate energy (MeV) after field size reduction and the gap between photon and electron field was 0.5cm.
Nephrotoxicity evaluation
Nephrotoxicity was observed according to RTOG radiation morbidity criteria through hematological and biochemical investigations and assessed weekly for the whole period of concurrent chemoradiotherapy and then every six weeks for four times.
Data collection and statistical analysis
After collection of all information, these data were checked, verified and edited for a finalized result. Continuous data was presented as mean +/-SD while categorical data was presented as frequency and percentage. 95% confidence intervals were calculated for these values. After editing and coding, the coded data was directly entered into the computer and processed and analyzed with the help of SPSS for windows software v-16 and Microsoft Excel-2007. To see the association between various variables chi-square test, Fisher’s Exact test and t-test were used. P value of 0.05 or less was considered as significant.
Results
The mean age of the arm A patients was 54.30 (SD±6.69) years and that of Arm B patients was 51.56 (SD±10.23) years. Among 60 patients 81.67% were male and 18.33% patients were female. Most of the patients were farmers by profession in both groups, 12% and 11% respectively. Most of the patients in both arms were of low socio-economic background with 66.67% and 60% respectively.
Table 1 shows that at first week of concurrent chemoradiotherapy 8.325% and 5.825% of patients developed nephrotoxicity in arm A and B respectively.
| Schedule of assessment | Renal Impairment | Weekly Cisplatin plus EBRT (Arm A) | Weekly Carboplatin plus EBRT (Arm B) | p-value | ||
| Grade | n | % | n | % | ||
| After 1 st week of CCRT | 1 | 7 | 23.3 | 5 | 16.7 | |
| 2 | 2 | 6.7 | 1 | 3.3 | 0.817 | |
| 3 | 1 | 3.3 | 1 | 3.3 | ||
| 4 | 0 | 0 | 0 | 0 | ||
| After 2 nd week of CCRT | 1 | 8 | 26.7 | 7 | 23.3 | |
| 2 | 6 | 20 | 4 | 13.3 | 0.666 | |
| 3 | 2 | 6.7 | 1 | 3.3 | ||
| 4 | 1 | 3.3 | 0 | 0 | ||
| After 3 rd week of CCRT | 1 | 10 | 33.3 | 5 | 16.7 | |
| 2 | 2 | 6.7 | 2 | 6.7 | 0.269 | |
| 3 | 1 | 3.3 | 1 | 3.3 | ||
| 4 | 2 | 6.7 | 0 | 0 | ||
| After 4 th week of CCRT | 1 | 11 | 36.7 | 9 | 30 | |
| 2 | 2 | 6.7 | 1 | 3.3 | 0.483 | |
| 3 | 4 | 13.3 | 1 | 3.3 | ||
| 4 | 1 | 3.3 | 1 | 3.3 | ||
| After 5 th week of CCRT | 1 | 12 | 40 | 12 | 40 | |
| 2 | 10 | 33.3 | 6 | 20 | 0.052 | |
| 3 | 5 | 16.7 | 3 | 10 | ||
| 4 | 3 | 10 | 2 | 6.7 | ||
| After 6 th week of CCRT | 1 | 18 | 60 | 15 | 50 | |
| 2 | 5 | 16.7 | 5 | 16.7 | 0.084 | |
| 3 | 3 | 10 | 3 | 10 | ||
| 4 | 4 | 13.3 | 1 | 3.3 | ||
| After 7 th week of CCRT | 1 | 17 | 56.7 | 15 | 50 | |
| 2 | 4 | 13.3 | 3 | 10 | 0.192 | |
| 3 | 2 | 6.7 | 1 | 3.3 | ||
| 4 | 4 | 13.3 | 1 | 3.3 | ||
| 1 st follow up | 1 | 5 | 16.7 | 1 | 3.3 | |
| 2 | 4 | 13.3 | 1 | 3.3 | 0.031 | |
| 3 | 2 | 6.7 | 0 | 0 | ||
| 4 | 1 | 3.3 | 0 | 0 | ||
| 2 nd follow up | 1 | 5 | 16.7 | 1 | 3.3 | |
| 2 | 3 | 10 | 1 | 3.3 | 0.044 | |
| 3 | 2 | 6.7 | 0 | 0 | ||
| 4 | 1 | 3.3 | 0 | 0 | ||
| 3 rd follow up | 1 | 3 | 10 | 0 | 0 | |
| 2 | 2 | 6.7 | 0 | 0 | 0.024 | |
| 3 | 1 | 3.3 | 0 | 0 | ||
| 4 | 0 | 0 | 0 | 0 | ||
| 4 th follow up | 1 | 3 | 10 | 0 | 0 | |
| 2 | 2 | 6.7 | 0 | 0 | ||
| 3 | 1 | 3.3 | 0 | 0 | 0.024 | |
| 4 | 0 | 0 | 0 | 0 |
On 2nd week follow-up 25% patients of arm A and 19.175% patients of arm B developed nephrotoxicity. The p-values over the whole period of chemoradiotherapy were not statistically significant (>0.05) but arm A patient who received Cisplatin showed more incidence of nephrotoxicity than arm B who received Carboplatin. Figure 1 shows that, in post chemoradiotherapy follow up, 10% patients of arm A and 1.65% patients of arm B retained some sorts of renal impairment but in 4th follow up 5% patients of arm A patient retained nephrotoxicity but on the other hand none of the arm B patients had nephrotoxicity at that time.
Figure 1. Incidence of Nephrotoxicity in Arm A and B.
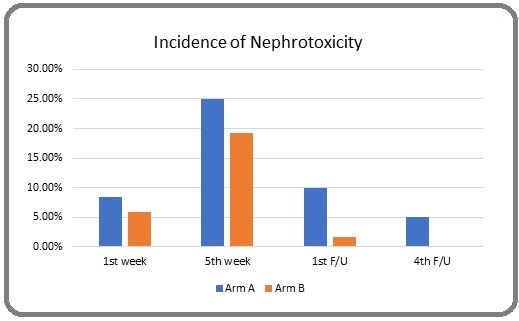
During post chemoradiotherapy period p-values between both the arms were statistically significant (<0.05).
Discussion
Cisplatin weekly with radiation is the standard agent however it causes nausea, vomiting, nephrotoxicity, mucositis, dermatitis and potentially permanent ototoxicity [4]. Moreover, Cisplatin has prolonged infusion time with monitoring of vigorous pre- and post-hydration and adequate potassium and magnesium replacement [11,12].
Carboplatin, though a platinum analogue having less nausea, vomiting, nephrotoxicity, ototoxicity and well tolerated by the patients but causes myelosuppression [13].
Cisplatin and Carboplatin both are excreted through kidney and may cause nephrotoxicity. In this study, after 1st week of concurrent chemoradiation 7 patients in arm A and 5 patients in arm B, 2 patients in arm Aand 1 patient in arm B and 1 patient in both arm A and B had grade 1, grade 2 and grade 3 toxicity, none of the patients in both arms had grade 4 toxicities. On subsequent follow up it is found that arm A patients developed more renal toxicity than arm B and after 7th week of CCRT, 13.3% patients of arm B had grade 1 toxicity whereas only 3.3% patients of arm B had grade 1 toxicity. During weekly follow up, p values are more than 0.05 that is statistically not significant though more patients in arm A had renal toxicity than arm B. After every 6 weeks following completion of concurrent chemoradiotherapy it is found that more patients of arm A had renal impairment than arm B and p values are 0.031, 0.044, 0.024 and 0.024 respectively and the differences are statistically significant. Meta-analysis comparing Cisplatin and Carboplatin based regimen in locally advanced squamous cell carcinoma of head-neck and Chemoradiation in locally advanced nasopharyngeal cancer comparing Cisplatin and Carboplatin also found statistically significant difference in renal impairment with Cisplatin [4,14].
Article published in Japanese Journal of Clinical Oncology in 2015 assessing safety and efficacy of concurrent Carboplatin plus radiotherapy in locally advanced head and neck cancers in 25 patients and found that concurrent Carboplatin plus radiotherapy is tolerated and may be an option in treating locally advanced squamous cell carcinoma of the head and neck patient’s ineligible for treatment with Cisplatin [15].
Carboplatin is currently in the WHO Essential Medicines List for Adults (2013, 18th Edition). Next to Carboplatin in the WHO List is a symbol that states that the listing of the drug indicates “similar clinical performance within a pharmacological class. The listed medicine should be the example of the class for which there is the best evidence for effectiveness and safety. Therapeutic equivalence is only indicated on the basis of reviews of efficacy and safety and when consistent with WHO clinical guidelines [16].
In conclusion, this study concludes that concurrent chemoradiotherapy with Carboplatin has less nephrotoxicity than Cisplatin in the treatment of locally advanced head and neck cancer hence can be used as an alternative radiosensitizing agent in locally advanced head and neck squamous cell cancer treatment.
References
- Devita, Hellman & Rosenberg’s Cancer: Principles and Practice of Oncology. 11th ed Devita VT , Lawrence TS , Rosenberg SA , editors . New York: Lippincott Williams & Wilkins.2019;:536-542.
- Principles and practice of radiation oncology. 7th ed Perez CA , Bradly LW . Philadelphia: Lippincott Williams & Wilkins.2019;:885.
- Head and neck cancers in developing countries Joshi Poonam, Dutta Sourav, Chaturvedi Pankaj, Nair Sudhir. Rambam Maimonides Medical Journal.2014;5(2). CrossRef
- A meta-analysis comparing cisplatin-based to carboplatin-based chemotherapy in moderate to advanced squamous cell carcinoma of head and neck (SCCHN) Guan Jian, Li Qinyang, Zhang Yue, Xiao Nanjie, Chen Min, Zhang Yaowei, Li Lu, Chen Longhua. Oncotarget.2016;7(6). CrossRef
- Evaluation of Cisplatin Induced Toxicity in Head and Neck Cancer and Cervical Cancer During Concurrent Chemoradiotherapy. Experience of National Institute of Oncology in Morocco Maghous A, Marnouche E, Loughlimi H, Rais F, Benhmidou . J Cancer Sci Ther.2017;9:314-318.
- Radical treatment of locally advanced head and neck cancer with concurrent chemoradiation-cisplatin versus carboplatin: A randomized comparative phase III trial Dutta S, Ghorai S, Choudhury K B , Majumder A. Clin Cancer Investig J.2013;2:122-127.
- Equivalence of cisplatin and carboplatin-based chemoradiation for locally advanced squamous cell carcinoma of the head and neck: a matched-pair analysis Wilkins A. C., Rosenfelder N., Schick U., Gupta S., Thway K., Nutting C. M., Harrington K. J., Newbold K., Bhide S. A.. Oral Oncology.2013;49(6). CrossRef
- Clinical recommendations for defining platinum unsuitable head and neck cancer patient populations on chemoradiotherapy: A literature review Ahn Myung-Ju, D'Cruz Anil, Vermorken Jan B., Chen Jo-Pai, Chitapanarux Imjai, Dang Huy Quoc Thinh, Guminski Alex, Kannarunimit Danita, Lin Tong-Yu, Ng Wai Tong, Park Keon-Uk, Chan Anthony Tak Cheung. Oral Oncology.2016;53. CrossRef
- Harrison’s Manual of Oncology.2nd ed Bruce A, Chabner , Dan L, Longo . New York: Mc Graw Hill Education.2014;:70.
- Combined Chemoradiation Therapy in the Treatment of Squamous Cell Carcinoma of the Head and Neck—An Evolving Paradigm Morris Z S , Mohindra P, Kruser T J . Oncology & Hematology Review.2013;9:115-121.
- South Asian Edition of Skeel’s Handbook of Cancer Therapy. 9th ed Khalif N. S , Rixe Olivier , Skeel T. R . Philadelphia: Lippincott Williams & Wilkins.2016;:112.
- Physicians’ Cancer Chemotherapy Drug Manual Chu E, DeVita T. V . Burlington: Jones and Bartlett Learning; 2019: 90..2019;:90.
- Manual of Clinical Oncology. 7th ed Denis A, Casciato , Mary C, Territo . Philadelphia: Lippincott Williams & Wilkins.2012;:64-65.
- Chemoradiation comparing cisplatin versus carboplatin in locally advanced nasopharyngeal cancer: randomised, non-inferiority, open trial Chitapanarux Imjai, Lorvidhaya Vicharn, Kamnerdsupaphon Pimkhuan, Sumitsawan Yupa, Tharavichitkul Ekkasit, Sukthomya Vimol, Ford Judith. European Journal of Cancer (Oxford, England: 1990).2007;43(9). CrossRef
- Safety and efficacy of concurrent carboplatin plus radiotherapy for locally advanced head and neck cancer patients ineligible for treatment with cisplatin Hamauchi Satoshi, Yokota Tomoya, Onozawa Yusuke, Ogawa Hirofumi, Onoe Tsuyoshi, Kamijo Tomoyuki, Iida Yoshiyuki, Nishimura Tetsuo, Onitsuka Tetsuro, Yasui Hirofumi. Japanese Journal of Clinical Oncology.2015;45(12). CrossRef
- Union for International Cancer Control, Review of Cancer Medicines on the WHO List of Essential Medicines 2014;:6.
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2022
Author Details