Expression of Vitamin D Receptor (VDR) in Urinary Bladder Carcinoma: Immunohistochemical and Histopathological Study
Download
Abstract
Background: Bladder cancer is the most common malignancy of the urinary tract. Calcitriol [1,25 (OH)2vitamin D3] has anticancer effects mediated through binding to vitamin D receptor (VDR). The expression of VDR is present in many normal and cancer tissues. But, there is little information about its expression in urinary bladder carcinoma. This study aimed to analyze VDR immunohistochemical expression in 74 Egyptian patients with urinary bladder carcinoma and to evaluate its association with different clinicopathological parameters.
Methods: Sections from formalin-fixed, paraffin-embedded tumor blocks were stained immunohistochemically using monoclonal anti-VDR antibody. VDR protein expression as well as its immunostaining patterns were recorded and scored separately in each case using semi-quantitative immunoreactive score.
Results: VDR was consistently expressed in the included histologically normal urothelium while tumor cells showed variable degrees of expression. Cytoplasmic/membranous VDR expression was common among the studied cases especially those with urothelial morphology (p = 0.076). While, the mean nuclear VDR was significantly (p = 0.007) higher in non-urothelial tumors. Nuclear VDR was significantly associated with muscle invasion (p = 0.000) and tumor stage (p = 0.001) in urothelial carcinoma. It was also statistically related to tumor grade, stage and muscle invasion in non-urothelial tumors (p = 0.002, 0.003 and 0.012, respectively).
Conclusion: there was a significant relation between nuclear VDR expression and prognostic markers suggesting its decrease as an indicator of a poorer prognosis. Vitamin D supplementation may represent a new treatment option for patients with bladder cancer.
Introduction
Incidence of urinary bladder cancer is steadily rising worldwide, particularly in developed countries [1]. It ranks as the 10th most common cancer with estimated 573,278 new cases diagnosed and 212,536 deaths in 2020, according to GLOBOCAN data [2]. In Egypt, urinary bladder tumors represent 14.31% of total malignancies with higher incidence among men Mokhtar et al. [3].
Urothelial carcinoma is certainly the most predominant histological type of bladder cancer [4]. But, other less common malignancies are encountered, including squamous cell carcinoma (2–5%), adenocarcinoma (0.5–2%), and small cell carcinoma (<1%) [5]. These subtypes are generally associated with worse clinical outcomes compared to urothelial carcinoma [6].
Both tumor grade and stage are important factors in directing treatment decisions [7]. Given the serious complications induced by traditional treatment options for bladder cancer patients, it is critical to provide novel therapies with less side effects and acceptable outcomes [8, 9].
Vitamin D is not only a hormone essential for calcium homeostasis [10]; it also produces various biological effects through both genomic and non-genomic pathways [11]. The genomic pathway is mediated via binding of Calcitriol (active form of vitamin D) to vitamin D receptor (VDR) that belongs to the steroid-thyroid-retinoid receptor gene superfamily. VDR is mainly a nuclear receptor and has been identified in many neoplastic and non-neoplastic tissues. In non-genomic pathway, vitamin D activates a number of cytoplasmic signaling pathways that affect cell proliferation, differentiation and apoptosis and may act with the classical genomic pathway to trans-activate VDR [12-14].
Many in vitro and in vivo studies have shown the anticancer effect of Calcitriol and VDR in a wide variety of malignancies including bladder carcinoma [15], head and neck cancer [16], colon, breast and lung [13], Thus, vitamin D supplementation, which is much less toxic and much more cost effective, deserves continued exploration for patients with bladder cancer [17].
In this viewpoint, we aimed to assess the immunohistochemical expression of VDR in different histologic subtypes of urinary bladder carcinoma and to evaluate the relation between VDR expression and the available clinicopathological characteristics.
Materials and Methods
Case selection
Tumor samples from 100 patients with histologically proven primary urinary bladder carcinoma were received at the Pathology laboratory at specialized medical Center, Faculty of Medicine, Beni-Suef University, Beni-Suef, Egypt between January 2019 and December 2020. All patients underwent a surgical procedure either radical cystectomy or transurethral resection of bladder tumor without receiving adjuvant chemo/radiotherapy before surgery. Out of them, 26 were excluded based on the following criteria: inadequacy, poor processing, extensive necrosis, absence of muscularis propria in biopsies with invasive tumor, and pT2 in biopsy.
So, this study consisted of 74 specimens of bladder cancer, obtained by transurethral resection (n= 34 cases) and radical cystectomy (n= 40 cases). Formalin-fixed, paraffin-embedded tissue blocks were retrieved from the archives of the Pathology Department.
The available clinicopathological data including age, sex, grade, muscle invasion and pathologic tumor stage of cases were collected from the pathology request sheets enclosed with the specimens. Patients’ data were completely anonymous and their names were replaced by numbers. This study was approved by Beni-Suef University Ethical Committee (CFM-BSUREC/01122019) and was performed in accordance with the Declaration of Helsinki.
Histopathology
Hematoxylin and Eosin slides for each case were reviewed independently by two pathologists to confirm tumor histology and grade according to the WHO histological classification of tumors of the urinary tract [18], pathologic stage according to the Tumor Node Metastasis (TNM) system of the American Joint Committee on Cancer (AJCC), 8th edition Amin et al. [19], and lymph node metastasis in radical cystectomy cases. Details concerning the demographic and histopathologic characteristics of the studied cases are given in (Table 1).
| Characteristics | Number of patients (%) | VDR expression | |
| Low (n=26) (%) | gh (n=48) (%) | ||
| Age | |||
| <60 y | 28 (37.8) | 10 (35.71) | 18 (64.29) |
| ≥60 y | 46 (62.2) | 16 (34.78) | 30 (65.22) |
| Gender | |||
| Males | 53 (71.6) | 17 (32.08) | 36 (67.92) |
| Females | 21 (28.4) | 9 (42.86) | 12 (57.14) |
| Histologic type: | |||
| Urothelial | 54 (73) | 19 (35.19) | 35 (64.81) |
| Non-urothelial: | 20 (27) | 7 (35) | 13 (65) |
| -Keratinizing SCC | 9 (12.1) | ||
| -Non-keratinizing SCC | 7 (9.5) | ||
| -Adenocarcinoma | 4 (5.4) | ||
| Tumor grade: | |||
| Low grade | 24 (32.4) | 8 (33.33) | 16 (66.66) |
| High grade | 50 (67.6) | 18 (36) | 32 (64) |
| Muscle invasion: | |||
| Absent | 34 (45.9) | 7 (20.59) | 27 (79.41) |
| Present | 40 (54.1) | 19 (47.50) | 21 (52.50) |
| LN metastasis: 1 | |||
| Absent | 20 (27.02) | 7 (35) | 13 (65) |
| Present | 20 (27.02) | 12 (60) | 8 (40) |
| Necrosis: | |||
| Absent | 43 (58.1) | 11 (25.58) | 32 (74.42) |
| Present | 31 (41.9) | 15 (48.39) | 16 (51.61) |
| LV invasion: 2 | |||
| Absent | 54 (73) | 16 (29.63) | 38 (70.37) |
| Present | 20 (27) | 10 (50) | 10 (50) |
| PN invasion: 3 | |||
| Absent | 57 (77) | 17 (29.82) | 40 (70.18) |
| Present | 17 (23) | 9 (52.94) | 8 (47.06) |
| Bilharziasis: | |||
| Absent | 48 (64.9) | 19 (39.58) | 29 (60.42) |
| Present | 26 (35.1) | 7 (26.92) | 19 (73.08) |
1LN, lymph node metastasis were assessed in radical cystectomy specimens only (n = 40); 2LV, lymphovascular; 3PN, perineural
VDR Immunohistochemistry (IHC)
Immunostaining was performed on 4μm thick sections of paraffin blocks using the streptavidin biotin peroxidase complex technique. Sections were incubated with ready to use mouse anti-VDR monoclonal antibody clone D6 (Medaysis, Catalog number #MC0304RTU7, San Francisco Bay Area, USA) for one hour at room temperature. In each staining session, a skin section was used as positive control for VDR antibody. Negative controls were done by replacing the primary antibody with phosphate buffer saline.
Evaluation of VDR immunostaining
Evaluation of immunostained sections was performed by two independent authors blinded to all clinicopathological information.
Expression of VDR was assessed semi-quantitatively with regards to the intensity and the proportion of immunoreactive tumor cells. We recorded the percentage of tumor cells expressing VDR (regardless the pattern) in relation to the whole tissue area and graded as: 0, <10%; 1, 11–30%; 2, 31-75%; 3, >75%. Staining intensity was scored at four intensity levels: nil (0), weak/buff (1), moderate/yellow (2) or strong / intense brown (3). Then, the immunoreactivity score (IRS) was calculated by multiplying the values of these two categories and ranged between 0-9. Cases were considered negative (IRS 0–1), low expression (IRS 2–4) or high expression (IRS 6–9) [20].
We also assessed the intensity of staining and percentage of positive staining of VDR expression in the nucleus and cytoplasm separately in each tumor using the same IRS.
Slide examination and imaging
All slides were viewed using light microscopy (Olympus model BX53) while the included photographs were taken by Leica digital pathology slide scanner (APERIO LV1) at Pathology lab, Beni-Suef University hospital, Beni-Suef, Egypt.
Statistical analysis
The collected data were coded and statistically described in terms of frequencies and percentages. Chi-square test was used for comparing categorical data and testing any significant correlation between VDR expression and other clinicopathological variables. P value of less than 0.05 was statistically significant. All analyses were performed using the statistical package for social sciences software for windows, SPSS version 18 (SPSS Inc, Chicago, IL, USA).
Results
Demographic and tumoral features
The mean ± SD age of the participants was 62.65±10.9 years with a range of 35-77 years. The majority of cases were males and male to female ratio was 2.5:1. Urothelial carcinoma was the most common histologic type (n= 54, 73%) including16 non-invasive low grade papillary carcinomas and 38 invasive high grade ones. Out of which, 17 were conventional (pure) infiltrating tumors, 15 with squamous differentiation and 6 showed other divergent differentiation and variants. Among the urothelial cases, both pTa and pT1 were the most frequent tumor stage (29.6% each), followed by tumors invading the perivesical tissue (pT3) (22.2%). On the other hand, 20 patients had non-urothelial malignant tumors, 85% of which were muscle invasive (pT2 and pT3).
Assessment of VDR expression
In this work, histologically normal urothelium was included in 29 cases. All showed high expression of VDR localized to the cell membrane and/or the cytoplasm (Figure 1).
Figure 1. VDR Expression in Histologically Normal Urothelium (A) and Urothelial Proliferative Changes (B) Shows Cytoplasmic/Membranous Immunostaining (IHC, Magnification A x 200, B x100).
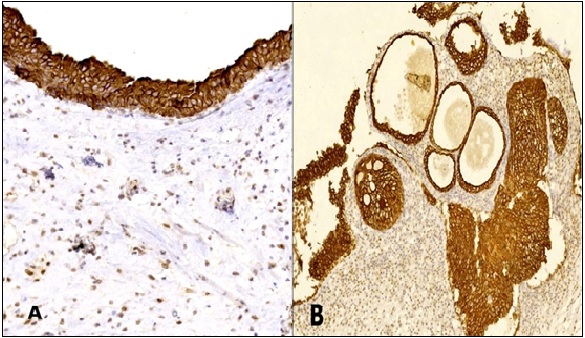
Meanwhile, all tumors expressed VDR in variable degree either in cell nucleus, cytoplasm and/or cell membrane with no recorded negative cases (Table 1). Chi-square test showed no significant association between VDR protein expression and any clinicopathological characteristics of urinary bladder carcinoma (p > 0.05).
Assessment of VDR immunostaining patterns
Overall, cytoplasmic/membranous and nuclear VDR expression were present in 68 (91.89%) and 45 (60.81%) cases, respectively. Strong immunoreactivity was commonly seen in cytoplasmic/membranous VDR expression (n= 35, 47.3 %,) as compared to 18 (24.3%) cases showed high expression of nuclear VDR (Table 2).
| Immunoreactivity score | Cytoplasmic/Membranous VDR (%) | Nuclear VDR (%) | |
| Negative | 0 | 6 (1.9) | 29 (46.3) |
| 1 | 4 (3.7) | 6 (11.1) | |
| Low | 2 | 9 (11.1) | 6 (11.1) |
| 3 | 14 (18.5) | 11 (11.1) | |
| 4 | 6 (9.3) | 4 (5.6) | |
| High | 6 | 13 (16.7) | 6 (7.4) |
| 9 | 22 (38.9) | 12 (7.4) | |
| n | 74 | 74 | |
| Mean ± STD | 4.93 ± 3.13 | 3.85 ± 3.27 |
Regarding the relation between tumor histology and VDR immunostaining patterns, the mean cytoplasmic/ membranous VDR expression was higher in urothelial tumors than that in non-urothelial ones, however, this difference was statistically not significant (p = 0.076).
The mean nuclear VDR expression was significantly (p = 0.007) higher in non-urothelial tumors (Table 3), (Figure 2 and 3).
| Tumor histology | All patients | VDR expression | |||
| n=74 (%) | Cytoplasmic/membranous | Nuclear | |||
| Mean ±STD | P * | Mean ±STD | P *4 | ||
| Urothelial | 54 (73) | 5.69 ± 2.99 | 0.076 | 2 ± 2.69 | 0.007 † 5 |
| Non-urothelial | 20 (27) | 2.85 ± 2.60 | 5.15 ± 3.63 |
4*p-value was calculated by mean test; 5† Statistically significant
Figure 2. VDR Expression in Urothelial Carcinoma Cases, Non-invasive Carcinoma Shows Strong Cytoplasmic, Membranous and Nuclear Staining (A), Weak Cytoplasmic Staining (B). Muscle-invasive Carcinoma Shows Strong Cytoplasmic Staining (C) and Weak Staining (D) (IHC, Magnification A, B, D x 200, C x100).
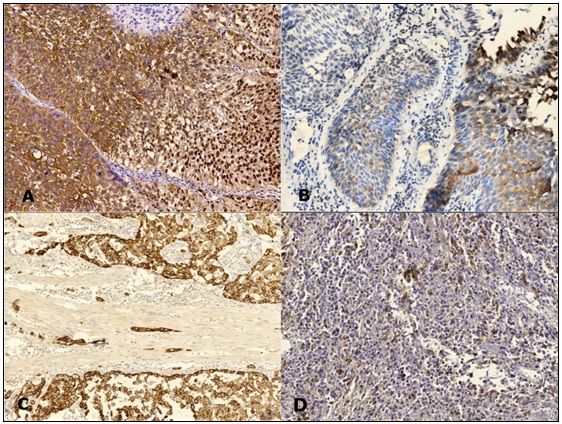
Figure 3. VDR Expression in Non-urothelial Carcinoma Cases, Squamous Cell Carcinoma Shows Strong Nuclear Staining (A), Weak Nuclear Staining (B) and High Cytoplasmic Expression (C). Primary bladder adenocarcinoma shows cytoplasmic/membranous expression (D) (IHC, magnification A, C, D x200, B x100).
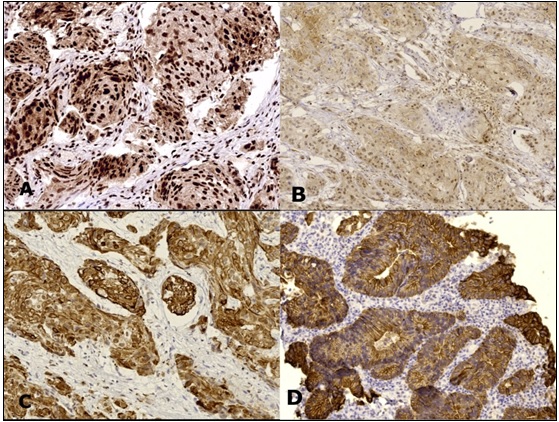
Among the urothelial carcinoma cases studied, there was a statistically significant association between muscle invasion, tumor extent and nuclear VDR expression (p = 0.000, p = 0.001, respectively). While, cytoplasmic VDR expression was not related to tumor grade, stage, muscle invasion or lymph node metastasis (Table 4).
| Characteristics | (n = 54) | VDR expression | ||||
| Cytoplasmic/membranous | Nuclear | |||||
| Mean ±STD | P *6 | Mean ±STD | P * | |||
| Tumor grade | 0.67 | 0.061 | ||||
| Low | 16 | 6.56 ±3.09 | 1.25 ±1.81 | |||
| High | 38 | 5.32 ± 2.91 | 2.32 ±2.95 | |||
| Muscle invasion | 0.905 | 0.000 † 7 | ||||
| Absent | 32 | 5.84 ±3.07 | 2.71 ±3.15 | |||
| Present | 22 | 5.45 ±2.94 | 0.95 ±1.29 | |||
| pT stage | 0.67 | 0.001 † | ||||
| pTa | 16 | 6.56 ±3.09 | 1.25 ±1.81 | |||
| pT1 | 16 | 5.13 ±2.37 | 4.19 ±3.56 | |||
| pT2 | 10 | 6.30 ±3.06 | 1 ± 1.05 | |||
| pT3 | 12 | 4.75 ±2.77 | 0.92 ±1.51 | |||
| LN metastasis ‡ 8 | 0.195 | 0.924 | ||||
| Negative | 9 | 7.00 ±3.04 | 1 ± 1.22 | |||
| Positive | 13 | 4.36 ±2.43 | 0.92±1.38 |
6p-value was calculated by mean test; 7† Statistically significant; 8‡ LN status was only assessed in radical cystectomies (n=22)
The mean nuclear VDR expression in moderately differentiated and superficial non-urothelial cancers was higher compared to poorly differentiated and more advanced tumors. The differences were statistically significant. Cytoplasmic VDR in non-urothelial cases was also compared according to tumor grade, stage and nodal status but no relation was found (Table 5).
| Characteristics | (n=20) | VDR expression | ||||
| Cytoplasmic/membranous | Nuclear | |||||
| Mean ±STD | P *9 | Mean ±STD | P * | |||
| Tumor grade | 0.765 | 0.002 † 10 | ||||
| G1 | 5 | 2.20±2.49 | 4.50±3.94 | |||
| G2 | 3 | 1.67±2.08 | 9.0±0.51 | |||
| G3 | 12 | 3.42±2.78 | 4.40±261 | |||
| Muscle invasion | 0.189 | 0.012 † | ||||
| Absent | 2 | 0.50±0.71 | 9.0±0.51 | |||
| Present | 18 | 3.11±2.61 | 4.72±3.58 | |||
| pT stage | 0.67 | 0.003 † | ||||
| pT1 | 2 | 6.56±3.09 | 9.0±0.05 | |||
| pT2 | 7 | 5.13±2.37 | 2.67±2.34 | |||
| pT3 | 11 | 6.30±3.06 | 5.45±3.75 | |||
| LN metastasis ‡ 11 | 0.142 | 0.904 | ||||
| Negative | 11 | 3.55±3.09 | 4.18±3.60 | |||
| Positive | 7 | 1.71±1.70 | 5.57±3.64 |
9*p-value was calculated by mean test; 10† Statistically significant; 11‡ LN status was only assessed in radical cystectomies (n=18)
Discussion
Several immunohistochemical studies, so far, have been published to assess the relation between VDR expression and different types of cancers with variable and conflicting outcomes. Few studies focused on urothelial carcinoma of the urinary bladder [21]. To our best knowledge; we are the first to report VDR expression in non-urothelial tumors, as well.
Consistent with a study of 100 patients with lung adenocarcinoma by Kim (2012), we observed no significant relations between VDR protein expression and all clinicopathological variables of urinary bladder carcinoma. While Anand et al. [20], McCain et al. [22] and Shi et al. [23], reported a significant decrease in VDR immunoreactivity score across the AJCC anatomic stage/ prognostic groups of patients with oral cancer, esophageal adenocarcinoma and colorectal carcinoma, respectively. All these studies did not evaluate the immunostaining patterns of VDR and their relation with histopathological parameters since several hypotheses found that the relationship between VDR expression and prognosis in cancer was mainly affected by the staining location.
This study revealed that VDR was consistently present in the cell membrane and the cytoplasm of normal urothelial cells. While cancer cells showed, in addition, nuclear immunostaining. Similar findings in normal colorectal cells were reported by Shi et al. [23]. Absent nuclear staining in normal gastric mucosa and the underlying gastric and fundic glands was also noticed by Trowbridge et al. [24]. However, Jóźwicki et al. [25] found nuclear and/or cytoplasmic localization of VDR in all normal urothelial samples. Other cancer studies by Salehin et al. [26] and Salomón et al. [27] revealed nuclear localization in non-pathological vulvar tissues (n= 44/48) and non-malignant brain tissue (n=3/3 positive samples), respectively, but in lower levels compared with the corresponding malignant tumors.
Overall, cytoplasmic/membranous VDR staining pattern was found to be more prevalent than the nuclear VDR among the studied cases. This observation agreed with those reported by Trowbridge et al. [24], Zhou et al. [28] and Shi et al. [23]. Moreover, there was a trend towards increased cytoplasmic VDR expression in urothelial tumors (5.69 ± 2.99) versus non-urothelial ones of 2.85 ± 2.60, though p value did not yield significant. In contrast, Jóźwicki et al. [25] found higher nuclear VDR levels (87.3%) in 71 patients with urothelial carcinoma. Different antibody clones used, different methods of staining, scoring and analyzing the VDR expression might explain the conflicting results.
Although VDR is mainly a nuclear receptor, it can be found in other subcellular structures as the cytoplasm and cell membrane. In the unliganded state, VDR remains in the cytoplasm [29]. Upon binding to Calcitriol, VDR translocation from the cytoplasm to the nucleus occurs with subsequent up or down regulation of hundreds of genes controlled by vitamin D [30]. Interestingly, VDR is also thought to mediate its molecular effect through a non- nuclear pathway in which VDR activates the MEK1/2/ ERK1/2 pathway. The extracellular signal-regulated kinase (ERK) pathway is one of the major signaling cascades of the MAPK signaling pathway [31] that seems to be associated with urothelial tumorigenesis [32]. In colorectal cancer, cytoplasmic VDR expression was found to be associated with KRAS and PI3K mutations [33]. The RTK/ RAS/PI3K pathway is altered in approximately 72% of cases with urothelial carcinoma [34].
For urothelial carcinoma cases, our work demonstrated that nuclear VDR expression was significantly reduced in muscle invasive and pT3 tumors compared with samples from early/superficial tumors. Meanwhile, a higher mean cytoplasmic VDR level was noted in non-invasive low grade tumors than that in higher grade advanced disease. This difference was statistically not significant. Jóźwicki et al. [25] reported the same finding but with a significant relation between cytoplasmic (not nuclear) VDR expression and tumor stage. Czogalla et al. [31] displayed significant correlations between cytoplasmic VDR staining and some prognostic factors in patients with ovarian cancer.
We found high nuclear VDR expression among squamous cell carcinomas and adenocarcinomas of urinary bladder. Furthermore, nuclear VDR showed statistically significant differences in relation to tumor grade, muscle invasion and p (T) stage indicating its loss with increasing malignant progression. No studies of non-urothelial bladder cancer have discussed this point to compare with our results. However, comparable findings were reported by Srinivasan et al. [21] where early and late stages of non- small cell lung carcinoma cases exhibited high nuclear VDR expression. Also, Salehin et al. [26] observed higher nuclear VDR expression in well differentiated vulvar squamous cell carcinoma than the cytoplasmic VDR.
Such above results highlight the role of the classic nuclear pathway of VDR in regulation of genes that control inhibition of tumor development and suggest nuclear VDR as a potential prognostic marker for bladder cancer either urothelial or non-urothelial. This hypothesis is supported by a pilot study of human cancers that measured nuclear VDR concentration by an immunoradiometric assay and showed altered nuclear VDR number when a cell undergoes malignant transformation [35]. Given the chemopreventive effect of vitamin D analogs shown on breast cancer cells [36], gastric carcinogenesis [37] and other cancer types [38] in animal models, these may be suitable for treating patients with bladder carcinoma particularly those expressing nuclear VDR.
Limitations in this study include relative small sample size, and absence of follow up data of patients to evaluate the association of VDR expression with survival in bladder carcinoma. Also, we did not study vitamin D status of patients, but some authors found no significant correlation between serum 25(OH)D3 values and corresponding immunohistochemical VDR expression [39, 16].
In conclusion, VDR is expressed in apparently normal urothelium and malignant tumors of urinary bladder; this may indicate an increased sensitivity to vitamin D-based therapeutic strategies. Nuclear localization of VDR was noted only in malignant cells. Nuclear VDR showed multiple significant relations with prognostic parameters in patients with urothelial and non-urothelial bladder cancer suggesting it as a potential biomarker for UBC.
Further larger studies correlated with patients’ serum level of vitamin D are needed to prove our results. More researches to investigate the role of VDR in premalignant bladder lesions are also recommended.
Acknowledgments
We express our sincere appreciation to departmental chair, all colleagues and technicians who helped us in this work. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for- profit sectors. The authors declare no conflict of interest.
References
- Epidemiology of Bladder Cancer Saginala K, Barsouk A, Aluru JS , Rawla P, Padala SA , Barsouk A. Medical Sciences.2020;8(1). CrossRef
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries Sung H, Ferlay J, Siegel RL , Laversanne M, Soerjomataram I, Jemal A, Bray F. CA: a cancer journal for clinicians.2021;71(3). CrossRef
- Cancer pathology registry 2000-2011. Cairo, Egypt: National Cancer Institute Cairo University; 2016 Mokhtar N, Salama A, Badawy O, Khorshed E, Mohamed G, Ibrahim M, Abdelazim H. ;:p18.
- Urinary schistosomiasis and the associated bladder cancer: update Zaghloul MS , Zaghloul TM , Bishr MK , Baumann BC . Journal of the Egyptian National Cancer Institute.2020;32(1). CrossRef
- Squamous cell carcinoma of the urinary bladder: Systematic review of clinical characteristics and therapeutic approaches Martin JW , Carballido EM , Ahmed A, Farhan B, Dutta R, Smith C, Youssef RF . Arab Journal of Urology.2016;14(3). CrossRef
- Oncologic outcomes in patients with nonurothelial bladder cancer Patel SG , Weiner AB , Keegan K, Morgan T. Indian journal of urology: IJU: journal of the Urological Society of India.2018;34(1). CrossRef
- Staging of bladder cancer Magers MJ , Lopez-Beltran A, Montironi R, Williamson SR , Kaimakliotis HZ , Cheng L. Histopathology.2019;74(1). CrossRef
- Clinical Spectrum of Complications Induced by Intravesical Immunotherapy of Bacillus Calmette-Guérin for Bladder Cancer Liu Y, Lu J, Huang Y, Ma L. Journal of Oncology.2019;2019. CrossRef
- Novel and emerging approaches in the management of non-muscle invasive urothelial carcinoma Yassaie O, Chehroudi C, Black PC . Therapeutic Advances in Medical Oncology.2021;13. CrossRef
- Functions of vitamin D in bone Goltzman D.. Histochemistry and Cell Biology.2018;149(4). CrossRef
- Vitamin D Receptor Polymorphism and Cancer: An Update Rai V, Abdo J, Agrawal S, Agrawal DK . Anticancer Research.2017;37(8). CrossRef
- Calcitriol in cancer treatment: from the lab to the clinic Beer T, Myrthue A. Molecular Cancer Therapeutic.2004;3(3):373-381.https://pubmed.ncbi.nlm.nih.gov/15026558/.
- Characterization of vitamin D receptor (VDR) in lung adenocarcinoma Kim SH , Chen G, King AN , Jeon CK , Christensen PJ , Zhao L, Simpson RU , Thomas DG , Giordano TJ , Brenner DE , Hollis B, Beer DG , Ramnath N. Lung Cancer (Amsterdam, Netherlands).2012;77(2). CrossRef
- Vitamin D receptor suppresses proliferation and metastasis in renal cell carcinoma cell lines via regulating the expression of the epithelial Ca2+ channel TRPV5 Chen Y, Liu X, Zhang F, Liao S, He X, Zhuo D, Huang H, Wu Y. PloS One.2018;13(4). CrossRef
- Effects of vitamin D (calcitriol) on transitional cell carcinoma of the bladder in vitro and in vivo Konety B. R., Lavelle J. P., Pirtskalaishvili G., Dhir R., Meyers S. A., Nguyen T. S., Hershberger P., Shurin M. R., Johnson C. S., Trump D. L., Zeidel M. L., Getzenberg R. H.. The Journal of Urology.2001;165(1). CrossRef
- Serum vitamin D levels of patients with oral squamous cell carcinoma (OSCC) and expression of vitamin D receptor in oral precancerous lesions and OSCC Grimm M, Cetindis M, Biegner T, Lehman M, Munz A, Teriete P, Reinert S. Medicina Oral, Patologia Oral Y Cirugia Bucal.2015;20(2). CrossRef
- Vitamin D receptor(s): In the nucleus but also at membranes? Zmijewski MA , Carlberg C. Experimental Dermatology.2020;29(9). CrossRef
- The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours Moch H, Cubilla AL , Humphrey PA , Reuter VE , Ulbright TM . European Urology.2016;70(1). CrossRef
- American Joint Committee on Cancer. AJCC Cancer Staging Manual. 8th Edition. New York, NY: Springer Cham 2018 Amin M, Gress D, Meyer Vega L, and Edge S. .
- Expression of vitamin D receptor and vitamin D status in patients with oral neoplasms and effect of vitamin D supplementation on quality of life in advanced cancer treatment Anand A, Singh S, Sonkar AA , Husain N, Singh KR , Singh S, Kushwaha JK . Contemporary Oncology (Poznan, Poland).2017;21(2). CrossRef
- Nuclear vitamin D receptor expression is associated with improved survival in non-small cell lung cancer Srinivasan M, Parwani AV , Hershberger PA , Lenzner DE , Weissfeld JL . The Journal of Steroid Biochemistry and Molecular Biology.2011;123(1-2). CrossRef
- Vitamin D receptor as a marker of prognosis in oesophageal adenocarcinoma: a prospective cohort study McCain S, Trainor J, McManus DT , McMenamin Ú, McQuaid S, Bingham V, James JA , Salto-Tellez M, Turkington RC , Coleman HG . Oncotarget.2018;9(76). CrossRef
- Decreased vitamin D receptor protein expression is associated with progression and poor prognosis of colorectal cancer patients Shi Q, Han X, Yu J, Peng H, Chen Y, Li F, Cui X. International Journal of Clinical and Experimental Pathology.2020;13(4).
- Vitamin D Receptor Expression in the Mucosal Tissue at the Gastroesophageal Junction Trowbridge R, Sharma P, Hunter WJ , Agrawal DK . Experimental and molecular pathology.2012;93(2). CrossRef
- Expression of Vitamin D Receptor (VDR) Positively Correlates with Survival of Urothelial Bladder Cancer Patients Jóźwicki W, Brożyna AA , Siekiera J, Slominski AT . International Journal of Molecular Sciences.2015;16(10). CrossRef
- Vitamin D receptor expression in patients with vulvar cancer Salehin D, Haugk C, Thill M, Cordes T, William M, Hemmerlein B, Friedrich M. Anticancer Research.2012;32(1).
- Vitamin D receptor expression is associated with improved overall survival in human glioblastoma multiforme Salomón DG , Fermento ME , Gandini NA , Ferronato MJ , Arévalo J, Blasco J, Andrés NC , Zenklusen JC , Curino AC , Facchinetti MM . Journal of Neuro-Oncology.2014;118(1). CrossRef
- Vitamin D receptor is highly expressed in precancerous lesions and esophageal adenocarcinoma with significant sex difference Zhou Z, Xia Y, Bandla Sa, Zakharov V, Wu S, Peters J, Godfrey TE , Sun J. Human Pathology.2014;45(8). CrossRef
- Prognostic role of vitamin D receptor in breast cancer: a systematic review and meta-analysis Xu H, Liu Z, Shi H, Wang C. BMC cancer.2020;20(1). CrossRef
- Role of vitamin D and vitamin D receptor (VDR) in oral cancer Fathi N, Ahmadian E, Shahi S, Roshangar L, Khan H, Kouhsoltani M, Maleki Dizaj S, Sharifi S. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie.2019;109. CrossRef
- Cytoplasmic VDR expression as an independent risk factor for ovarian cancer Czogalla B, Deuster E, Liao Y, Mayr D, Schmoeckel E, Sattler C, Kolben T, Hester A, Fürst S, Burges A, Mahner S, Jeschke U, Trillsch F. Histochemistry and Cell Biology.2020;154(4). CrossRef
- The Emerging Molecular Landscape of Urothelial Carcinoma Solomon JP , Hansel DE . Surgical Pathology Clinics.2016;9(3). CrossRef
- Vitamin D receptor expression is associated with PIK3CA and KRAS mutations in colorectal cancer Kure S, Nosho K, Baba Y, Irahara N, Shima K, Ng K, Meyerhardt JA , Giovannucci EL , Fuchs CS , Ogino S S. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology.2009;18(10). CrossRef
- Pathogenesis of Urothelial Carcinoma. In: Wojcik, E., Kurtycz, D., and Rosenthal, D. Sundling K, Antic T, Pambuccian S. (eds.) The Paris System for Reporting Urinary Cytology, 2nd ed. Springer-Cham; 2022.;:1-5.
- 1,25-Dihydroxyvitamin D3 receptors in human carcinomas: a pilot study Sandgren M., Danforth L., Plasse T. F., DeLuca H. F.. Cancer Research.1991;51(8).
- Calcium plus vitamin D supplementation and the risk of breast cancer Chlebowski RT , Johnson KC , Kooperberg C, Pettinger M, Wactawski-Wende J, Rohan T, Rossouw J, Lane D, O'Sullivan MJ , Yasmeen S, Hiatt RA , Shikany JM , Vitolins M, Khandekar J, Hubbell FA . Journal of the National Cancer Institute.2008;100(22). CrossRef
- Pathogenic roles of alterations in vitamin D and vitamin D receptor in gastric tumorigenesis Du C, Yang S, Zhao X, Dong H. Oncotarget.2017;8(17). CrossRef
- Role of Vitamin D Metabolism and Activity on Carcinogenesis Wu X, Zhou T, Cao N, Ni J, Wang X. Oncology Research.2014;22(3). CrossRef
- Predicted 25(OH)D score and colorectal cancer risk according to vitamin D receptor expression Jung S, Qian ZR , Yamauchi M, Bertrand KA , Fitzgerald KC , Inamura K, Kim SA , Mima K, Sukawa Y, Zhang Xu, Wang M, Smith-Warner SA , Wu K, Fuchs CS , Chan AT , Giovannucci EL , Ng K, Cho E, Ogino S, Nishihara R. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology.2014;23(8). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2022
Author Details