CDX2 and Ki-67 Expression in Gastric Carcinoma and Its Association with Clinocopathological Parameters
Download
Abstract
Background: CDX2 has been established as a good prognostic marker for colorectal carcinomas. Differentiated adenocarcinomas are characterized by higher CDX2 expression than undifferentiated tumors with stronger reactivity in intestinal phenotypes. There is a close correlation between the degree of tumor differentiation and the Ki-67 score. It was also observed that strong CDX2 expression was associated with low Ki-67 index whereas negative or dim CDX2 expression was associated with high Ki-67 index.
Methods: Gastric biopsies and gastrectomy specimens with gastric carcinoma were evaluated clinicopathologically and processed for immunohistochemistry (IHC) staining to assess CDX2 and Ki-67 expression. The relationship between 2 markers and each clinicopathological parameter was evaluated. Data was statistically analysed. P<0.05 were taken for statistical significance.
Results: The study was done on 57 gastric adenocarcinoma cases with mean age 56.12 years. No significant correlation was found between CDX2 & Ki-67 with clinical, gross & microscopic parameters except for tumor location and depth of invasion. Significant correlation was detected between CDX2 (p=0.04) & Ki-67 (p=0.03) with tumor location. Depth of tumor invasion was significantly associated with Ki-67 (p=0.013). No significant association between CDX2 and Ki-67 was observed.
Conclusion: The statistical correlation between CDX2 & Ki-67 with clinicopathological parameters proves that CDX2 & Ki-67 to be the independent markers with respect to tumor site and depth of invasion (in case of Ki-67). But due to lack of association of CDX2 with Ki-67 further studies need to be done with higher sample size.
Introduction
Gastric cancer is the fourth most common malignancy throughout the world after lung, breast and colorectal neoplasms, and the second and fourth reason of malignancy associated death in men and women, respectively [1]. In India gastric cancer remains 5th most common among males and 7th most common cancer among females [2]. Understanding the current burden of stomach cancer and the differential trends across various locations is essential for formulating effective preventive strategies [3]. Due to paucity of clinical symptoms, most patients with tumor diagnosed at advanced stages have poor prognosis. Hence best strategy proven to improve patient survival is early diagnosis and resection at early (pT1) stage which have significantly improved 5year survival rates to over 90%. [4,5].
CDX2 is an intestinal transcriptional factor that plays an important role in the proliferation and differentiation of intestinal epithelial cells. The protein, CDX2 is encoded by CDX2 gene known as caudal type homeobox 2 [4]. Due to its restrictiveness to intestinal epithelium, it is a very useful marker of adenocarcinoma. CDX2 is considered as an important prognostic biomarker in gastric cancer and several studies have shown that there is a positive association between CDX2 and favorable treatment outcomes including long survival [1, 6]. CDX2 has been observed to be significantly higher in incomplete type IM than complete IM (P=0.045) and also in H.pylori positive group of patients (P=0.045) [7].
Ki-67 is an antigen that corresponds to nuclear non- histone protein expressed by cells in proliferative phases G1, G2, M and S [1]. Ki-67 is a good marker to detect cell proliferative fraction. It’s a nuclear proliferation marker associated antigen expressed in growth and synthesis phases of cell cycle but not in resting phase, thus providing information about proportion of active cells in a cell cycle. Also, a close relation between degree of tumor differentiation and Ki-67 score (P=0.001) was observed. The characteristics of cellular kinetics reflect aggressiveness of tumor and prognosis, demonstrating correlation between marked proliferation activity and an unfavorable prognosis [8]. Gastric cancer patients with CDX2 positive expression had a less invasive pattern of gastric carcinoma and better outcomes. Patient with CDX2 positive expression showed a higher survival rate than CDX2 negative expression (P=0.038), therefore useful in predicting prognosis of gastric carcinomas [1].
Hence the present study was conducted to evaluate clinicopathological features of gastric carcinomas, expression of CDX2 and Ki-67 and their correlation with each other. Expression of CDX2 indicates better prognosis, whereas Ki-67 indicates aggressiveness. This inverse relation will combinedly aid in overall treatment mode and prognosis.
Materials and Methods
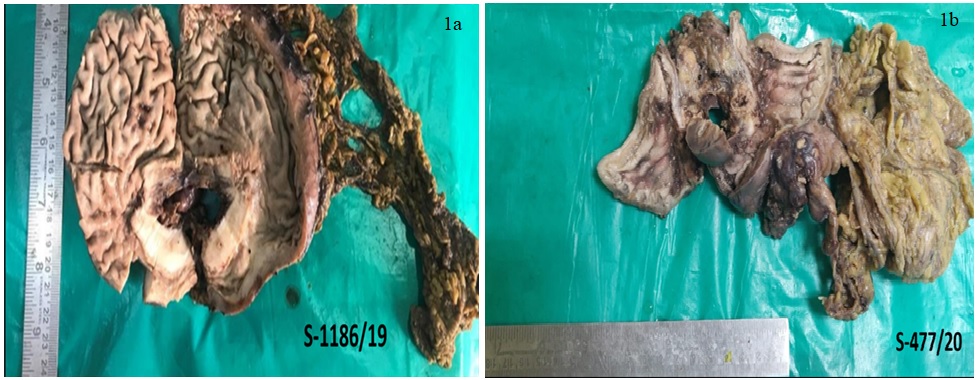
This cross-sectional study was conducted on all gastric biopsies and gastrectomy specimens (Figure 1A and 1B) received for routine histopathological evaluation in a tertiary care centre over a period of 1 year and 11 months.
Figure 1. a; Radical Gastrectomy Specimen. The Cut Surface Shows an Ulceloproliferative Grey White Growth with Loss of Mucosal Folds in the Surrounding Area. b; Distal Gastrectomy Specimen. Cut Surface Showing Ulceroinfiltrative Grey White Area at Pylorus, Extending Upto Lesser Curvature.
57 cases were reported as primary gastric tumors clinically, radiologically and histopathologically. Radical and biopsy specimens from patients aged above 18 years with histopathological diagnosis of primary gastric malignancy were included in the study. Non neoplastic lesions of the stomach, cases with extensive tumor necrosis without sufficient viable tumor cells for accurate evaluation of the IHC results; inadequate specimens were excluded.
Statistical analysis
Data was entered into Microsoft excel datasheet and was analyzed using SPSS 22 version software. Categorical data was represented in the form of frequencies and proportions. Chi-square test or Fischer’s exact test (for 2x2 tables only) was used as test of significance for qualitative data. Continuous data was represented as mean and standard deviation. Independent t test was used as test of significance to identify the mean difference between two quantitative variables. ANOVA was used as test of significance to identify the mean difference between more than two quantitative variables. For graphical representation of data, MS Excel and MS word was used to obtain various types of graphs. p value <0.05 was considered as statistically significant after assuming all the rules of statistical tests. MS Excel, SPSS version 22 (IBM SPSS Statistics, Somers NY, USA) was used to analyze data.
Method of study
Informed consent was obtained from participants >18years of age. The detailed clinical history and results of relevant investigations (endoscopic findings) were obtained from the participants. The gastric biopsies and gastrectomy specimens were received in the Pathology Department in 10% formalin. In every case the standard protocol for surgical grossing of gastric specimens was followed. The specimen was kept for fixation for 24 hours. After detailed macroscopic description of external surface and cut surface findings, multiple representative areas were sampled. After conventional processing in a Leica 120 model histokinette and embedding in paraffin wax, sections of 5µm thickness were cut using Leica JUNG RM 2025 model rotatory microtome and stained using hematoxylin and eosin (H&E) for histopathological study. In addition, 3-5µm sections were cut from a paraffin block of tumor tissue and taken on 2 glass slides coated with adhesive poly-L-lysine for immunohistochemistry. For each case 2 sections were taken, one for CDX2 and other for Ki-67.3-5µm sections were cut from a paraffin block of tumor tissue and taken on 2 glass slides coated with adhesive poly-L-lysine for immunohistochemistry. Slides were treated with a primary rabbit monoclonal antibody (EP25) against CDX2 (Diagnostic BioSystems) and primary monoclonal antibody (SP6) against Ki- 67 (Diagnostic BioSystems). Further treatment with peroxidase and DAB was performed. Positive controls were run with each batch.
Scoring for CDX2
Scoring for CDX2 expression was based on the proportion of tumor cells exhibiting distinct nuclear immunopositivity. Colon adenocarcinoma was taken as positive control. The CDX2 scoring results were calculated as follows: Score 0: 0%-5% positive tumor cells. Score 1: >5%- 35% positive tumor cells. Score 2: >35%-65% positive tumor cells. Score 3: >65% positive cells. Samples with score 0 were considered negative.
Ki-67 indexing
Ki-67 is interpreted as labelling index (Ki-67 LI)
Ki-67 = Number of nuclei showing positive staining (brown colour) X 100/Total number of nuclei
≤20% was considered as low Ki-67 and >20% as high Ki-67. Tonsil was taken as positive control.
CDX2 and Ki-67 expression was correlated with clinicopathological parameters. In addition, in cases of radical gastrectomies, CDX2 & Ki-67 expression was studied in relation to tumour size, pattern of growth, depth of invasion, perineural invasion, lymph node metastasis etc.
Results
We studied 57 patients of gastric carcinoma, the mean age being 56.12 years and mean age group 50-60 years with the youngest patient being 24 years and the oldest 84 years. Males (43/57) were most commonly diagnosed with gastric carcinoma. Non-vegetarianism (46/57) and smoking (34/57) were found to be the most common predisposing factors. H Pylori infection was discerned in 44 cases. 48 cases were clinically diagnosed with gastric carcinoma.40 cases showed mucosal ulceration on endoscopy. Ulceroinfiltrative pattern (7/20) was the most common type of gross appearance. Antrum was the most common tumor location (42/57). Tumor size between 3.1- 6cm (9/20) was noticed maximum with intestinal type the most common histologic type (36/57) as compared to diffuse type (21/57). Moderately differentiated grade was observed in 26 cases. Tumor invading muscularis propria was identified in 10 specimens. PNI & LNI were present in 11 & 16 specimens respectively. Tumors were mostly found in stage III (10/20).
CDX2 expression with clinicopathological parameters
When gender was compared with CDX2, males showed more common tendency of expressing CDX2. High score of CDX2 was almost similar in both gender (p=0.23). Irrespective of diet, CDX2 was not expressed in majority of population. CDX2 score 3 was observed only in non-vegetarian and absent in vegetarian (p=0.77). A minimal percentage of cases showed high score of CDX2 in relation to smoking(p=0.36). No statistically significant difference was found between age group, gender, diet, smoking, clinical diagnosis and CDX2 score (Table 1).
| Clinical Parameters | CDX2 Score | P Value | Ki-67 Index | P Value | |||||
| 0 | 1 | 2 | 3 | >20 | <20 | ||||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||||
| Age Group | 20-40yrs | 4 (80.0) | 0 | 1 (20.0) | 0 | 0.59 | 1 (20.0) | 4 (80.0) | 0.881 |
| 41-60yrs | 21 (65.6) | 6 (18.8) | 2 (6.3) | 3 (9.4) | 5 (15.6) | 27 (84.4) | |||
| 61-80yrs | 15 (83.3) | 0 | 2 (11.1) | 1 (5.6) | 2 (11.1) | 16 (88.9) | |||
| >80yrs | 2 (100) | 0 | 0 | 0 | 0 | 2 (100.0) | |||
| Total | 42 (73.7) | 6 (10.5) | 5 (8.8) | 4 (7.0) | 8 (14.0) | 49 (86.0) | |||
| Sex | Female | 8 (57.1) | 3 (21.4) | 1 (7.1) | 2 (14.3) | 0.237 | 1 (7.1) | 13 (92.9) | 0.664 |
| Male | 34 (79.1) | 3 (7.0) | 4 (9.3) | 2 (4.7) | 7 (16.3) | 36 (83.7) | |||
| Total | 42 (73.7) | 6 (10.5) | 5 (8.8) | 4 (7.0) | 8 (14.0) | 49 (86.0) | |||
| Diet | Non vegetarian | 33 (71.7) | 5 (10.9) | 4 (8.7) | 4 (8.7) | 0.775 | 40 (87.0) | 6 (13.0) | 0.659 |
| Pure vegetarian | 9 (81.8) | 1(9.1) | 1 (9.1) | 0 | 9 (81.8) | 2 (18.2) | |||
| Total | 42 (73.7) | 6 (10.5) | 5 (8.8) | 4 (7.0) | 8 (14.0) | 49 (86.0) | |||
| Smoking | Non-Smoker | 16 (69.6) | 3 (13.0) | 1 (4.3) | 3 (13.0) | 0.365 | 4 (17.4) | 19 (82.6) | 0.702 |
| Smoker | 26 (76.5) | 3 (8.8) | 4 (11.8) | 1 (2.9) | 4 (11.8) | 30 (88.2) | |||
| Total | 42 (73.7) | 6 (10.5) | 5 (8.8) | 4 (7.0) | 8 (14.0) | 49 (86.0) | |||
| Clinical Diagnosis | Normal | 1 (50.0) | 0 | 0 | 1 (50.0) | 0.157 | 0 | 2 (100.0) | 0.844 |
| Gastritis | 4 (57.1) | 2 (28.6) | 1 (14.3) | 0 | 1(14.3) | 6 (85.7) | |||
| Carcinoma | 37 (77.1) | 4 (8.3) | 4 (8.3) | 3 (6.3) | 7(14.6) | 41 (85.4) |
CDX2 low scores (73.7%) were seen irrespective of type of endoscopic findings. But as the scoring increases in CDX2, the endoscopic findings appear to decrease (7%). Majority of cases irrespective of their gross appearance have been CDX2 negative (85%). Both gross appearance and endoscopic findings were not significantly correlated with CDX2.With regards to tumor location, majority showed no CDX2 expression (85%). Only one case in pylorus exhibited high score3 of CDX2. Significant correlation was reported between CDX2 and tumor location(p=0.04). Greater the tumor size lesser CDX2 expression and higher the CDX2 score there is minimal expression of CDX2.CDX2 expression was detected in 28% of cases (16/57) and intestinal- type of carcinomas (27.8%) had higher expression as compared to diffuse-type carcinomas (23.8%). With respect to histologic grade, most of the tumors were CDX2 negative. 8 out of 57 cases were reported as signet ring cell carcinoma. Maximum of PNI was associated with CDX2 score0. Also no immunoreactivity in case of CDX2 w.r.t lymph node involvement in majority of cases. Only one case with LNM expressed CDX2 score3. None of the microscopic parameters were significantly correlated with CDX2. Histologic grade with CDX2 was of marginal significance (Table 2).
| Histopathological Parameters | CDX2 Score | P Value | Ki-67 Index | P Value | |||||
| 0 | 1 | 2 | 3 | >20 | <20 | ||||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||||
| Endoscopic Findings | Red discolouration on mucosal surface | 11 (68.8) | 3 (18.8) | 1 (6.3) | 1 (6.3) | 0.717 | 3 (18.8) | 13 (81.3) | 0.567 |
| Nodular Surface | 10 (83.3) | 1 (8.3) | 0 | 1 (8.3) | 1 (8.3) | 11 (91.7) | |||
| Mucosal ulcerations | 21 (72.4) | 2 (6.9) | 4 (13.8) | 2 (6.9) | 4 (13.8) | 25 (86.2) | |||
| Total | 42 (73.7) | 6 (10.5) | 5 (8.8) | 4 (7.0) | 8 (14.0) | 49 (86.0) | |||
| Gross Appearance | Ulcero-infiltrative | 6 (85.7) | 1 (14.3) | 0 | 0 | 0.604 | 0 | 7 (100.0) | 0.295 |
| Ulcero-proliferative | 4 (80.0) | 0 | 0 | 1 (20.0) | 0 | 5 (100.0) | |||
| Flat, plaque like lesions without infiltration | 4 (80.0) | 1 (20.0) | 0 | 0 | 1 (20.0) | 4 (80.0) | |||
| Diffuse infiltration without ulcers | 3 (100.0) | 0 | 0 | 0 | 1 (33.3) | 2 (66.7) | |||
| Total | 17 (85.0) | 2 (10.0) | 0 | 1 (5.0) | 2 (10%) | 18 (90%) | |||
| Tumor Location | Antrum + Pylorus | 4 (100.0) | 0 | 0 | 0.04 | 2 (50.0) | 2 (50.0) | 0.031 | |
| Antrum + Pylorus, Lesser curvature | 2 (66.7) | 1 (33.3) | 0 | 0 | 3 (100.0) | ||||
| Gastro-oesophageal junction | 0 | 1 (100.0) | 0 | 0 | 1 (100.0) | ||||
| Pylorus | 11 (91.7) | 0 | 1 (8.3) | 0 | 12 (100.0) | ||||
| Total | 17 (85.0) | 2 (10.0) | 1 (5.0) | 2 (10) | 18 (90) | ||||
| Tumor Size | <3cm | 3 (75.0) | 1 (25.0) | 0 | 0 | 0.745 | 4 (22.2) | 0 | 0.745 |
| 3.1-6cm | 7 (77.8) | 1 (11.1) | 0 | 1 (11.1) | 9 (50.0) | 1 (50.0) | |||
| 6.1-9cm | 5 (100.0) | 0 | 0 | 0 | 5 (27.7) | 1 (50.0) | |||
| >9cm | 2 (100.0) | 0 | 0 | 0 | 2 (11.1) | 0 | |||
| Histologic Type | Diffuse | 16 (76.2) | 2 (9.5) | 1 (4.8) | 2 (9.5) | 0.809 | 5 (23.8) | 16 (76.2) | 0.13 |
| Intestinal | 26 (72.2) | 4 (11.1) | 4 (11.1) | 2 (5.6) | 3 (8.3) | 33 (91.7) | |||
| Histologic Grade | G1 | 5 (62.5) | 0 | 2 (25.0) | 1 (12.5) | 0.05 | 2 (25.0) | 6 (75.0) | 0.233 |
| G2 | 19 (73.1) | 4 (15.4) | 2 (7.7) | 1 (3.8) | 1 (3.8) | 25 (96.2) | |||
| G3 | 15 (100.0) | 0 | 0 | 0 | 3 (20.0) | 12 (80.0) | |||
| G3, S | 3 (37.5) | 2 (25.0) | 1 (12.5) | 2 (25.0) | 2 (25.0) | 6 (75.0) | |||
| DOI | lamina propria | 1 (100.0) | 0 | 0 | 0 | 0.5 | 1 (100.0) | 0 | 0.013 |
| Submucosa | 2 (66.7) | 1 (33.3) | 0 | 0 | 0 | 3 (100.0) | |||
| Muscularis propria | 8 (80) | 1 (10.0) | 0 | 1 (10) | 0 | 10 (100.0) | |||
| Serosa | 5 (83.3) | 0 | 0 | 1 (16.7) | 1 (16.7) | 5 (83.3) | |||
| PNI | Absent | 9 (100.0) | 0 | 0 | 0 | 0.236 | 2 (22.2) | 7 (77.8) | 0.189 |
| Present | 8 (72.7) | 2 (18.2) | 0 | 1 (9.1) | 0 | 11 (100.0) | |||
| Total | 17 (85.0) | 2 (10.0) | 0 | 1 (5.0) | 2 (10.0) | 18 (90.0) | |||
| Lymph Node | Absent | 3 (75.0) | 1 (25.0) | 0 | 0 | 0.488 | 0 | 4 (100.0) | 0.456 |
| Present | 14 (87.5) | 1 (6.3) | 0 | 1 (6.3) | 2 (12.5) | 14 (87.5) | |||
| Total | 17 (85.0) | 2 (10.0) | 0 | 1 (5.0) | 2 (10.0) | 18 (90.0) |
Ki-67 expression with clinicopathological parameters
Low Ki-67 was more common in all age groups, especially between age groups of 40-80years. Younger and older age groups showed only low Ki-67. Males expressed Ki-67 more as compared to females. Both vegetarians and non-vegetarians expressed high Ki67 index. Around 86% of cases reported low Ki-67 index and 14% with high Ki67 index irrespective of smoking history. No significant association found between Ki-67 and clinical parameters. Around 86% of cases were observed to express low K-67 in endoscopic findings. Maximum cases (90%) expressed low Ki-67 in all growth pattern types. Given that higher the Ki-67 greater the cell proliferation and poorer the prognosis. Majority of Ki-67 expression was exhibited in low Ki-67 activity (90%) w.r.t tumor location. Tumor location was statistically significant with Ki-67, implying more distal the tumor, low Ki-67 index will be found. High Ki-67 activity was found most in between tumor sizes 3.1-6cm.
Ki-67expression was noted in all cases and high Ki-67 in 8.3% of intestinal-type of carcinomas and 23.8% in diffuse-type carcinomas. Most of the tumors were reported to show low Ki-67 index w.r.t histologic grade. Significant association detected between Ki-67 and depth of tumor invasion. Majority of the cases expressed low Ki-67, i.e deeper the tumor, lower the Ki-67 activity. High proliferative index are associated minimal invasion. Maximum of PNI was associated with low Ki. With respect to LNM, maximum cases expressed low Ki (87.5%) and only 2 cases had high Ki. All of the node- negative cases (100%) demonstrated low Ki (Table 2). In our current study, we evaluated 57 cases of gastric carcinomas. CDX2 was negative in 71.9% cases, 8.8% cases with score1, 10.5% cases with score2, and 8.8% cases with score 3. 86% cases demonstrated high Ki-67 and 14% showed low Ki-67. Majority (75%) of cases did not express CDX2 among low Ki-67 cases and only 25% demonstrated score3 CDX2 when Ki-67 index was low (Figure 2 and 3).
Figure 2. (a) H&E of Moderately Differentiated Intestinal Type of Adenocarcinoma of Stomach under100x. (b) IHC Staining of Intestinal Type Adenocarcinoma Showing Negative CDX2 Expression: Score 0 Under 400X. (c) IHC Staining of Intestinal Type Adenocarcinoma Showing High Ki-67 Activity Under 400X.
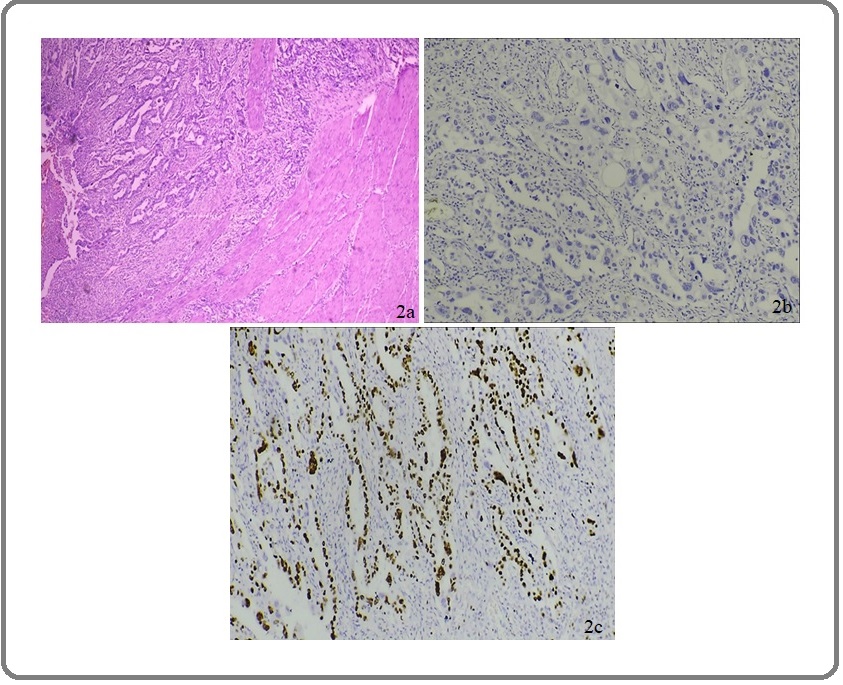
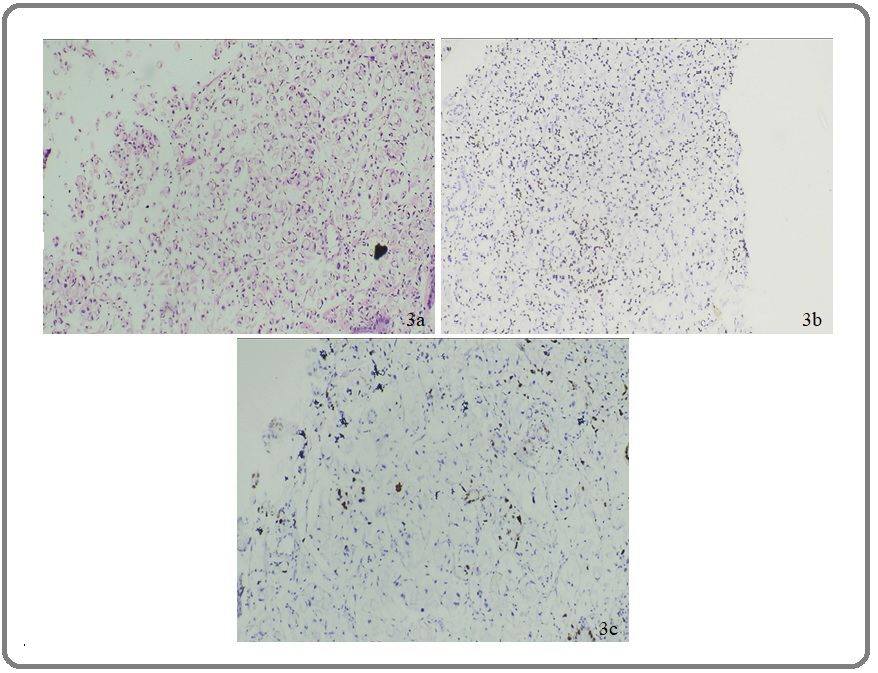
Figure 3. (a) H&E of Signet Ring Adenocarcinoma of Stomach Under 100X. (b) IHC Staining of Signet Ring Adenocarcinoma of Stomach Showing CDX2 Expression: Score 2 Under 100X. (c) IHC Staining of Signet Ring Adenocarcinoma of Stomach Showing Low Ki-67 Index 100X.
Conversely, it was noted that high Ki-67 was associated with negative CDX2 (71.5%) (Figure 4), thus indicating an inverse relationship between these two markers. p value 0.107.
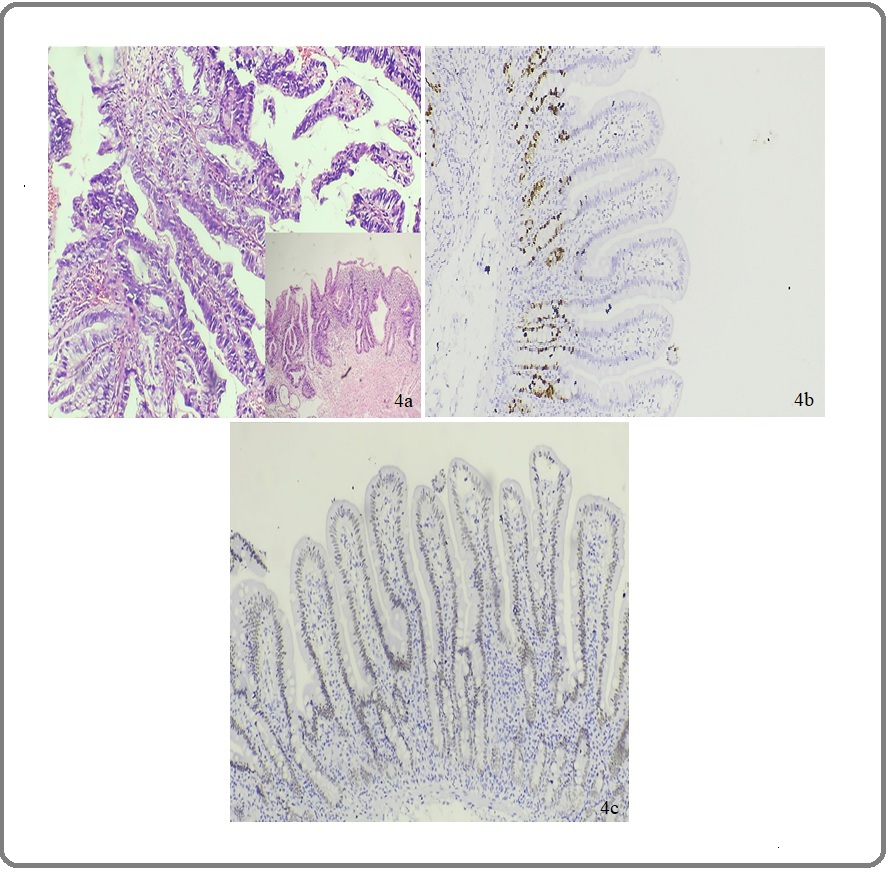
Figure 4. (a) H&E of Papillary Adenocarcinoma of Stomach (Outset: Under 400X). Inset: 100X View of the Same. (b) IHC Staining of Papillary Adenocarcinoma of Stomach Showing CDX2 Expression: Score 1 Under 400X. (c) IHC Staining of Papillary Adenocarcinoma of Stomach Showing High Ki-67 INDEX Under 400X.
There was no statistically significant association found between CDX2 score and Ki-67 index (Table 3).
| Ki-67 Index | CDX2 Score | Total | ||||
| 0 | 1 | 2 | 3 | |||
| >20 | 6 (75.0) | 0 | 0 | 2 (25.0) | 8 (100.0) | |
| <20 | 36 (73.5) | 6 (12.2) | 5 (10.2) | 2 (4.1) | 49 (100.0) | |
| Total | 42 (73.7) | 6 (10.5) | 5 (8.8) | 4 (7.0) | 57 (100.0) |
Discussion
Stomach cancer incidence rates are much lower in India than elsewhere, but the stomach remains one of the 10 leading sites of cancer in both sexes in most of the metropolitan registries [9]. Gastric carcinoma constitutes the 4th most common malignancy worldwide with it being 2nd and 4th most common reason of malignancy associated death in men and women respectively [1].
Early detection plays a pivotal role in prognosis. Lauren classification divides gastric cancer into two major histological types- intestinal and diffuse type. CDX2 has been established as a good prognostic marker for colorectal carcinomas, unlike the derth in knowledge regarding its role in outcome of gastric carcinoma [3]. Differentiated adenocarcinomas are characterized by higher CDX2 expression than undifferentiated tumors with stronger reactivity in intestinal phenotypes. Some of the latest studies have shown inverse relation between CDX2 expression and depth of invasion and lymphnode metastasis [1]. There is a close correlation between the degree of tumor differentiation and the Ki-67 score (p<0.001) [5]. Increased CDX2 expression was more frequently associated with adenomatous type of gastric epithelial dysplasia. CDX2 expression also gradually decreased from gastric epithelial dysplasia, to early and advanced gastric cancers [6]. It was also observed that strong CDX2 expression was associated with low Ki-67 index whereas negative or dim CDX2 expression was associated with high Ki-67 index. Many factors help in prognosis, important being the biomarkers such as: c-ERBB-2, p53,cathepsin. CDX2 expression has been evaluated in a few studies and found to be more consistent to be used as a prognostic markers [3][7]. Therefore this study was conducted to evaluate the expression of CDX2 and Ki-67 labelling index in different histological types of gastric carcinoma by immunohistochemistry.
Majority of the patients in this study belonged to the age group 50-60 years. Similar observations were reported by Rao et al.,(2002) [9], although an epidemiological survey conducted by Theuer et al., (1996) [10] revealed younger population (<40years) being at higher risk of gastric carcinoma. Majority of the patients in this study suffered from vague abdominal discomfort, like the study conducted by Barad et al., (2014) [2]. The difference between the clinical diagnosis and CDX2 (p=0.157) & Ki-67 (p=0.844) expression was insignificant in this study.
In our study 70% cases showed mucosal ulceration.
Both CDX2 and Ki-67 have been found to most commonly express low score (73.7%) and low proliferation index (86%) respectively irrespective of type of endoscopic findings. Inverse relationship has been observed, but it was not statistically significant [11]. Majority of the tumors were ulcero-infiltrative (35%) and ulcero-proliferative (25%). Present study, majority of cases irrespective of their gross appearance have been CDX2 negative (85%). Few studies say that CDX2 expression relates to good prognosis. Thus it can be inferred that very few cases have good prognosis. At the same time when the gross appearance was correlated with Ki-67, maximum cases (90%) expressed low Ki-67 in all growth pattern types. [12,13].
In our study, the least size reported was 1.8cm and greatest being 10cm (10%). The tumor size association with CDX2 & Ki-67 was insignificant. This in agreement with the study conducted by Yokota et al., (2002) [14] and Zhao et al., (2015) [15]. Greater number of tumors were located in antrum (42%), followed by pylorus (23%). A similar frequency was found in the study conducted by Miomir et al (2004) who found that 73.34% tumors were located in antrum. Majority of tumors expressed score 0 (85%) and <20% (90%) for CDX2 and Ki-67 respectively. Only one case in pylorus exhibited score 3 of CDX2 and high Ki-67 was obsereved antral + pylorus location in 2 cases. Thus, CDX2 loss and low Ki-67 are significantly associated with tumor site [14,15].
Majority of the tumors were intestinal type (63%). CDX2 was expressed in 27.8% of intestinal type & 23.8% of diffuse type. The association between the histological type of tumor and CDX2 expression was statistically insignificant. Similar observations were reported by Roessler et al., (2005). High Ki-67 in 8.3% of intestinal-type and 23.8% in diffuse-type. This association was not of significance in our study [8] [16,17]. A study by Ko GH et al (2017) [18] also gives similar report w.r.t to Ki-67.
When depth of tumor invasion was correlated with CDX2 expression, itwas found that majority of the cases either did not express CDX2 or showed only minimal expression (score 1); only 10% cases expressed score 3. Depth of invasion was notsignificantly associated with CDX2.In our study there was a significant association found between low Ki-67 and depth of tumor invasion. Majority of the cases expressed low Ki-67. A study conducted by Halder A et a l (2018) [19] reported high Ki67 was associatedwith depth of tumor invasion . Thus, a significant inverse association between Ki-67 expression and depth of tumor invasion was detected in the current study [20, 21].
Maximum of PNI was linked with CDX2 negativity and low KI. We also correlated CDX2 and Ki-67 with the lymph node metastasis. Only one case with LNM expressed CDX2 score3. 87.5% cases expressed low Ki-67 and only 2 cases had high KI. All of the node negative cases (100%) demonstrated low Ki. Similarly, majority of the node negative cases either demonstrated no immunoreactivity or minimal expression of score1 in case of CDX2. Both LNM & PNI association however was observed to be not significant statistically with CDX2 & Ki-67 [22, 23].
CDX2 was negative in 71.9% of cases, 8.8% cases with score1, 10.5% cases of score2 and 8.8% cases of score3. 86% of cases were demonstrating high Ki-67 and 14% showed low Ki-67. Majority (75%) of cases did not express CDX2 among low Ki-67 cases and only 25% demonstrated score 3 CDX2 when Ki-67 index was low. Conversely, it was noted that high Ki-67 was associated with negative CDX2 (71.5%). Thus, indicating an inverse relationship between these two markers. A study by Halder et al (2018) (19) reported CDX2 expression correlation with Ki 67 index. Strong CDX2 expression was related with low Ki 67 index and negative or low CDX2 expression was related with high Ki 67 index. The correlation was statistically significant (P < 0.0048). Seno et al (2002) [24], in his study observed that CDX2 positive GC cases showed significantly low Ki-67. This multivariate analysis revealed that the CDX2 positive GC patients survived significantly longer than the CDX2 negative patients. These results collectively determine that CDX2 expression in GC might be a novel prognostic marker for patient survival. Despite the demonstration of inverse association between CDX2 and Ki-67 in our study, which is similar to other studies [19], [24], there was no significant association between CDX2 and Ki-67. Small sample size, lack of molecular studies to quantify the CDX2 & Ki-67 expression and also to understand the reason for lack inverse relationship of CDX2 & Ki-67 are few limitations of this study. Future studies that overcome these limitations would help in understanding the CDX2 & Ki-67 expression pattern in a better manner. Gastric cancers are on the rise in the recent past in younger age group individuals too; so chose this topic for study to try to find out a prognostic marker which would help time treatment.
In conclusion this lack of statistically significant correlation between CDX2 and Ki-67 with various clinicopathological prognostic parameters, except for tumor site and depth of invasion (w.r.t Ki-67); suggests that CDX2 &Ki-67 immunoexpression may not be a significant prognostic marker in GC. Also, the lack of significant inverse correlation between CDX2 and Ki-67 suggests that CDX2 & Ki-67 may not be applied together for predicting GC prognosis. However, further studies using larger sample size, various geographical areas and more sensitive methods of detection of CDX2 & Ki-67 may help evaluate the role of CDX2 & Ki-67 in GC prognosis. Also, follow up studies with correlation between CDX2, Ki-67 and cancer specific 5-year survival statistics need to be done for definite assessment of prognostic value of CDX2 & Ki-67 in gastric carcinoma.
Approval
Part of an approved student thesis.
Ethical Declaration
Institutional ethical clearance taken. MSRMC/EC/ AP-2/10-2019.
References
- Association of CDX2 expression and overall survival and clinicopathologic disease manifestations in patients with gastric cancer in Northwest of Iran Akbari F, Ghoreishi Z, Asghari Jafarabadi M, Rezazadeh K, Nejati B, Esfhanai A. Immunopathologia Persa.2019;5. CrossRef
- Gastric cancer-a clinicopathological study in a tertiary care centre of North-eastern India Barad A K , Mandal S K , Harsha H S , Sharma B M , Singh T S . Journal of Gastrointestinal Oncology.2014;5(2). CrossRef
- The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease study 2017 Etemadi A. The Lancet. Gastroenterology & Hepatology.2020;5(1). CrossRef
- Low risk of lymph node metastasis in 495 early gastric cardiac carcinomas: a multicenter clinicopathologic study of 2101 radical gastrectomies for early gastric carcinoma Huang Q, Cheng Y, Chen L, Mingzhan D, Wang Y, Xu G, Shi J, et al . Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc.2018;31(10). CrossRef
- Explaining gastric cancer survival differences among European countries Verdecchia A, Corazziari I, Gatta G, Lisi D, Faivre J, Forman D. International Journal of Cancer.2004;109(5). CrossRef
- An immunohistochemical study of primary signet-ring cell carcinoma of the stomach and colorectum: III. expressions of EMA, CEA, CA19-9, CDX-2, p53, Ki-67 antigen, TTF-1, vimentin, and p63 in normal mucosa and in 42 cases Terada T. International Journal of Clinical and Experimental Pathology.2013;6(4).
- CDX1 and CDX2 expression in intestinal metaplasia, dysplasia and gastric cancer Kang JM , LeeBH , Kim N, Lee HS , Lee HE , Park JH , Kim JS , Jung HC , Song IS . Journal of Korean Medical Science.2011;26(5). CrossRef
- Ki-67 expression in gastric cancer. Results from a prospective study with long-term follow-up Lazăr D, Tăban S, Sporea I., Dema A, Cornianu M, Lazăr E, Goldiş A, Vernic C. Romanian Journal of Morphology and Embryology = Revue Roumaine De Morphologie Et Embryologie.2010;51(4).
- A case-control study of stomach cancer in Mumbai, India Rao DN , Ganesh B, Dinshaw KA , Mohandas KM . International Journal of Cancer.2002;99(5). CrossRef
- Gastric adenocarcinoma in patients 40 years of age or younger Theuer C. P., Virgilio C., Keese G., French S., Arnell T., Tolmos J., Klein S., Powers W., Oh T., Stabile B. E.. American Journal of Surgery.1996;172(5). CrossRef
- Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation Forman D., Newell D. G., Fullerton F., Yarnell J. W., Stacey A. R., Wald N., Sitas F.. BMJ (Clinical research ed.).1991;302(6788). CrossRef
- Handbuch der Speziellen Pathologischen Anatomic und Histologie; Springer: Berlin, Germany,4 Borrmann R, Henke F, Lubarsch O. 1926.
- Are Borrmann's Types of Advanced Gastric Cancer Distinct Clinicopathological and Molecular Entities? A Western Study Díaz Del Arco C, Ortega Medina L, Estrada Muñoz L, Molina Roldán E, Cerón Nieto MA , García Gómez de Las Heras S, Fernández Aceñero MJ . Cancers.2021;13(12). CrossRef
- Is tumor size a prognostic indicator for gastric carcinoma? Yokota T, Ishiyama S, Saito T, Teshima S, Yamada Y, Iwamoto K, Takahashi M, Murata K, Yamauchi H. Anticancer Research.2002;22(6B).
- Prognostic Significance of Tumor Size in 2405 Patients With Gastric Cancer: A Retrospective Cohort Study Zhao L, Zhang W, Chen X, Yang K, Chen X, Liu K, Zhang B, Chen Z, Chen J, Zhou Z, Hu J. Medicine.2015;94(50). CrossRef
- Clinicopathological importance of Ki-67, p27, and p53 expression in gastric cancer Çalik M, Demirci E, Altun E, Çalik İ, Gündoğdu Ö, Gürsan N, Gündoğdu B, Albayrak M. Turkish Journal of Medical Sciences.2015;45(1). CrossRef
- Study on Ki-67 immunoreactivity as a prognostic indicator in patients with advanced gastric cancer Manzoni G., Verlato G., Tomezzoli A., Guglielmi A., Pelosi G., Ricci F., Di Leo A., Cordiano C.. Japanese Journal of Clinical Oncology.1998;28(9). CrossRef
- Prognostic impact of Ki-67 in patients with gastric cancer-the importance of depth of invasion and histologic differentiation Ko GH , Go S , Lee WS , Lee JH , Jeong SH , Lee YJ , Hong SC , Ha WS . Medicine.2017;96(25). CrossRef
- CDX2 Expression in Gastric Carcinoma: A Clinicopathological Study Halder A, Kundu M, Das R, Chatterjee U, Datta C, Choudhuri M, Chatterjee B. Indian Journal of Medical and Paediatric Oncology.2018;39. CrossRef
- Prognostic utility of Ki-67 in gastric carcinoma Almabrouk NM , El-Maraghy MN , Badr AME , Meckawy GR , Shakweer MM . Immunopathologia Persa.2021;8(1). CrossRef
- Low Ki-67 proliferation index is an indicator of poor prognosis in gastric cancer Lee HE , Kim MA , Lee BL , Kim WH . Journal of Surgical Oncology.2010;102(3). CrossRef
- Clinicopathologic significance and prognostic value of Ki-67 expression in patients with gastric cancer: a meta-analysis Luo G, Hu Y, Zhang Z, Wang P, Luo Z, Lin J, Cheng C, Yang Y. Oncotarget.2017;8(30). CrossRef
- Ki67 labelling index in gastric carcinomas. An immunohistochemical study using double staining for the evaluation of the proliferative activity of diffuse-type carcinomas Ramires M., David L., Leitão D., Seixas M., Sansonetty F., Sobrinho-Simões M.. The Journal of Pathology.1997;182(1). CrossRef
- CDX2 expression in the stomach with intestinal metaplasia and intestinal-type cancer: Prognostic implications Seno H., Oshima M., Taniguchi M.-A., Usami K., Ishikawa T.-O., Chiba T., Taketo M. M.. International Journal of Oncology.2002;21(4). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2022
Author Details