The Effectiveness of PAlliative Split COurse RAdiotherapy (PASCORA) Regimen in Non-metastatic Head and Neck Cancer Patients who are Treated with Palliative Intent- A Retrospective Single Centre Study
Download
Abstract
Introduction: We at our centre practice a PAlliative Split COurse RAdiotherapy (PASCORA) of 22.5Gy in 5 fractions followed by a gap of 4 weeks and then again repeat 22.5Gy in 5 fractions for locally advanced squamous cell carcinoma patients treated with a palliative intent. Aim was t0 assess the symptomatic relief at 3 months following PASCORA regimen.
Materials & Methods: 49 Patients with LAHNSCC between January 2014 to January 2021, planned for PASCORA regimen were evaluated. Symptomatic relief was assessed on an objective scale. OS was determined using Kaplan Meir survival curves.
Results: Median age was 61 years, multiple comorbidities (37%) were the most commonly documented reason for these patients being treated with a palliative intent. 25% of our patients had an excellent symptomatic relief, 26% of our patients had a good symptomatic relief and 31% had a partial relief. Median OS was 38 months in patients who had an excellent symptomatic relief and 3-8 months in patients with no or partial symptomatic relief ( p value=0.000) 6% of our patients had Grade 3 /4 RTOG toxicity.
Conclusion: PASCORA regimen offers a good symptomatic relief with good local control rates and acceptable level of toxicity and comparable OS.
Introduction
According to the GLOBOCON 2020 data, head and neck (HN) cancers are the most common malignancies (approximately 1.9 Lakhs cases per year) seen in India [1]. The locally advanced HNC patients are treated with a combination of surgery, radiotherapy (RT) and chemotherapy. Many randomized controlled trials have shown benefit with the use of chemoradiotherapy (CTRT) or anti Epidermal Growth Factor receptor (EGFR) therapy combined with RT resulting in better local control rates and an overall survival rates [2-4]. However, these treatment regimens are directed towards patients intended for curative intent. For patients who are not fit to undergo the proposed radical treatment there isn’t any available evidence based guidelines to offer standard of care. A patient can be deemed unfit for a radical intent treatment either due to poor performance status or due to locally advanced stage at presentation. Usually such patients are offered a multitude of palliative Radiotherapy (RT) regimens followed by supportive therapy. The supportive care including pain medications, airway protection and feeding tubes are most commonly provided for such patients. However, there are no standardised RT regimens which are recommended in these patients.
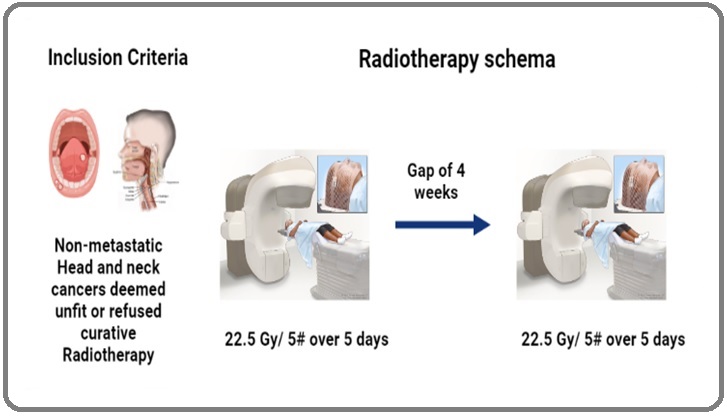
In our centre, we commonly practice PAlliative Split COurse Radiotherapy (PASCORA) which is 22.5 Grays (Gy) in 5 fractions on 5 days with one fraction per day.
Then a treatment gap of 4 weeks followed by second dose of 22.5Gy/5 fractions on 5 days with one fraction per day. In this study we have retrospectively analysed the efficacy of this regimen in patients who were unfit for radical intent treatment.
Materials and Methods
Between January 2014 to January 2020, the patients with histologically proven squamous cell carcinoma of non-metastatic HN cancer, deemed unfit to receive radical intent treatment and planned to be treated by the PASCORA regimen were identified from our RT data base. The patient’s hospital records were accessed after ETHICS committee approval from Medical Records Department (MRD) of our institute. All patients were staged using American Joint Committee on Cancer (AJCC) 7th Edition [5]. The patients demographics, tumour staging, and performance status were documented.
Radiotherapy Procedure
All patients were offered RT according to the department protocol. They were immobilized in supine position with a HN mould and neck placed in neutral position. The image acquisition was done by a non-contrast CT scan of HN region, 3 mm slice thickness i.e. from vertex to 2 cm below the clavicular head. The Gross Tumour Volume (GTV) was defined as all radiologically visible gross disease including the lymph nodes. Prophylactic nodal irradiation was avoided. A margin of 1 cm was given around the GTV to generate PTV 1 (Planning Target Volume 1) in 1st split course. The patients were prescribed a radiation dose of 22.5Gy in 5 fractions to 95% iso dose and delivered over 5 consecutive days with 1 fraction per day. This was followed by a 4-week gap and a second session of 22.5Gy in 5 fractions to 95% iso dose PTV 2 (Planning Target Volume 2) in 2nd split course and was delivered over a period of 5 consecutive days with 1 fraction per day. The patients were planned by 6MV photon beams using 3DCRT technique. The dose constraint used for spinal cord was Dmax (maximum dose to spinal cord) 30Gy with EQD2 of 36.56Gy for late effects with alpha/ beta ratio of 2 (Figure 1).
Figure 1. rt Schema Representation.
Radiobiology
The calculated Biological Equivalent Dose (BED) for this regimen with an alpha/beta of 10 was calculated to 65.23Gy. The biologically 2 Gy equivalent dose (EQD2) was calculated to be 54.38 Gy.
Assessments
The PTV volumes were assessed during both the phases of RT. PTV1 and PTV2 were recorded and the difference between the two was calculated. The patients were assessed clinically before each phase of split course of RT. If there was increase in the volume (in cc) of PTV2 compared to PTV1, the disease was defined as progressive disease. If there was no increase in the volume when compared to PTV1, the disease was defined as stable disease. If there was decrease in volume of PTV2 when to compared to PTV1 by 50% or less, it was termed as partial response. If there was a decrease in the volume of PTV2 when compared to PTV1 by 50-70%, it was termed as good response. If there was a decrease in the volume of PTV2 when compared to PTV1 by more than 70% it was termed as excellent response. After the completion of phase 2 of PASCORA the patients were followed up on a monthly basis for the first 3 months and 3 monthly subsequently. The patients were assessed for symptomatic relief by objective scale (Table 1) and clinically for tumour response on each follow up visit. RT toxicity profile was assessed based on Radiation Therapy Oncology Group (RTOG)/European Organization for Research and Treatment of Cancer (EORTC) acute and late morbidity scoring criteria [6].
| Extent of symptom relief (scale 0-100%) | |
| 0 | No relief |
| Less than 50 | Partial relief |
| 50-75 | Good relief |
| More than 75 | Excellent relief |
Statistics
The statistical analysis was carried using SPSS version 26. The overall survival (OS) was defined as the time duration from the date of diagnosis of the disease till the time the patient was alive from any cause. OS was determined using Kaplan Meir survival curves. The patients who had a disease progression or recurrence were censored. Log Rank test was used to determine the differences in Survival based on several factors. The statistical significance was declared at 10%.
Results
We analysed a total 144 patients with non metastatic HN cancers who were treated with a palliative intent from January 2014 to January 2020. Of these, 84 patients were treated with other regimens of palliative radiotherapy such as 20Gy in 5 fractions or 30 Gy in 10 fractions and thus excluded from the study. Finally, 60 patients planned for PASCORA regimen were included in our study. Of the 60, 11 patients were excluded from the study (3 had disease progression after 1st course of RT, 2 patients defaulted after 2 fractions of RT and 4 patients follow up data was not available).
The patient demographics are summarised in Table 2.
| Number | Percentage (%) | |
| Gender | ||
| Male | 39 | 79 |
| Female | 10 | 21 |
| Age | ||
| Less than 70 years | 41 | 84 |
| More than 70 years | 8 | 16 |
| ECOG –Performance score | ||
| 1 | 35 | 71 |
| 2 | 10 | 20 |
| 3 | 4 | 9 |
| Addiction | ||
| Tobacco alone | 14 | 28 |
| Alcohol alone | 1 | 2 |
| Tobacco + Alcohol | 26 | 53 |
| None | 8 | 17 |
| Comorbidities | ||
| Single | 24 | 49 |
| Multiple | 18 | 37 |
| None | 7 | 14 |
| Site | ||
| Oral Cavity | 7 | 14 |
| Oropharynx | 14 | 28 |
| Laryngopharynx | 11 | 22 |
| Hypopharynx | 14 | 28 |
| Metastasis of Unknown Origin | 3 | 8 |
The median age of patients was 61 years (36-92 years). The ECOG performance status was 2 or more in 14 patients (29%). Majority of the patients had one or more co-morbidities (86%). The most common sites were oropharynx (28%) and hypopharynx (28%) followed by laryngopharynx (22%). The tumour characteristics have been elaborated in Table 3.
| N1 | N2 | N3 | Total | |
| T2 | 0 | 5 | 5 | 10 |
| T3 | 0 | 6 | 15 | 21 |
| T4 | 6 | 9 | 0 | 15 |
| MUO | 0 | 1 | 2 | 3 |
| Total | 6 | 21 | 22 | 49 |
All patients had advanced HNC cancers AJCC stage IVA - 27 (55.2%) and Stage IVB - 22 (44.8%).
Unknown primary of the neck was seen in 3 patients (6%). The various reasons for palliative intent offered to these patients were firstly, the presence of multiple co-morbidities (18 patients, 37%), secondly due to poor performance status (14 patients, 28%), thirdly patient refusal of radical intent treatment (9 patients, 19%) and finally due to advanced age i.e. >70 years (8 patients, 16%) (Table 4).
| Reason | Number | Percentage |
| Multiple Co-Morbidities | 18 | 37% |
| Performance status | 14 | 28% |
| Patients refusing Radical intent | 9 | 19% |
| Advanced Age | 8 | 16% |
All 49 patients completed the planned PASCORA regimen without any treatment delays. The PTV1 and PTV 2 were analysed and compared. 10% of our patients had increase in size of the PTV 2 when compared to PTV 1. About 31% of patients had a less than 30% reduction in size of PTV 2. 47% of our patients had a 30%-50% reduction in size of PTV 2. About 10% of our patients had a reduction of 50 to 75% in PTV 2. While 2% of our patient showed a response of more than 75%. These findings are summarised in Table 5.
| Response | Number | Percentage (%) |
| Increase in size | 5 | 10 |
| <30% size reduction | 15 | 31 |
| 30-50% reduction in size | 23 | 47 |
| 50%-75% reduction in size | 5 | 10 |
| More than 75% reduction in size | 1 | 2 |
Radiation Toxicity
There were no deaths noted amongst the study population. RT induced toxicities are summarised in Table 6.
| Grade | Number | Percentage |
| Grade 1/ 2 | 49 | 100 |
| Grade 3/ 4 | 3 | 6% |
Grade 1 dermatitis were seen in all of our patients at the end of the regimen. 2 patients developed a Grade 3 mucositis following which they required nasogastric tube insertion. 1 patient developed Grade 3 oesophageal toxicity which was managed conservatively. 1 patient developed Grade 4 dermatitis which was managed by a short hospital admission.
Clinical Response
The clinical responses were documented based on symptomatic relief and clinical examination along with indirect laryngoscopic assessment. The findings are summarised in Table 7.
| Type of response | Number | Percentage (%) |
| No relief | 9 | 18 |
| Less than 50% relief-Partial Relief | 15 | 31 |
| 50-75% relief- Good relief | 13 | 26 |
| More than 75% relief- Excellent Relief | 12 | 25 |
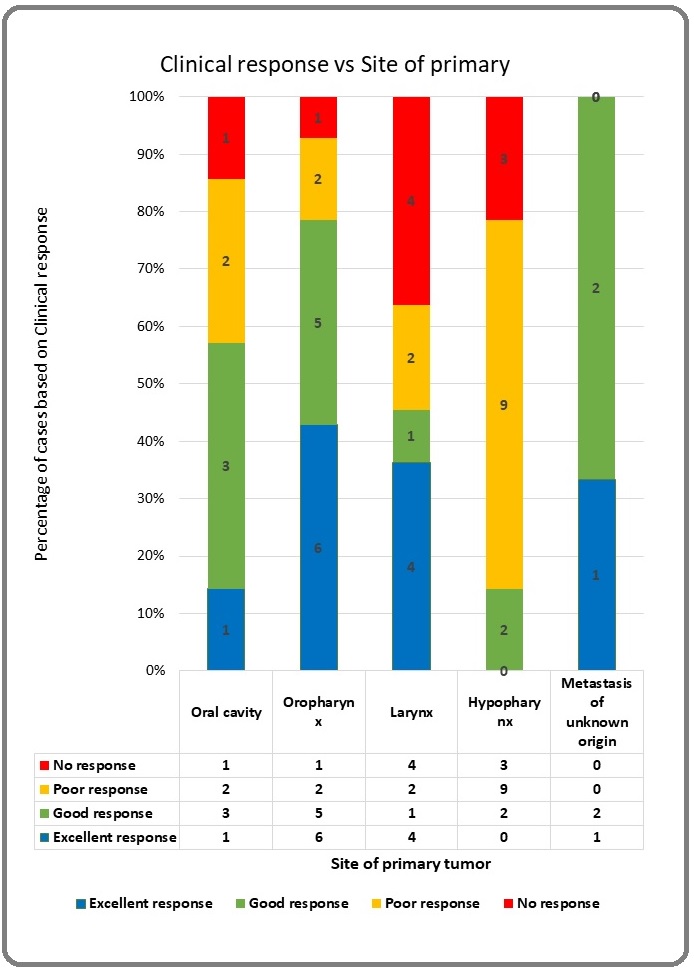
In our study we found that 25% of patients had an excellent symptomatic relief (>75% relief), 26% had a good symptomatic relief, 31% had a partial symptomatic relief (<50% relief) and 18% of had no relief in their symptoms. The extent of symptom relief on univariate analysis was found to be determined to be affected by ECOG status, site of primary and nodal (N) stage. ECOG 1 patients had better symptomatic relief (23/35) when compared to ECOG 2 (1/9) and ECOG 3 (1/3) patients (p value = 0.004). Patients with primary at oropharynx and unknown primary neck had the best clinical relief (78.57% and 100% respectively) when compared to those with hypopharynx primary (14. 28%) (p value = 0.005). Patients with N2 disease (80.95%) had better clinical relief compared to those with N3 disease (27.27%) (p value = 0.001).
Survival Data
The estimated median overall survival is 25 months for a median follow up of 22 months (Figure 2).
Figure 2. Kaplan Meir Survival Curves based on Symptomatic Relief with Life Table.
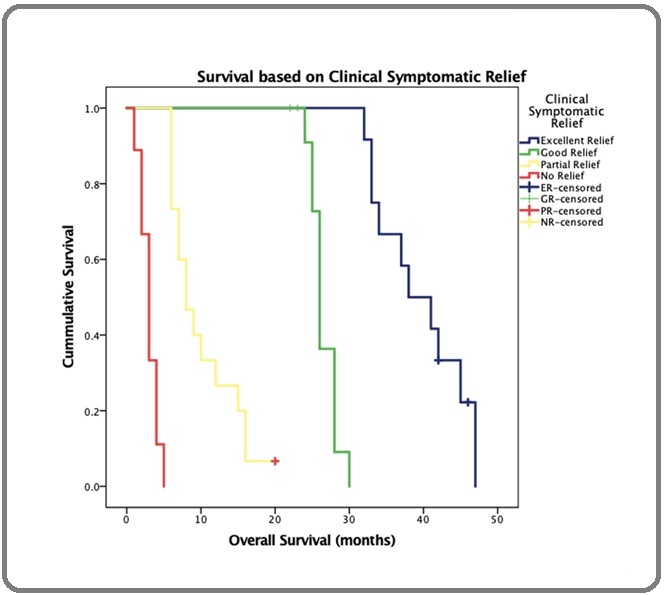
On univariate analysis the overall survival was found to be influenced by site of primary tumour, nodal stage and extent of symptom relief. On Cox regression analysis, extent of symptom relief was the only significant variable with a median survival of 38 months in patients having excellent symptom relief while those with no or partial symptom relief had survival of only 3 to 8 months respectively. This was found to be statistically significant (p value = 0.000) (Table 8 and Figure 3).
| Type of response | Median Overall Survival in months |
| No relief | 3 |
| Less than 50% relief-Partial Response | 8 |
| 50-75% relief- Good response | 26 |
| More than 75% relief- Excellent Response | 38 |
| Overall | 25 |
Figure 3. Clinical Response vs Site of Primary.
Discussion
The 5-year survival rates in locally advanced HNC are reported to be as high as 40-60% [7]. Unfortunately not all patients however will be eligible for a curative intent.
There are several reasons due to which such patients are treated with a palliative intent. The predominant ones being multiple co morbidities, poor performance status, advanced age at presentation and patients refusal of radical intent of treatment due to socio-economic constraints. Either one or a combination of these factors play a crucial role in deeming these patient as palliative intent.
A patient who is offered a palliative intent of treatment usually suffer with an array of symptoms due to local infiltration of the disease. These can be pain, bleeding from primary tumour site, dysphagia, stridor & mental distress. There are no fixed set guidelines to manage a patient intended for palliative treatment. The treatment modalities offered should be able to alleviate the patient’s symptoms without causing additional morbidities or mortalities. Surgery as a palliative option for a patient is not offered since a complete excision of the lesion would not be possible without causing significant morbidity in a locally advanced malignancy [8,9]. Several trials have tried to address the palliative symptoms with chemotherapy alone [10]. Most of these trials included patients with metastasis or recurrence HN cancers and the response rates have been as low as 20-30% [11]. Majority of the patients (approximately 60%) in India fall under the below poverty line (BPL) category [12]. The affordability of these chemotherapy drugs thus becomes a huge challenge for them. In this scenario it is very essential to have a palliative treatment option which is more affordable and less toxic, at the same time comparable to other modalities with respect to symptom relief. RT used in a palliative setting is affordable when compared to other modalities such as surgery or chemotherapy. The tumour control rates are superior and with acceptable toxicity profile when compared to chemotherapy.
One of the most widely used palliative RT dose regimen is 20Gy in 5 daily fractions [13]. Although it offers a good alternative to palliative chemotherapy. In the AIIMS trial it was seen that the patients who had a 50% or more objective regression had received a total dose of 70Gy. However, in a scenario where the patient is treated with a palliative intent, it is unsure if the dose escalation to 70Gy is warranted.
The biggest hurdle for using a split course regimen in a general setup is increase in the total treatment time. The traditional principles of radiobiology state that as the treatment time increases it invariably leads to a decrease in local tumour control. However excellent local control was achieved in our study as demonstrated by significant symptom relief ( 51% patients having good to excellent relief and 31% having partial relief) and the same translating to an increased overall survival (26 - 38 months in good and excellent relief patients respectively).
Also the advantage of using a split course regimen is that it reduces the acute radiation toxicity and yet at the same time delivers a high dose of RT to the tumour. The EQD2 in our study was calculated to be 54.38 Gy. With a gap of 4 weeks and using the radiation dose lost per day was calculated to be only 4.5Gy. Additionally the patient didn’t have to incur added charge for delivering the second palliative course, thus being cost effective.
With the intent of treatment being to alleviate the symptoms rather than cure, to achieve more than 50% symptomatic relief in more than half of our study patients was significant. It was achieved without any financial burden or repeated hospital visits. With this regimen it was found that the RT dose was adequate to achieve a good tumour control rates and symptomatic relief rates.
Split course Radiotherapy
Historically the most commonly used palliative radiotherapy regimen (RT0G 8502 regimen- QUAD SHOT) has been 3 cycles of 14Gy-14.8Gy given over 2 days with twice daily fractions (3.7Gy/fraction BID) followed by a gap of 3-4 weeks between each cycle. Paris et al [14], evaluated 33 patients using this regimen and reported an average survival of 4.5 months. Lok et al [15], evaluated 75 patients on this regimen and reported a median OS of 5-7 months. However, in this study about 45% of patients were non squamous cell carcinoma patients. Corry et al [16] evaluated 30 patients with this regimen and reported a median OS of 5.7 months and PFS of 3.1 months. However, only 53% of patients were able to complete all 3 cycles of QUAD shot regimen in this study. Also in the treatment QOL which was used to assess the patient’s satisfaction after the completion of 3 courses of RT, only 10 out of the 30 felt the treatment course was worthwhile. In our study all 49 patients planned for PASCORA regimen completed the planned course of radiation.
Porceddu et al [17] reported a split course RT in which 30 patients were evaluated. The patients received a dose of 30Gy in 5 fractions with each fraction being at least 3 days apart. The patients who responded well received an addition dose of 6Gy as boost. They reported an objective response of 80%. But there was also a higher incidence of grade 3 mucositis 26% and grade 3 dysphagia 11%.
Kancherla et al [18] retrospectively evaluated 33 patients who received a palliative split course RT of 20Gy in 5 fractions over a week followed by a gap of 2 weeks and then a second course of 20Gy in 5 fractions over a week. They reported a median OS of 9 months.
Murthy et al [19] evaluated 126 patients with a palliative radiotherapy dose of 32 Gy in 8 fractions given over 2 weeks and patients who responded well were given an additional dose of 20Gy in 4 fractions. It was noted in this study that only 26% of the total patients planned were eligible for the additional radiotherapy. Also of note is that out of the original 126 patients only 43 patients were available for the first follow up. This reflects that these RT regimens might have resulted in significant morbidity.
Split Course Radiotherapy with Chemotherapy Minnatel et al [20] evaluated 58 patients with 50Gy/10
fractions of RT given over a split course regimen of 25Gy in 10 fractions with a gap of 2 weeks and then another 25Gy in 10 fractions. Injection Bleomycin 30mg was given concurrently over twice a week for first 3 weeks of treatment. They reported grade 3 mucositis in 27 of 58 patients (47%), which is a significant number considering the fact that the intent was to palliative with a lower rates of toxicity.
Carrascosa et al [21], evaluated 20 patients in QUAD shot regimen of RT along with administration of intravenous Paclitaxel 1 hour before every course of RT. They reported the grade 3 mucositis rate of 14%.
Gamez et al [22], analysed 21 patients with QUAD shot regimen of palliative RT along with administration of concurrent Carboplatin or Cetuximab 1 hour prior to each course of RT. Of the 21 patients 6 were metastatic malignancies, 7 had recurrent diseases. 35% of these patients had grade 2 mucositis which again is a significantly higher number.
From the above trials it’s clear that combining a chemotherapy regimen to a split course RT regimen results in more toxicities overshadowing the cure rates. In a financially constrained country like India the affordability of drugs like Paclitaxel, Carboplatin or Cetuximab is an issue which cannot be over looked. Moreover, these patients are unable to complete the entire regimen due to the toxicities related to the concurrent chemotherapy.
Single course Radiotherapy (10 or less than 10 fractions)
Mohanti et al [13], analysed 574 non metastatic HN cancer patients retrospectively. All patients were given a palliative RT dose of 20Gy in 5 fractions over 1 week. They reported a median OS of 6.7 months and partial response in 37% of the patients.
Saikat et al [23], evaluated 33 inoperable HN cancer patients with a palliative RT dose of 40Gy in 10 fractions with 2 fractions per week. They reported a median overall survival of 7 months and symptomatic relief in 88% of their patients. It was observed that 27% of the patients could not completed the entire course of RT. The total duration of the treatment was 5 weeks. 27% of patients also required radiation breaks in order to recover from the toxicity. There was no comment on the actual duration of treatment in the patients who needed treatment breaks. Although tumoricidal dose was delivered, there was also the risk of treatment delays and potential increase in toxicity which would invariably translate to higher hospital admissions and more financial burden.
Paliwal et al [24], evaluated 50 patients treated with palliative intent with RT of 20Gy in 5 daily fractions. The patients were assessed for symptomatic relief after 1 month of treatment. They reported 92% symptomatic relief in their patients. The short follow up may not be adequate to determine the local response or radiation induced toxicity in such patients.
Fortin et al [25], in a phase 2 study evaluated 32 patients who were planned for palliative RT with a dose of 25Gy in 5 fractions. They reported a median overall survival of 6.5 months.
Single course Radiotherapy (more than 10 fractions)
Agarwal et al [26], evaluated 110 patients with palliative RT of 40Gy in 16 fractions. They reported a PFS at 12 months to be 55.1%. Complete response (CR) was seen in 10% of patients. 63% of patients developed grade III and 3% with grade IV mucositis. It was also seen that 14% patients had grade III dermatitis and grade 2 xerostomia in 54% of the patients.
Al-mamgani et al [27] evaluated 158 patients with palliative RT of 50Gy in 16 fractions. They reported an overall response rate of 71% and OS at 3 years with 17%. It should be noted that 16 patients received induction chemotherapy. Grade III mucositis was seen in 65% of patients and grade III dermatitis was seen in 71% of patients.
As we can see there have a wide array of RT regimens which have been offered when it comes to palliative RT in HN cancers. Each regimen has its own advantage and disadvantage which have been summarised in Table 9.
| Study | Number of participants | PRT dose regimen | Results | Drawbacks |
| 1) Split course Regimens | ||||
| Velu et al (this study) | 49 | 22.5Gy in 5#-->4 wk gap 22.5Gy in 5# | Median Survival 25 months | -No standard QOL questionnaire used for symptom relief since this was retrospective study -Objective assessment of response |
| Paris et al [14] | 33 | 3.7Gy BID (14.4Gy /4 Fr) for 2 daysà (3-4 weeks gap × 3 cycles) | Average survival-4.5 months | -Only 40-50% patients were able to complete the entire course of RT -Increase in treatment time compromised the tumor control probability |
| Lok et al [15] | 75 | Median Survival-5-7 months | ||
| Corry et al [16] | 30 | Median OS-5.7 months, PFS- 3.1 months | ||
| Porceddu et al [17] | 30 | 6Gy in 1 fraction (Fr) à3 days gap ×5 cycles if good response + 6Gy boost | Objective response -80% | Grade III mucositis-26% Grade III dysphagia- 11% |
| Kancherla et al [18] | 33 | 20Gy in 5 Frà 2 weeks gap 20Gy in 5 Fr | Median OS- 9 months | |
| Murthy et al [19] | 126 | 4Gy/ Fr every alternate day upto 8 fractions (32Gy/8Fr) if good response 20Gy in 4Fr | 3.2% CR ,41%PR for primary 3.2%CR for node | -Only 26% patients were eligible for the boost phase -Only 43 of the 126 patients data were available during the first follow up |
| 2) Split Course Regimens with Chemotherapy | ||||
| Minnatel et al [20] | 58 | 25Gy/10Frà 2 week gapà 25Gy/10Fr with Concurrent Inj Bleomycin | Median OS-7mo 80% symptom relief | Grade III mucositis -47% of patients |
| Carascosa et al [21] | 20 | 3.7Gy BID (14.4Gy / 4 Fr) for 2 daysà(3-4 weeks gap × 3 cycles with Inj Paclitaxel 1 hour before every course | Median OS- 4 months, CR in 14% | Grade 3 mucositis in 14% |
| Gamez et al [22] | 21 | 3.7Gy BID (14.4Gy / 4 Fr) for 2 daysà(3-4 weeks gap × 3 cycles with Inj Cetuximab or Carboplatin 1 hour before each course of RT | Median OS- 7 Mo, 24% CR | -35% had Grade II mucositis -Included recurrent and metastatic patients |
| 3) Single course of PRT ( 10 or less than 10 Fractions) | ||||
| Mohanti et al [13] | 574 | 20Gy in 5 Fr 4 weeks gap if >50% responseà convert to Radical dosing | Median OS-6.7 Mo | 100% Grade 2 mucositis in all the treated patients |
| Saikat et al [23] | 33 | 40Gy 10 Fr with 2 Fr/week for a total of 4 weeks | Median OS -7Mo 88% symptom relief | 27% of patients needed treatment breaks to recover from the toxicity |
| Paliwal et al [24] | 50 | 20Gy in 5 Fr | 92% symptom relief after 1 month Follow Up | Only 1 month follow up period |
| Fortin et al [25] | 32 | 25Gy in 5 Fr | Median OS-6.5 Mo | 14% of Grade III toxicity |
| 4) Single course of PRT ( More than 10 Fractions) | ||||
| Agarwal et al [26] | 110 | 40Gy in 16 Fr | Median OS 12 Mo, CR in 10% | 63% Grade III mucositis, 3% with Grade IV mucositis Grade II xerostomia 54% |
| Al-mamgani [27] | 158 | 50Gy in 16 Fr | OS at 3 years-17%, Response rate-71% | 16 patients received Induction chemotherapy Grade III mucositis- 63% Grade III dermatitis-71% |
Ideally a non-metastatic HN cancer patient who is treated with a palliative intent should receive a regimen which offers a good local control rate, has the least toxicity profile and results in an acceptable overall survival.
The shortcomings of our study are that the patients were not evaluated for symptom relief with a standard QOL questionnaire since this was retrospective study. Hence the response evaluation maybe biased. Also an objective assessment of response evaluation by imaging was not done due to financial burden on our patients.
In conclusion, with this study we would like summarize that with the PASCORA regimen of RT of 22.5Gy in 5 fractions followed by a break of 4 weeks and a second course of 22.5Gy in 5 fractions, offers a very good local control rates with acceptable level of toxicity and better overall survival rates. However, to further prove our claim a prospective trial will have to be conducted.
References
- https://gco.iarc.fr/today/data/factsheets/populations/356-india-fact-sheets.pdf .
- Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients Pignon J, Maître A, Maillard E, Bourhis J. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology.2009;92(1). CrossRef
- Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck Bonner JA , Harari PM , Giralt J, Azarnia N, Shin DM , Cohen RB , Jones CU , et al . The New England Journal of Medicine.2006;354(6). CrossRef
- Role of radiotherapy fractionation in head and neck cancers (MARCH): an updated meta-analysis Lacas B., Bourhis J., Overgaard J., Zhang Q., Grégoire V., Nankivell M., Zackrisson B., et al . The Lancet Oncology.2017;18(9). CrossRef
- The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM Edge SB , Compton CC . Annals of surgical oncology.2010;17(6). CrossRef
- Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Cox J. D., Stetz J., Pajak T. F.. International Journal of Radiation Oncology, Biology, Physics.1995;31(5). CrossRef
- Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis Pulte D, Brenner H. The Oncologist.2010;15(9). CrossRef
- Palliative surgery for head and neck cancer with extensive skin involvement Jang DW , Teng MS , Ojo B, Genden EM . The Laryngoscope.2013;123(5). CrossRef
- The role of surgery in the palliation of head and neck cancer Roland NJ , Bradley PJ . Current Opinion in Otolaryngology & Head and Neck Surgery.2014;22(2). CrossRef
- Palliative chemotherapy in head and neck cancer: balancing between beneficial and adverse effects Rajendra A, Noronha V, Joshi A, Patil VM , Menon N, Prabhash K. Expert Review of Anticancer Therapy.2020;20(1). CrossRef
- [Palliative chemotherapy of head and neck cancer: present status and future development] Hennemann B.. Laryngo- Rhino- Otologie.2006;85(3). CrossRef
- https://www.business-standard.com/article/economy-policy/coronavirus-impact-over-100-million-indians-could-fall-below-poverty-line-120041700906_1.html .
- Short course palliative radiotherapy of 20 Gy in 5 fractions for advanced and incurable head and neck cancer: AIIMS study Mohanti BK , Umapathy H, Bahadur S, Thakar A, Pathy S. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology.2004;71(3). CrossRef
- Phase I-II study of multiple daily fractions for palliation of advanced head and neck malignancies Paris K. J., Spanos W. J., Lindberg R. D., Jose B., Albrink F.. International Journal of Radiation Oncology, Biology, Physics.1993;25(4). CrossRef
- Palliative head and neck radiotherapy with the RTOG 8502 regimen for incurable primary or metastatic cancers Lok BH , Jiang G, Gutiontov S, Lanning RM , Sridhara S, Sherman EJ , Tsai CJ , McBride SM , Riaz N, Lee NY . Oral Oncology.2015;51(10). CrossRef
- The 'QUAD SHOT'--a phase II study of palliative radiotherapy for incurable head and neck cancer Corry J, Peters LJ , Costa ID , Milner AD , Fawns H, Rischin D, Porceddu S. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology.2005;77(2). CrossRef
- Hypofractionated radiotherapy for the palliation of advanced head and neck cancer in patients unsuitable for curative treatment--"Hypo Trial" Porceddu SV , Rosser B, Burmeister BH , Jones M, Hickey B, Baumann K, Gogna K, Pullar A, Poulsen M, Holt T. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology.2007;85(3). CrossRef
- The role of split-course hypofractionated palliative radiotherapy in head and neck cancer Kancherla K. N., Oksuz D. C., Prestwich R. J. D., Fosker C., Dyker K. E., Coyle C. C., Sen M.. Clinical Oncology (Royal College of Radiologists (Great Britain)).2011;23(2). CrossRef
- Twice-weekly palliative radiotherapy for locally very advanced head and neck cancers Murthy V., Kumar D. P., Budrukkar A., Gupta T., Ghosh-Laskar S., Agarwal J.. Indian Journal of Cancer.2016;53(1). CrossRef
- Combined radiotherapy and bleomycin in patients with inoperable head and neck cancer with unfavourable prognostic factors and severe symptoms Minatel E., Gigante M., Franchin G., Gobitti C., Mascarin M., Bujor L., Barzan L., Trovò M. G.. Oral Oncology.1998;34(2). CrossRef
- Palliation of pelvic and head and neck cancer with paclitaxel and a novel radiotherapy regimen Carrascosa LA , Yashar CM , Paris KJ , Larocca RV , Faught SR , Spanos WJ . Journal of Palliative Medicine.2007;10(4). CrossRef
- Hypofractionated Palliative Radiotherapy with Concurrent Radiosensitizing Chemotherapy for Advanced Head and Neck Cancer Using the "QUAD-SHOT Regimen" Gamez ME , Agarwal M, Hu KS , Lukens JN , Harrison LB . Anticancer Research.2017;37(2). CrossRef
- Hypofractionated Palliative Radiotherapy in Locally Advanced Inoperable Head and Neck Cancer: CMC Vellore Experience Das S, Thomas S, Pal SK , Isiah R, John S. Indian Journal of Palliative Care.2013;19(2). CrossRef
- Palliative Hypo-fractionated Radiotherapy in Locally Advanced Head and Neck Cancer with Fixed Neck Nodes Paliwal R, Patidar A, Walke R, Hirapara P, Jain S, Raj-Bardia M. Iranian journal of cancer prevention.2012;5.
- Palliative Radiation Therapy for Advanced Head and Neck Carcinomas: A Phase 2 Study Fortin B, Khaouam N, Filion E, Nguyen-Tan PF , Bujold A, Lambert L. International Journal of Radiation Oncology, Biology, Physics.2016;95(2). CrossRef
- Hypofractionated, palliative radiotherapy for advanced head and neck cancer Agarwal JP , Nemade B, Murthy V, Ghosh-Laskar Sa, Budrukkar A, Gupta T, D'Cruz A, Pai P, Chaturvedi P, Dinshaw K. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology.2008;89(1). CrossRef
- Hypofractionated radiotherapy denoted as the "Christie scheme": an effective means of palliating patients with head and neck cancers not suitable for curative treatment Al-mamgani A, Tans L, Van rooij PHE , Noever I, Baatenburg de jong RJ , Levendag PC . Acta Oncologica (Stockholm, Sweden).2009;48(4). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2022
Author Details