A Rare Presentation of Primary Plasma Cell Leukemia: A Case Report and Review of Literature
Download
Abstract
Background: The primary plasma cell leukemia (pPCL) is an aggressive plasma cell neoplasm. It is diagnosed by the presence of an absolute plasma cell count of >2 × 109/L or 20% plasma cells in the peripheral blood. pPCL is rare and reported to be <1 in a million. Hence, our case report is a rare opportunity to describe clinical presentation and management of pPCL.
Case Details: A 72-year-old male patient with benign prostatic hyperplasia, presented to the hospital for cough, breathlessness, and intermittent fever of one month. On arrival, he was tachypnoeic with altered sensorium and rales in his chest. Laboratory examination showed anemia, leucocytosis, and thrombocytopenia. The peripheral blood smears revealed 25-30% circulating atypical plasmacytoid cells. Flow cytometry on the peripheral blood revealed 58.7% lymphoid cells. Out of the total lymphoid cells, 73.6 % cells were characterized by expression of CD38, CD138, CD19, CD49d, CD43, CD27, CD81 (96.9% dim positives) CD 56 with dim kappa light chain restriction suggestive of PCL. Furthermore, serum protein electrophoresis and serum immunofixation showed an M-band (0.53g) of IgG kappa subtype. However, the patient developed lower respiratory tract infections with multi-organ dysfunction and he succumbed to the same.
Conclusion: The prognosis of pPCL is very poor and the high risk of infective complications. Early diagnosis and optimal chemotherapy would be the key to the management. Detailed peripheral blood film examination and characterization of abnormal cells with immune phenotyping are of utmost importance in diagnosing pPCL.
Introduction
Plasma cell leukemia (PCL) is a rare, but one of the most aggressive variants of multiple myeloma (MM) characterized by a very high number of plasma cells in the blood and bone marrow [1]. The incidence of PCL ranges between 2 and 4% of patients with MM [2].
The first PCL was reported in 1906 by Gluzinski and Reichenstein in a patient who presented with bone pain, a palpable rib mass, rib fractures, anemia, and splenomegaly [3]. Traditionally, PCL has been diagnosed with the help of Kyle’s criteria which required the presence of 2 × 109/μL peripheral blood clonal plasma cells or >20% plasma cells in the peripheral blood. It accounts for about 0.6%-4% of all plasma cell neoplasms [4]. Of them, 60–70% of PCL is primary and 30–40% is secondary.
The primary plasma cell population is of “de novo” origin with no evidence of previous MM and in secondary PCL it is observed as a leukemic transformation of relapsed or refractory disease in patients with previously diagnosed with MM [2, 5]. Although PCL and MM are closely related disease entities, the prognosis of PCL patients is poor with the median survival ranging from 6 to 11 months with up to 28 % dying within the 1st month after diagnosis [3].
The best therapeutic plan for pPCL remains unknown so far. Patients treated with standard chemotherapy showed an overall response rate of <50%, with a median survival of a few months [6]. Treatment includes a combination approach with proteasome inhibitors including bortezomib, immunomodulatory drugs like lenalidomide, and autologous stem cell transplantation, but the outcomes are not promising. However, even after treatment, median survival after rigorous chemotherapy and transplant procedures may partially improve but not more than three years [3, 4]. There are a few case reports which showed effective use of newer agents like Daratumumab in PCL.
Case Report Chief complaints
A 72-year-old male patient presented to the local
hospital in Pune for cough, breathlessness, and intermittent fever for a month. He was treated as left lower lobe consolidation and received multiple antibiotics like Meropenem, Piperacillin tazobactam, and Clarithromycin empirically. Afterward patient developed altered sensorium and worsening respiratory distress and therefore he was referred to Deenanath Mangeshkar Hospital, Pune, India for further management.
History of past illness
He was a known case of Benign Prostatic Hyperplasia (BPH) on medical management. He denied any history of smoking and did not have any significant family history. There was no history of Diabetes Mellitus, hypertension, bronchial asthma, obstructive lung disease, or coronary artery disease.
Physical examination
On arrival, he was afebrile, drowsy & arousal with bilateral basal crepitations. His vital signs were stable except for tachypnoea and physical exam was otherwise unremarkable. He was able to maintain oxygenation with venti mask.
Laboratory examination
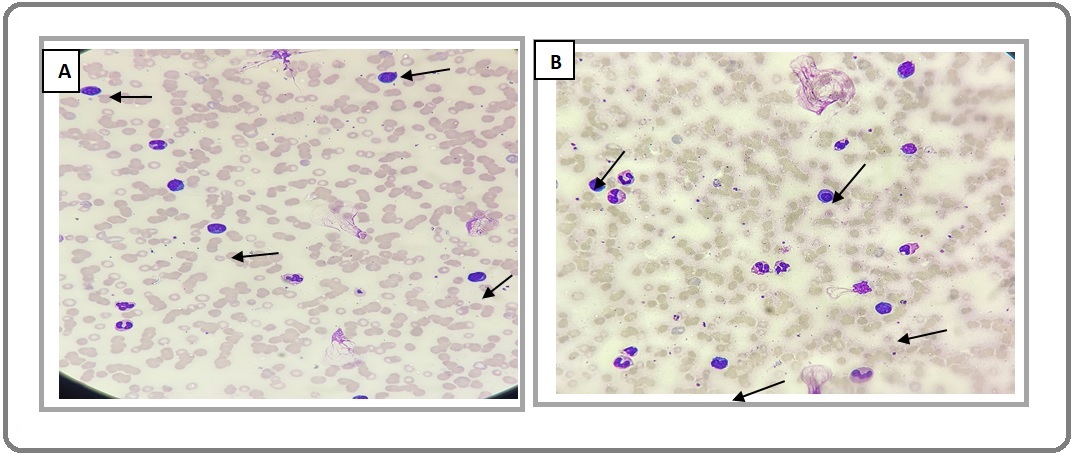
His laboratory evaluation revealed anemia (Hb of 8.7 mg/dL), leucocytosis with a total leucocyte count of 38700/c.mm), and thrombocytopenia (39000/c.mm). The peripheral blood smear showed 35-40% circulating atypical plasmacytoid lymphoid cells of medium size with eccentric nuclei and deep blue cytoplasm, which were confirmed from flow cytometry (Figure 1 A, B).
Figure 1. (A) and (B) 35-40% Atypical Plasmacytoid Lymphoid Cells, Small to Medium in Size with Eccentric Nuclei and Deep Blue Cytoplasm (Black arrows).
Biochemistries were significant for sodium of 127 Meq/L, creatinine of 1.19 mg/dL, serum uric acid level of 11.69 mg/dl, blood urea level of 115 mg/dl and calcium of 7.70 mg/dL. The liver enzymes were normal with a total bilirubin of 1.8 mg/dL and reversal of albumin globulin ratio with elevated globulins up to 6.03g/dl (Table 1).
| Blood component | Result |
| Haemoglobulin | 8.7 mg/dl |
| Total leucocyte counts | 38700/Cmm |
| Platelet count | 39000/Cmm |
| Neutrophils | 41.20% |
| Lymphocytes | 39.30% |
| Eosinophils | 3% |
| Monocytes | 16.10% |
| Basophils | 0.40% |
| Peripheral smear | 35-40% atypical plasmacytoid Lymphoid cells |
| Serum Calcium | 7.70mg/dl |
| Serum Phosphorus | 6.60 mg/dl |
| Uric Acid | 11.69 mg/dl |
| Serum Creatinine | 1.19mg/dl |
| Blood urea level | 115mg/dl |
| Albumin | 1.90 g/dl |
| Globulin | 6.06 g/dl |
| Serum LDH | 673 IU/L |
Imaging findings: (Chest X ray)/ CT chest)
A chest x-ray showed left lower lobe haziness. Computed tomography (CT) scan of the chest was ordered to characterize the lung lesions better and the same revealed left lower lobe pneumonia. As the patient showed agitation with altered sensorium, a CT scan of the brain was performed to rule out any intracranial pathology, which showed age-related cerebral atrophy with chronic white matter ischemic changes. By the time diagnosis of plasma cell leukemia was made, the patient was not fit enough to undergo PET CT.
Further diagnostic work-up: Protein electrophoresis and Flow cytometry (FCM)
Serum protein electrophoresis (SPEP) revealed a total protein level of 8.80 g/dL (normal range: 6.40–8.20 g/dL), albumin level of 2.36 g/dL (normal range: 3.50–5.60 g/dL), α1- globulin level of 0.32 g/dL (normal range: 0.10–0.30 g/dL), α2-globulin level of 0.35 g/dL (normal range: 0.20–1.00 g/dL), β-globulin level of 0.88 g/dL (normal range: 0.50–1.10 g/dL), γ-globulin level of 4.89 g/dL (normal range: 0.50–1.50 g/dL) and albumin/globulin ratio of 0.37 (normal ratio: 0.90–2.00) (Table 2).
| SPEP Examination | |
| Fraction | Patients value |
| Albumin | 2.36 g/dL |
| α1- globulin | 0.32 g/dL |
| α2-globulin | 0.35 g/dL |
| β-globulin | 0.88 g/dL |
| γ-globulin | 4.89 g/dL |
| Total Sr. Protein | 8.80 g/dL |
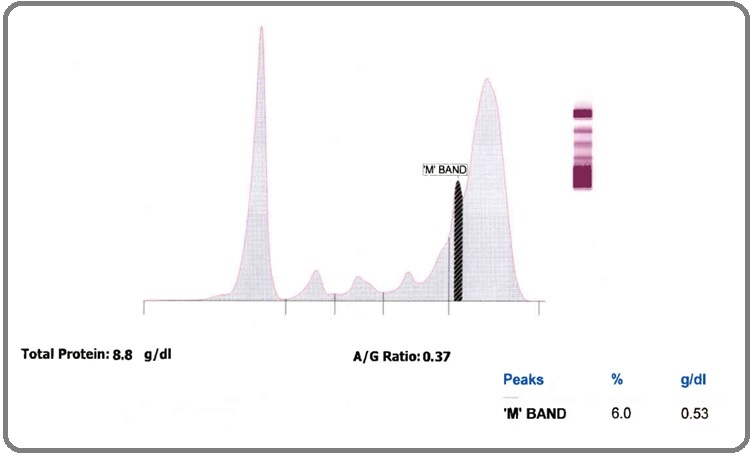
Also, the thick “M band” (0.53g/dl) were seen at the junction of beta & gamma region results in polyclonal increase in gamma globulin.
Serum-free light chain assay revealed the elevated kappa free light chains at 488.00 mg/L (normal range: 6.7–22.4 mg/L) (Figure 2), free lambda light chain at 482mg/dl (normal range: 8.3–27.0 mg/L) and a kappa/ lambda ratio of 1.01 (normal ratio: 0.26–1.65).
Figure 2. Serum Protein Electrophoresis (SPEP) Showed M' BAND (0.53 g/dl) at the Junction of Beta and Gamma Region with Background Polyclonal Hypergammaglobulinemia.
Serum β2-microglobulins were markedly raised at 13,606ng/ mL (normal range: 670–2,143 ng/mL). Furthermore, he showed the marked increase in all immunoglobulins IgG level of 4430 mg/dl (normal range; 700-1600mg/dl), IgA level of 792 mg/dl (normal range; 70-400mg/dl), and IgM level of 323mg /dl (normal range; 40-230 mg/dl). His serum lactic dehydrogenase (LDH) was elevated at 673 IU/L (normal range: 125-220 IU/L). The bone marrow aspiration was avoided, as the patient was not co-operative with altered sensorium and severe breathlessness.
Flow cytometry (FCM)
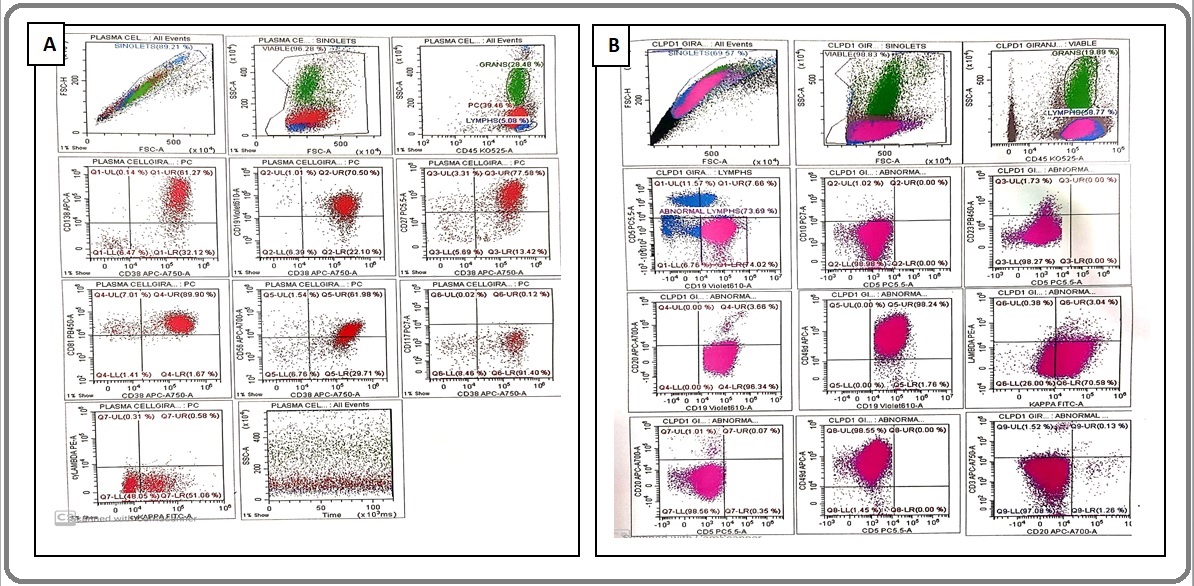
FCM on the peripheral blood revealed 58.7% lymphoid cells. Out of the total lymphoid cells, 73.6 % cells were characterized by expression of CD38, CD138, CD19, CD49d, CD43, CD27, CD81 (96.9% dim positives) CD 56 with dim kappa light chain restriction (cytoplasmic as well as surface staining). These gated cells are negative for CD5, CD23, CD3, CD10, CD20, FMC-7, CD11c, CD25, CD200, CD103, CD117, lambda light chain (cytoplasmic as well as surface staining) suggestive of PCL (Figure 3).
Figure 3. Flow Cytometry (A) Shows 73.6% of Atypical Cells Expressing CD38 (+), CD138 (+), CD56 (+) with Dim Cykappa, CD19 (+), CD81 (+) and CD117(-). (B) shows atypical cells lacking expression of CD5, CD10, CD23 and CD20.
Final Diagnosis
Based on the findings of the peripheral smear flow cytometric analysis, quantitative serum immunofixation electrophoresis, a final diagnosis of primary plasma cell leukemia (pPCL) was made.
Treatment
Following confirmation of the diagnosis of pPCL, the patient was treated with 4 drug-based regimen- Cyclophosphamide (300mg IV on Day 1,8,15,22), Bortezomib (2mg iv push on Day 1,4,15,22) Dexamethasone (40mg PO for 4 days/week) and Thalidomide (100 mg OD). Supportive therapy was given with hydration, allopurinol, antiviral prophylaxis with acyclovir, zolendronic acid, blood, and platelet transfusion.
After chemotherapy patient’s sensorium deteriorated further and he needed sedation with fentanyl infusion. His general condition worsened with hypertensive left ventricular failure which was managed with Nitroglycerin infusion, diuretics and, Dexmedetomidine infusion. The trial of weaning from the ventilator failed. 2D ECHO showed severe pulmonary hypertension which was normal at the time of admission. Sonography of abdomen and pelvis revealed the splenomegaly with possible evolving abscess. He developed multi-organ failure following this and could not be revived from the same.
Discussion
PCL is identified by the presence of > 20% circulating plasma cells or an absolute plasma cell count > 2 × 109/L on peripheral smear [4]. This criteria is still followed as the WHO criterion for the diagnosis of PCL. It is a rare and aggressive form of plasma cell dyscrasia, characterized by a poor prognosis with rapidly fatal outcomes. The median survival with chemotherapy is 2–8 months, but complications usually lead to death within the first few months of diagnosis. The outcome is relatively poor because of the absence of effective treatment options and infective complications of available options for this condition [4, 7].
The incidence and prevalence of pPCL are relatively low because most of the clinical data come from isolated case reports and small retrospective studies. Therefore the incidence of pPCL is believed to be less than 1 case per million population. From the surveillance, epidemiology, and end results (SEER) database it is observed that there are no significant differences based on gender, age, or race when compared with patients with MM. PCL exist in all races and all geographic locations. As compared to MM, PCL is more common in African Americans and blacks from Africa than whites [5, 7]. Patients with PCL are usually younger (aged 50-59 years) as compared with MM (aged 65-70 years) [4, 5]. Our patient was aged 70 years; this shows the wide range of presentation of pPCL. The pPCL shows a more aggressive clinical presentation than MM, including a higher tumor burden. Generally, patients manifest similar symptoms of MM due to profound anemia, hypercalcemia, and thrombocytopenia. On physical examination, patients generally exhibit more prevalence of organomegaly with the involvement of the liver, spleen, lymph nodes, with possible pulmonary manifestation like pleural effusions. Also, neurological deficits related to central nervous system involvement. Our patient had splenomegaly. On blood analysis, anemia, leukocytosis, and thrombocytopenia will be visible. Also, PCL patients will have an increased level of lactic dehydrogenase (LDH) and beta 2 microglobulin. Immunophenotypically, plasma cells in PCL present a wide spectrum of monoclonal gammopathy [5, 7]. However, our patient had hypergammaglobulinemia with an increase in IgM and IgA as well. Similar features were also reported by Singh S. et al [8]. The diagnostic evaluation of a patient with suspected PCL is similar to that of MM. It includes a review of the peripheral blood smear, bone marrow aspiration and biopsy, serum protein electrophoresis with immunofixation, urine protein electrophoresis, and peripheral blood plasma cell assessment by flow cytometry [5, 7]. Our present case also had profound anemia, leukocytosis, thrombocytopenia, hypercalcemia, and elevated serum LDH, creatinine, and blood urea. Our patient did not have characteristic clinical features of myeloma like bone pains and acute renal failure. The diagnosis of plasma cell leukemia was incidental due to abnormal lab parameters and it accounts for about 1% cases of PCL [9].
There are two types of pPCL i.e. secretory and non-secretary. In the secretary type of pPCL M-protein is detected in serum electrophoresis while in non-secretary type M-protein is absent. Our case was a secretary type of PCL showing a thick M band in serum electrophoresis. This feature was also seen in the case series by Gangadhar P. et al. While in patients with non-secretary PCL a monoclonal protein is absent in the serum and urine due to (i) low synthetic capacity for immunoglobin (nonproducers) (ii) an inability to excrete immunoglobin (nonsecretors) (iii) or rapid extracellular degradation of abnormal immunoglobins (iv) increased intracellular degradation [5].
How to use flow cytometry (FCM) for diagnosis of PCL
The plasma cells in peripheral blood can be characterized by immunophenotyping. The expression of cluster differentiation factors differs between myeloma and plasma cell leukemia. PCL tends to express CD20 more commonly compared to myeloma and express CD56 less often [10]. With progress in the treatment of myeloma, now all hematologists aim for stringent complete remission (CR) and, hence the use of FCM to demonstrate clonality of plasma cells has gained popularity. Multiparameter flow cytometry (MFC) is a routine tool for measurable residual disease assessments (MRD) in many tertiary care centers. With very specific markers or their combination, it is now possible to differentiate clonal and reactive plasma cells. The same technique can be used to diagnose and find aberrancies in PCL. Although clonal plasma cells in myeloma and PCL express CD38 and CD138 alike, expression various other markers like CD27, CD28 vary between them. HLA DR, CD9, CD56 and, CD117 are expressed low and CD20 may be expressed high in PCL compared to MM. Similarly, CD28 and CD27 are more frequently seen in PCL and their acquisition in tumor cells gives them survival advantage by helping in cell proliferation and by activating the NFkB pathway respectively.
The prognosis of pPCL is very poor as there are no standard curative therapies. The treatments usually given are derived from those used in the management of MM [6]. During treatment, the most important prognostic factor is the response to treatment, as patients presenting with the disease showed resistance to initial therapy, results in a poor outcomes [11]. Induction therapy with novel triplet therapy using immunomodulators and proteasome inhibitors such as VRd (Bortezomib, Lenalidomide, and Dexamethasone) or KRd (Carfilzomib, Lenalidomide, and Dexamethasone), has shown a relatively good response in both primary and secondary PCL. In those patients who have an aggressive form of pPCL, more aggressive combination regimen can be used, like VDT-PACE (Bortezomib, Dexamethasone, Thalidomide or Lenalidomide, Cisplatin, Doxorubicin, Cyclophosphamide, and Etoposide) or HyperCVAD (High dose Cyclophosphamide, Vincristine, Adriamycin and, Thalidomide or Lenalidomide), as in proliferative disease cyclophosphamide and doxorubicin are particularly effective [6, 7]. Also, induction therapy includes some alternative options with Bortezomib-based regimens such as VDT (Bortezomib, Thalidomide, and Dexamethasone), VRD (Bortezomib, Lenalidomide, and Dexamethasone), VCD (Bortezomib, Cyclophosphamide, and Dexamethasone), VAD (Bortezomib, Doxorubicin, and Dexamethasone), or VMP (Bortezomib, Melphalan, and prednisone) [6, 7]. With advancements in medical science, immunotherapy and, targeted therapy with monoclonal antibody- anti CD38 antibody Daratumumab and soon Isatuximab would the backbone of managing plasma cell leukemia, both in transplant and non-transplant patients [12].
After induction therapy, an autologous stem cell transplant is recommended as the consolidative modality (for transplant-eligible pPCL patients) to achieve prolonged disease control. In the immunocompromised patients standard antiviral and bacterial prophylaxis should be used. The tumor lysis syndrome may also develop in these patients, so appropriate prophylaxis with allopurinol and appropriate hydration is indicated. Along with this adequate antithrombotic prophylaxis as well as platelet, red blood cell monitoring, and replacement is also important. Osteolytic bone lesions are observed, though less common than MM. Therefore, all patients with pPCL should be started on bisphosphonate therapy along with vitamin D and calcium supplements [4].
The International Myeloma Working Group have proposed the seven categories of PCL remission based on the following four main parameters: (1) peripheral blood plasma cell count; (2) bone marrow plasma cell count; (3) serum and urinary M-protein levels; and (4) the assessment of extramedullary disease. In general, a patient is considered to be incomplete remission if plasma cells are absent from the peripheral blood and there are <5% of plasma cells in the bone marrow. Moreover, relapse is denoted by a >10% increase in bone marrow plasma cells with the reappearance of peripheral blood plasma cells and M-protein, along with evidence of extramedullary disease [8].
In conclusion, our case is unique in different ways. (1) Clinical presentation- (incidental detection while evaluating for pneumonia. (2) Use of peripheral blood flow cytometry to characterize the plasma cells instead of bone marrow. (3) Hypergammaglobulinemia. So the take-home message is attention into peripheral blood film and wise utilization of modern diagnostic armamentarium like FCM is going to help patients and doctors to make an early diagnosis of this rare disease.
Conflicts of interest
The authors declare that they have no conflict of interest.
Acknowledgments
Dr.Vijaya Gadage, MD Pathology,Consultant Hematopathology, at Deenanath Mangeshkar Hospital,Pune who reported the FCM.
Dr.Sayali Jadhav, DNB Pathology trainee, at Deenanath Mangeshkar Hospital, Pune who help in getting colorful images.
References
- Primary plasma cell leukemia: A case report and review of the literature Ngu S, Asti D, Valecha G, Thumallapally N, Pant M, Bershadskiy A. Clinical Case Reports.2019;7(9). CrossRef
- Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group Fernández de Larrea C., Kyle R. A., Durie B. G. M., Ludwig H., Usmani S., Vesole D. H., Hajek R., et al . Leukemia.2013;27(4). CrossRef
- A case report of a young female with plasma cells leukemia Rashid NG , Jalal SD . Iraqi Journal of Hematology.2017;6(2). CrossRef
- Plasma cell leukemia - one in a million: A case report Jain AG , Faisal-Uddin M, Khan AK , Wazir M, Shen Q, Manoucheri M. World Journal of Clinical Oncology.2019;10(3). CrossRef
- Primary plasma cell leukemia: A report of two cases of a rare and aggressive variant of plasma cell myeloma with the review of literature Gangadhar P, Ahmed Z, Pai MR , Sandhya I. Indian Journal of Pathology & Microbiology.2016;59(4). CrossRef
- Frontline chemotherapy with bortezomib-containing combinations improves response rate and survival in primary plasma cell leukemia: a retrospective study from GIMEMA Multiple Myeloma Working Party D'Arena G., Valentini C. G., Pietrantuono G., Guariglia R., Martorelli M. C., Mansueto G., Villani O., et al . Annals of Oncology: Official Journal of the European Society for Medical Oncology.2012;23(6). CrossRef
- Plasma cell leukemia Chauhan S, Jaisinghani P, Rathore J, Tariq H, Galan Y, Madhavan A, Rana H, Frenia D. Journal of Family Medicine and Primary Care.2018;7(2). CrossRef
- Primary Plasma Cell Leukaemia: Case report and review of the literature Singh S, Rath A, Yadav S. Sultan Qaboos University Medical Journal.2018;18(3). CrossRef
- Plasma Cell Leukemia: Definition, Presentation, and Treatment Gundesen MT , Lund T, Moeller HEH , Abildgaard N. Current Oncology Reports.2019;21(1). CrossRef
- Primary plasma cell leukemia: clinical, immunophenotypic, DNA ploidy, and cytogenetic characteristics García-Sanz R., Orfão A., González M., Tabernero M. D., Bladé J., Moro M. J., Fernández-Calvo J., Sanz M. A., Pérez-Simón J. A., Rasillo A., Miguel J. F.. Blood.1999;93(3).
- Primary Plasma Cell Leukemia: Identity Card 2016 Musto P, Simeon V, Todoerti K, Neri A. Current Treatment Options in Oncology.2016;17(4). CrossRef
- CD38: A Target for Immunotherapeutic Approaches in Multiple Myeloma Morandi F, Horenstein A; , Costa F, Giuliani N, Pistoia V, Malavasi F. Frontiers in Immunology.2018;9. CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2022
Author Details