Study of Mismatch Repair Protein Expression by Using Immunohistochemistry in Various Carcinomas with Special Reference to Colorectal Adenocarcinomas - At a Tertiary Referral Laboratory in India
Download
Abstract
Background: DNA mismatch repair (MMR) is an important process during the DNA replication for correcting DNA replication errors. Deficient MMR (dMMR) leads to increased mutational burden and has been associated with several carcinomas and cancer syndromes like Lynch syndrome. The absence of MMR protein by immunohistochemistry is a surrogate marker for microsatellite instability. The aim of the study is to present our datas of MMR deficient tumors and the pattern of MMR protein loss by using immunohistochemistry,also to highlight the technical issues of tissue processing that interfere in result interpretation.
Material & Method: 318 cases of various carcinomas were analysed for mismatch repair proteins viz. MLH1, MSH2, MSH6 and PMS2 using immunohistochemistry.
Result: Out of total 318 cases,47 cases showed deficient MMR proteins. Among the MMR deficient cases colonic adenocarcinoma cases has the highest percentage with loss of MMR proteins. Regarding pattern of MMR protein loss the simultaneous loss of both MLH1 and PMS2 is the most common pattern. In 5 cases (1.5%) of total cases we could not interpret the result. In compare to MMR proficient colorectal adenocarcinomas, the MMR deficient tumors are predominantly right sided and on histopathology they shows high grade histology,intratumoral lymphocytic infiltration, peritumoral lymphocytic infilatration,,mucinous and signet cell components.
Conclusion: Evaluation of MMR proteins using immunohistochemistry is relatively easy to institute in the routine testing as it is a useful predictive and prognostic marker in various carcinomas,also it helps screening the cases of lynch syndrome. This study also highlight the need of using standard protocols for tissue fixation and processing before evaluating for MMR proteins.
Introduction
During the cell cycle, DNA mismatch repair (MMR) is a critical step to repair errors in DNA replication [1]. Due to mutation in MMR genes there is loss of function of the MMR pathway. Failure to repair replication-associated errors due to a defective MMR system allows persistence of mismatch mutations all over the genome, but especially in regions of repetitive unit of DNA known as microsatellites this causes instability in microsatellite region giving rise to the phenomenon of microsatellite instability (MSI) which results in accumulation of additional mutations at a rapid rate that leads to development of carcinoma. Such microsatellite instability is the hallmark of the most common form of hereditary susceptibility to CRC, known as Lynch syndrome, but is also observed in approximately 15-20% of sporadic colonic cancer.Patients diagnosed with Lynch syndrome are prone to develop many different carcinomas like carcinomas of colorectum, endometrium, ovary, prostate, small bowel, stomach, bladder, ureter, urethra, brain, kidney, biliary tract and gallbladder and sebaceous adenomas/carcinomas [2-4].
The four most commonly mutated DNA mismatch repair genes are MLH1, MSH2, MSH6 and PMS2. They function as heterodimer. MLH1 and PMS2 are heterodimer where MLH1 is the dominant one i.e loss of MLH1 leads to degradation of PMS2 but not the vice versa, similarly MSH2 and MSH6 are heterodimer where MSH2 is the dominant one i.e loss of MSH2 leads to degradation of MSH6 but not the vice versa. Defective MMR gene can be detected via immunohistochemistry. Absence of MMR protein by immunohistochemistry is a surrogate marker for microsatellite instability.Testing for MSI (thus classifying microsatellite stable, MSI–H and MSI–L) is done by molecular testing of at least five loci recommended by National cancer institute using PCR or NGS technique. In the year 1996, first monoclonal antibodies against MMR proteins became available; first came antibodies to MSH2 and then to others. Such antibodies rendered IHC detection of MMR protein possible, providing an alternative methodology for detecting MMR deficiency [5]. Furthermore it is helpful in identifying the specific defective genes within the microsatellite instable tumors. Also detection of MMR using IHC is cost effective and easily available. Specific staining is performed on tumor tissue for each of the 4 mismatch repair proteins, mutations in MMR genes typically lead to nonfunctional proteins that do not stain by IHC suggestive of defective DNA mismatch repair within the tumor. Also an abnormal immunostain can guide the choice of genes to be analysed.
In colon cancer Up to 15% of sporadic colon cancers are also associated with microsatellite instability, usually due to somatic hypermethylation of the MLH1 promoter and more than 50% of them harbor BRAF V600E mutation. Thus detection of BRAF V600 E excludes possibility of Lynch syndrome. In the colon, BRAF mutations are a reasonable surrogate for the presence of MLH1 promoter hypermethylation, but a similar molecular pairing is not seen in the gynecologic tract [6-11]. Thus BRAF mutation testing has exceedingly rare or no role in endometrial carcinomas [6-8].
Identification of the tumors with deficient MMR proteins have clinical importance as these tumors have prognostic value [12], a predictive marker of response to 5FU chemotherapy specially in colorectal carcinomas [13,14] and screening tool for lynch syndrome.
Again the application of the immunotherapy in cancer is limited because of variation of its response and efficacy to certain tumor. Hence the uses of biomarker testing like deficient MMR protein or MSI-h, PDL1 expression, tumor infiltrating lymphocytes, tumor mutational burden are useful in selecting patients and predict therapeutic response to immune check point blockade (ICB) therapies.one importatnt aspect of dMMR or MSI-H is it is independent of tumor location and type of tumor while identifying responders.The US food and drug administration (FDA) approved the uses of ICB therapies in all dMMR solid tumors. Pembrolizumab,an anti-programmed death 1 (PD1) antibody is showing effective in mismatch repair deficient tumors specially in colo-rectal carcinomas [15].
Even though analysis of MMR protein and subsequently the MSI testing has great preventive and therapeutic significance, in our region, there is not much data available on the MMR deficient genes and tumors. In this study, we aim to present our datas of MMR deficient tumors and also the pattern of MMR protein deficiency by using immunohistochemical techniques and compare our datas with those of the reported literatures.
Materials and Methods
It was a retrospective study,total 318 cases were analysed in two year duration from November 2019 to October 2021.The datas of the MMR testing which was done when requested by the referring treating physician in primary gross specimens and biopsies as well as on the blocks submitted for review in our centre were analysed. The patients demographic records, clinical details were retrieved from our digital archive.
For the immunohistochemistry stain, slides from the formalin fixed paraffin embedded tumor tissues were used. The monoclonal antibodies which were used in this study are FLEX Monoclonal Rabbit Anti-Human MutS Protein Homolog 6 Clone EP49 for MSH6, FLEX Monoclonal Mouse Anti-Human MutS Protein Homolog 2 Clone FE11 for MSH2, FLEX Monoclonal Mouse Anti-Human Mut L Protein Homolog 1 Clone ES05 for MLH1, FLEX Monoclonal Rabbit Anti-Human post meiotic segregation increased 2, clone EP51 for PMS2. Staining was done on the VENTANA Benchmark ULTRA IHC platform. Normal colonic mucosa, lymphocytes and stromal cells used as positive control.
The H&E slides for morphological features and the IHC results were interpreted by the consultant pathologists using olympus CX33 microscope. Reporting was done following the CAP protocol of bio marker testing for MMR protein. Any amount of intact nuclear staining by IHC was considered as positive(intact expression), cases with weak and focal nuclear positive were also considered as positive but noted separately. The loss of nuclear staining by IHC with positive internal control (lymphocytes, normal colonic mucosa and stromal cells) was considered as loss of expression.Again cases with negative internal control after repeat testing was considered not interpretable.
In the cases where MLH1 expression was lost in colorectal carcinomas, BRAF V600 mutation testing was advised to look for somatic methylation of the MLH1 promoter.
Results
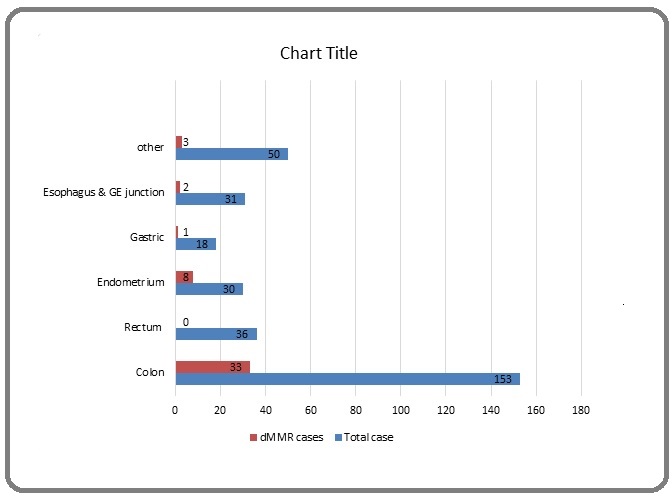
There are total 318 cases of different tumors analysed for MMR protein deficient(dMMR). The detail of the different site and diagnosis of the tumors analysed for the dMMR is listed in the Table 1.
| Site | Diagnosis | Number |
| Colon | Adenocarcinoma | 153 |
| Rectum | Adenocarcinoma | 36 |
| Gastric | Adenocarcinoma | 18 |
| Esophagus & GE junction | Adenocarcinoma | 31 |
| Endometrium | Endometrial adenocarcinoma | 30 |
| Other tumor* | 50 | |
| Total cases | 318 |
*Other tumor includes adenocarcinomas of Prostate, Ileum, Pancreas, Cervix, Gallbladder, Lung, Cholangiocarcinoma, Urothelial carcinoma & Mesothelioma.
Out of the total 318 cases analysed , total 47 cases were found to be deficient in one or more of the MMR proteins. Moreover 5 cases of the total cases which includes 2 cases each of colonic and endometrial adenocarcinoma and 1 case of prostate adenocarcinoma were not interpretable. The site wise distribution of the MMR deficient cases and pattern of MMR protein loss were summarised in Table 2, Figure 1.
| Site | No of case | dMMR cases | Not Interpretable cases | % age of dMMR case | Pattern of MMR deficiency | |||||
| MLH1- PMS2- | MSH+ MSH6- | MSH2- MSH6- | MLH1- PMS2-MSH6- | MLH+ PMS2- | MLH1-PMS2- MSH2-MSH6- | |||||
| Colon | 153 | 33 | 2 | 21.5 | 24 | 5 | 2 | 1 | 0 | 1 |
| Rectum | 36 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gastric | 18 | 1 | 0 | 5 | 1 | 0 | 0 | 0 | 0 | 0 |
| Esophagus and GE junction | 31 | 2 | 0 | 6.4 | 2 | 0 | 0 | 0 | 0 | 0 |
| Endometrium | 30 | 8 | 2 | 28.5 | 5 | 2 | 1 | 0 | 0 | 0 |
| Other tumor** | 50 | 3 | 1 | 6.2 | 3 | 0 | 0 | 0 | 0 | 0 |
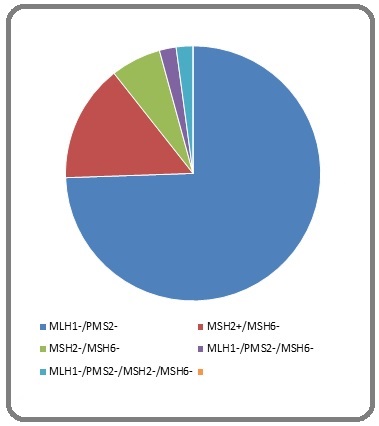
| Total | 318 | 47 | 5 (1.4%) | 14.7 | 35 | 7 | 3 | 1 | 0 | 1 |
** The othe dMMR tumor group includes one case of each pancreatic adenocarcinoma, gall bladder adenocarcinoma and cervical adenocarcinoma
Figure 1. Distribution of Total Cases & dMMR Cases.
Out of the total 47 MMR deficient cases, majority of the cases shows loss of MLH1 & PMS2 (Figure 2) followed by individual loss of MSH6 protein (Figure 3), loss of MSH2 & MSH6 protein (Figure 4), loss of MSH6, MLH1&PMS2 and loss of all 4 protein (Figure 5).
Figure 2. Immunohistochemistry Staining with Antibodies Against MSH2, MSH6, MLH1, PMS2 on a Colorectal adenocarcinoma. The normal colonic crypt, lymphocytes and stromal cells serve as internal positive control. Absence of nuclear expression observed for MLH1, PMS2. This is the most common abnormal staining pattern. BRAF V600e test was subsequently advised for such patient to determine somatic or germline mutation.
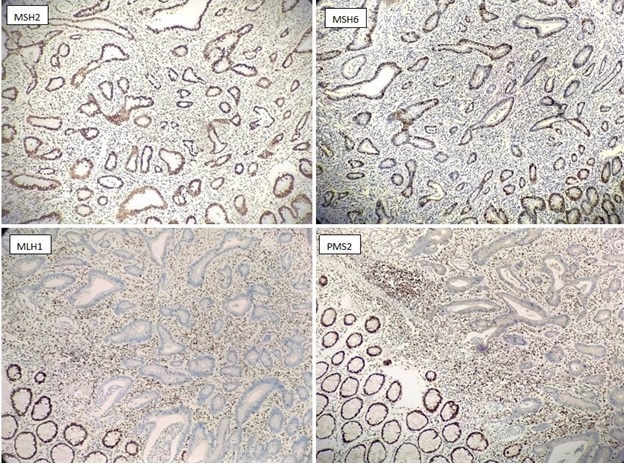
Figure 3. Immunohistochemistry staining with antibodies against MSH2, MSH6, MLH1, PMS2 on a colonic adenocarcinoma. The normal colonic crypt, lymphocytes and stromal cells serve as internal positive control. Absence of nuclear expression observed for MSH6.
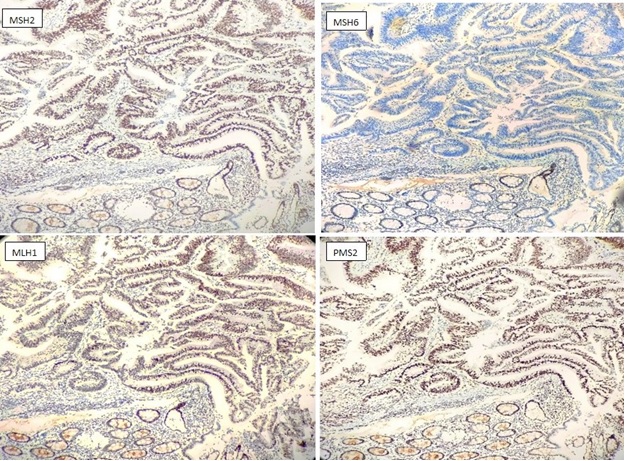
Figure 4. Immunohistochemistry Staining with Antibodies against MSH2, MSH6, MLH1, PMS2 on a Colonic Adenocarcinoma. The normal colonic crypt, lymphocytes and stromal cells serve as internal positive control. Absence of nuclear expression observed for MSH2, MSH6.
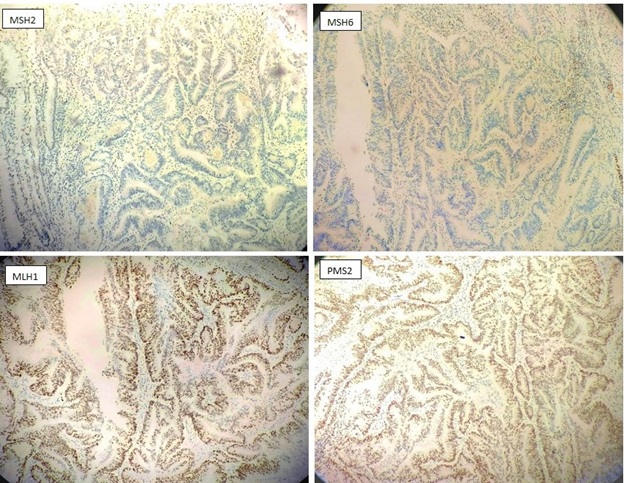
Figure 5. Pie Chart Showing Pattern of Loss of MMR Protein.
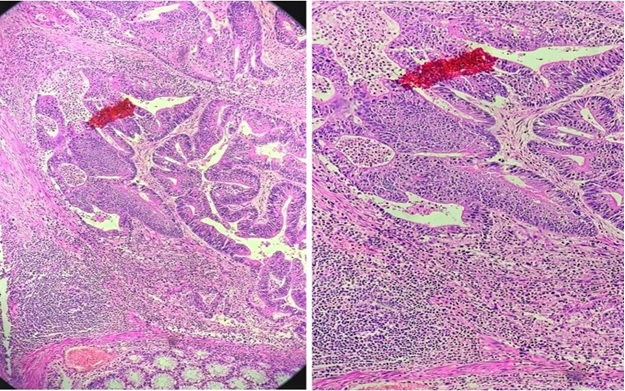
Out of total 318 cases there were 189 cases of colorectal carcinoma analysed for dMMR. Among them 33 cases were deficient of MMR proteins. The demographical data, clinical characteristics and histopathological features (Figure 6) are summarized in the Table 3.
| MMR proficient (%) | MMR deficient (%) | ||
| Age | More than 60 year | 74 (47.4) | 15 (45.4) |
| Less than 60 year | 82 (52.6) | 18 (54.6) | |
| Sex | Male | 102 (65.3) | 19 (57.5) |
| Female | 54 (34.7) | 14 (42.5) | |
| Sites | Right colon | 43 (27.4) | 25 (75.7) |
| Left colon | 56 (35.6) | 5 (15.2) | |
| Transverse colon | 1 (0.6) | 0 (0) | |
| Sigmoid colon | 21 (13.4) | 3 (9.1) | |
| Rectum | 36 (23.0) | 0 | |
| Tumor differentiation | Well – moderate | 140 (89.7) | 24 (72.7) |
| Poor | 16 (10.3) | 09 (27.3) | |
| Mucinous and signet ring cell component | 20 (12.8) | 08 (24.2) | |
| Intra tumoral lymphocytes | 08 (5.1) | 5 (15.1) | |
| Peri tumoral lymphocytes | 25 (16) | 12 (36.4) |
Furthermore among those 33 MMR deficient colonic adenocarcinoma cases, in 6 cases (where MLH1 expression is loss) we performed BRAF V600 mutation testing to look for somatic methylation of the MLH1 promoter.
Figure 6. Histopathology of MMR Deficient Colonic Adenocarcinoma. There is prominent intratumoral and peritumoral lymphocytic infiltrates seen (Low power and high power).
Among those 6 cases in 4 of the cases we found BRAF V600 mutated.
Discussion
In our study out of 318 carcinoma cases from all the sites 47 cases (14.7%) were MMR deficient, data in the study by sarode et al [16] they found 12.7% cases with loss and indeterminate IHC expression of MMR protein in 479 patients of colorectal and endometrial carcinomas. In our study out of the 153 colonic adenocarcinoma cases (excluding the rectal carcinoma cases) 33 cases i.e 21.7% of colonic adenocarcinoma were MMR deficient. In Indian scenario Pandey et al [17] using 2 marker MLH1 & MSH2 found 15.7% of colorectal carcinomas cases with deficient MMR. Singh et al [18] performed 2 marker MLH1 & MSH2 and observed MMR deficient in 63.3% cases. Nayak et al [19] performed IHC in total 236 cases of colorectal carcinomas using all 4 MMR protein and has found 22.9% cases with MMR deficient. Interestingly none of our rectal carcinoma cases showed deficiency of MMR protein.
In our study 28.5% of endometrial carcinoma had deficient MMR protein. In the study by sarode et al16 they have found 21.2% of endometrial carcinoma with loss and indeterminate IHC expression of MMR protein. Regarding pattern of MMR protein loss, in our study the loss of nuclear expression of MLH1 and PMS2 is the most common MMR protein lost, followed by isolated loss of MSH6 expression and loss of both MSH2 and MSH6 expression, which is similar to most other studies [20-22].
In one case we observed loss of expression of 3 MMR protein i.e MLH1, PMS2, MSH6. south christopher et al20 reported similar pattern in one case. In another case there is absence of all 4 MMR protein such “null IHC” phenotype is very rare, wang et al [23] reported one such colorectal carcinoma case which was associated with concurrent somatic inactivation of MLH1 and MSH2,Hagen et al [24] also reported one such “null pattern” IHC in a colorectal carcinoma which was associated with germline MSH2 mutation and somatic MLH1 hypermethylation. Furthermore in the 6 cases of colonic adenocarcinoma where we went ahead and performed BRAF V600 mutation analysis,4 cases were observed to have BRAF V600 mutation. Harman et al have observed MLH1 hypermethylation to be the most common cause of deficient MMR protein in US population [25].
In this study total 5 cases i.e. 1.5% demonstrated indeterminate expression, which is possibly due to delayed fixation of the tissues which leads to autolysis of the luminal aspect of the colon and endometrium specimens and lack of standard protocol in using the fixatives. It is one of the major problem in assessment of MMR protein by IHC. Sarode et al [16] in their study observed 3 % cases with indeterminate expression. In Fadhil and Ilyas [26] study comparing assessment of MMR protein by IHC in biopsy specimens and resection specimen of the same samples, they observed it is easier to interpret MMR protein in biopsy samples as biopsy sample usually have more uniform and complete fixation compared to resection specimens. Furthermore post neoadjuvant chemoradiation, the expression of MMR proteins specially MSH6 tends to have reduced expression, hence it is preferable to evaluate treatment status prior to assessment of MMR protein by IHC. Bao et al [27] observed post neoadjuvant reduced but not complete loss of MSH6 expression. In rare instances there may be “constitutional MMR deficiency”.
In the study, the MMR deficient colon carcinomas comparing to MMR proficient colon carcinomas has more cases with right sided colon involvement and in microscopy this MMR deficient tumor has more cases showing high grade histology, intra tumoral lymphocytic infiltration, peritumoral lymphocytic infiltration and mucinous and signet cell components. The similar findings has been demonstrated in other studies [16,19,28].
In conclusion, the evaluation of MMR proteins by using immunohistochemistry is relatively easy to institute in the routine testing as it is a useful predictive and prognostic biomarker in various carcinomas, also it helps in screening the cases of Lynch syndrome. There is need of using standard protocols for tissue fixation and processing before evaluating for MMR proteins using IHC so that there is no loss of antigen due to technical errors. With the introduction of immune check point blockade therapy there is a need of more widespread testing of MMR protein using IHC for better management of the patient,also to screening for lynch syndrome and to identify the families with predisposed germ line mutation.
Conflict of Interest
The authors declare no conflicts of interest.
Source of Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not for profit sectors.
References
- DNA mismatch repair protein immunohistochemistry - an illustrated guide Bateman AC . Histopathology.2021;79(2). CrossRef
- Hereditary colorectal cancer Lynch HT , Chapelle A. The New England Journal of Medicine.2003;348(10). CrossRef
- Cancer risk in mutation carriers of DNA-mismatch-repair genes M Aarnio , R Sankila , E Pukkala , R Salovaara , La Aaltonen , A de la Chapelle , P Peltomäki , Jp Mecklin , Hj Järvinen . International journal of cancer.1999;81(2). CrossRef
- Extracolonic manifestations of lynch syndrome Bansidhar BJ . Clinics in Colon and Rectal Surgery.2012;25(2). CrossRef
- Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry Shia J. The Journal of molecular diagnostics: JMD.2008;10(4). CrossRef
- Molecular Characterization of Endometrial Cancer: A Correlative Study Assessing Microsatellite Instability,: MLH1: Hypermethylation, DNA Mismatch Repair Protein Expression, and: PTEN: ,: PIK3CA: ,: KRAS: , and: BRAF: Mutation Analysis Peterson LM , Kipp BR , Halling KC , Kerr SE , Smith DI , Distad TJ , Clayton AC , Medeiros F. International Journal of Gynecological Pathology.2012;31(3). CrossRef
- Analysis of Lynch Syndrome Mismatch Repair Genes in Women with Endometrial Cancer Rubio I, Ibáñez-Feijoo E, Andrés L, Aguirre E, Balmaña J, Blay P, Llort G, González-Santiago S, Maortua H, Tejada MI , Martinez-Bouzas C. Oncology.2016;91(3). CrossRef
- Tumour MLH1 promoter region methylation testing is an effective prescreen for Lynch Syndrome (HNPCC) K Newton , Nm Jorgensen , Aj Wallace , Dd Buchanan , F Lalloo , Rf McMahon , J Hill , Dg Evans . Journal of medical genetics.2014;51(12). CrossRef
- Correlation of tumour BRAF mutations and MLH1 methylation with germline mismatch repair (MMR) gene mutation status: a literature review assessing utility of tumour features for MMR variant classification Parsons MT , Buchanan DD , Thompson B, Young JP , Spurdle AB . Journal of Medical Genetics.2012;49(3). CrossRef
- MLH1 promoter methylation frequency in colorectal cancer patients and related clinicopathological and molecular features Li X, Yao X, Wang Y, Hu F, Wang F, Jiang L, Liu Y, Wang D, Sun G, Zhao Y. PloS One.2013;8(3). CrossRef
- Promoter hypermethylation frequency and BRAF mutations distinguish hereditary non-polyposis colon cancer from sporadic MSI-H colon cancer McGivern A., Wynter C. V. A., Whitehall V. L. J., Kambara T., Spring K. J., Walsh M. D., Barker M. A., Arnold S., Simms L. A., Leggett B. A., Young J., Jass J. R.. Familial Cancer.2004;3(2). CrossRef
- Systematic review of microsatellite instability and colorectal cancer prognosis Popat S., Hubner R., Houlston R. S.. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2005;23(3). CrossRef
- Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer Ribic CM , Sargent DJ , Moore MJ , Thibodeau SN , French AJ , Goldberg RM , Hamilton SR , Laurent-Puig P, Gryfe R, Shepherd LE , Tu D, Redston M, Gallinger S. The New England Journal of Medicine.2003;349(3). CrossRef
- Does microsatellite instability predict the efficacy of adjuvant chemotherapy in colorectal cancer? A systematic review with meta-analysis Des Guetz G, Schischmanoff O, Nicolas P, Perret G, Morere J, Uzzan B. European Journal of Cancer (Oxford, England: 1990).2009;45(10). CrossRef
- First FDA Approval Agnostic of Cancer Site - When a Biomarker Defines the Indication Lemery S, Keegan P, Pazdur R. The New England Journal of Medicine.2017;377(15). CrossRef
- Screening for Lynch Syndrome by Immunohistochemistry of Mismatch Repair Proteins: Significance of Indeterminate Result and Correlation With Mutational Studies Sarode VR , Robinson L. Archives of Pathology & Laboratory Medicine.2019;143(10). CrossRef
- Assessment of microsatellite instability in colorectal carcinoma at an Indian center Pandey V, Prabhu JS , Payal K., Rajan V, Deepak C., Barde S, Jagannath P., Borges A, Sridhar T. S.. International Journal of Colorectal Disease.2007;22(7). CrossRef
- Clinico-Histopathological Features Of Colonic Adenocarcinoma In Young (< 50years) Patients With Special Reference To Microsatellite Instability Status Singh P, Sharma K, Tewari R, Misra V, Dwivedi M, Misra SP . Indian J Med Res Pharm Sci.2015;2:9-18.
- Prevalence estimation of microsatellite instability in colorectal cancers using tissue microarray based methods - A tertiary care center experience Nayak SS , Roy P, Arora N, Arun I, Roy MK , Banerjee S, Mallick I, Mallath MK . Indian Journal of Pathology & Microbiology.2018;61(4). CrossRef
- APC Mutations Are Not Confined to Hotspot Regions in Early-Onset Colorectal Cancer Aitchison A, Hakkaart C, Day RC , Morrin HR , Frizelle FA , Keenan JI . Cancers.2020;12(12). CrossRef
- Immunohistochemistry for PMS2 and MSH6 alone can replace a four antibody panel for mismatch repair deficiency screening in colorectal adenocarcinoma Hall G, Clarkson A, Shi A, Langford E, Leung H, Eckstein RP , Gill AJ . Pathology.2010;42(5). CrossRef
- Heterogeneous Staining for Mismatch Repair Proteins during Population-Based Prescreening for Hereditary Nonpolyposis Colorectal Cancer Watson N, Grieu F, Morris M, Harvey J, Stewart C, Schofield L, Goldblatt J, Iacopetta B. The Journal of molecular diagnostics : JMD.2007;9(4). CrossRef
- Immunohistochemical null-phenotype for mismatch repair proteins in colonic carcinoma associated with concurrent MLH1 hypermethylation and somatic MSH2 mutations Wang T, Stadler ZK , Zhang L, Weiser MR , Basturk O, Hechtman JF , Vakiani E, Saltz LB , Klimstra DS , Shia J. Familial cancer.2018;17(2). CrossRef
- “Null pattern” of immunoreactivity in a Lynch syndrome-associated colon cancer due to germline MSH2 mutation and somatic MLH1 hypermethylation Hagen CE , Lefferts J, Hornick JL , Srivastava A. The American journal of surgical pathology.2011;35(12):1902-1905.
- Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma Herman J. G., Umar A., Polyak K., Graff J. R., Ahuja N., Issa J. P., Markowitz S., Willson J. K., Hamilton S. R., Kinzler K. W., Kane M. F., Kolodner R. D., Vogelstein B., Kunkel T. A., Baylin S. B.. Proceedings of the National Academy of Sciences of the United States of America.1998;95(12). CrossRef
- Immunostaining for mismatch repair (MMR) protein expression in colorectal cancer is better and easier to interpret when performed on diagnostic biopsies Fadhil W, Ilyas M. Histopathology.2012;60(4). CrossRef
- Neoadjuvant therapy induces loss of MSH6 expression in colorectal carcinoma Bao F, Panarelli NC , Rennert H, Sherr DL , Yantiss RK . The American Journal of Surgical Pathology.2010;34(12). CrossRef
- Screening for Microsatellite Instability in Colorectal Cancer and Lynch Syndrome - A Mini Review Wang M, Kanehira K, Chen F. North American Journal of Medicine and Science.2016;9(1).
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2022
Author Details