Long Term Culture of Mesenchymal Stem Cells: No Evidence of Chromosomal Instability
Download
Abstract
Introduction: Mesenchymal stem cells (MSCs) technology has opened promising roads toward different aspects of medicine and biology. However, possible tumorgenic potential of MSCs has raised many concerns in using them in regenerative medicine. In the present study, we aimed to investigate the safety of MSCs by in-vitro culture and karyotype analysis.
Materials and Methods: MSCs were successfully cultured in DMEM medium including 3.5 ng/ml recombinant human basic fibroblast growth factor (bFGF) and 20 % fetal bovine serum (FBS) within 25 passages.
Results: No significant abnormality was observed in basic karyotype and morphology of MSCs in different passages of culture.
Conclusions: Using of EGF free medium containing low bFGF concentration are possible explanations for observed integrity in karyotype and morphology of MSCs. Our findings would be further convincing evidence on the safety of MSCs under suggested conditions to guarantee using of them in regenerative medicine and disease treatment.
Introduction
Mesenchymal stem cell (MSCs) as the major type of adult stem cell has been frequently studied in regenerative medicine of various non-hematologic diseases. High proliferation rate and multipotent capacity of MSCs besides their availability in bone marrow (BM), adipose tissue, amniotic fluid or placenta are satisfactory reasons to use them in various clinical trials. Inevitable demand for expansion of MSCs in long term culture has raised many concerns regarding their morphologic and karyotype transformation leading to cancer. Similar to fibroblast cells, normal MSCs have small and flat cell body containing apparent and large nucleus with a few long and thin cell processes [1]. Adherence and contact inhibition characteristics of MSCs make long term expansion of them very difficult in a monolayer culture and, therefore, require specific optimal culture protocol. Multiple culture expansion makes MSCs to reach senescence phase therein instead of mitosis the cells changed to be large and gain the so called fried egg morphology with limited differentiation potential [2, 3]. In the other hand, human mesenchymal stem cells (MSCs) need sufficient growth factors to be vigorously cultivated within culture medium [4].
MSCs secrete some cytokines and growth factors that promote tissue regeneration through modulating the proliferation and differentiation processes [5]. It was demonstrated that immunomodulatory and growth factors secreted by MSCs can enhance survival and success of tissue engraftment [6-8]. However, in contrast to human embryonic stem (hES) cells, genetic instability and some physiological dysfunctions have shown to be inevitable in multiple MSCs passages [9]. Therefore, development of an optimal culture protocol would be critical in harvesting large amount of MSCs before using in various aspects of regenerative medicine. European regulatory authorities have declared that karyotype analysis is necessary before using of MSCs and more detailed cytogenetic assessment are recommended in the presence of recurrent genetic aberrations. It has been demonstrated that mouse MSCs could be cytogenetically stable up to the passage 11 and even 25 of extension [10, 11]. Although formation of tumors following more than 10 passages of mouse derived MSCs has been attributed to the sensitivity of these cells to mutations of cell cycle control genes as well as p53, it was also shown to be held for human MSCs (hMSCs) in some studies [12, 13]. Rubio et al have initially reported that hMSCs would be transformed to malignant cells after 4-5 months of culture [14]. In vitro culture of hMSCs within 106 weeks also has shown malignant transformation in around 46% of these bone marrow derived cells in a separate bi-center study [15].
In addition, it was described that the expression level of growth factors has changed from donor to recipient during culture passage that can change the possibility of tumor formation in recipient. It was shown that decreased expression of fibroblast growth factor-4 (FGF-4), hepatocyte growth factor (HGF) and epidermal growth factor (EGF) were associated with reduction of stemness in MSCs and may cause autophagy and senescence in serial passage [5].
In general, there are limited number of assays which have focused on the most appropriate method of MSCs culture in order to be as less tumorgenic as possible.
The present work was aimed to evaluate the effect of the long-term culture using lower concentration of human basic FGF (bFGF) on MSCs expansion through karyotype and cell morphology analysis.
Materials and Methods
MSCs culture
The Bone Marrow (BM) samples were provided from 5 healthy individuals whom were selected to be donor for BM transplantation. The names of the enrolled donor’ samples were blind to us and they filled the consent form according to the protocol of the Ethical Review Board of Tehran University of medical sciences. hMSCs were isolated from BM samples through centrifugation in 700g for 15 minutes at 4 °C and using a Ficoll– Hypaque gradient (Sigma, St. Louis, MO) [16]. Isolated mononuclear cells were then cultured in Dulbecco’s modified Eagle’s medium (DMEM, GIBCO, Boston, USA). Culture medium was supplemented with 3.5 ng/ml recombinant human basic FGF (Upstate, USA), 20 % fetal bovine serum (FBS, GIBCO, USA), 100 U/mL penicillin, 100 lg/mL streptomycin, 1 mM sodium pyruvate and 4 mM L-glutamine. Culture was incubated at 37˚C in a humidified atmosphere containing 5% CO2 and 95% air. Non-adherent cells including hematopoietic stem cells (HSCs) were removed from culture medium through washing with 1X PBS and also DMEM medium on the 3th day and the medium of adherent cells was changed with fresh medium. Morphology, proliferation rate of hMSCs were examined under invert microscope and the culture medium was freshen every 4-6 days until the complete monolayer of cells have been appeared. When the hMSCs reached to 70% confluency, they were subculture using 0.05% Trypsin-EDTA to make single cell suspension.
Karyotype analysis
One flask of each passage series (5-25) was subjected to colchicine (0.5 µg/ml) for 1.5 hours to analyze the karyotype of hMSCs’s chromosomes. Both right chromosome staining and Giemsa chromosome banding was performed on chromosome spreads. Over 100 metaphases were undergone morphologic and karyotype analysis in every 5 samples.
All the karyotype analysis was performed by Zeiss Microscopy (Germany) and the cell spread pictures was taken using Motic´s new AE2000 Inverted Microscope (Spain) (10 ×10 and 10 ×20 magnification).
Results
We successfully removed HSCs from the culture medium to eliminate their inhibitory effect on the proliferation rate of MSCs. Within several weeks, normal and pure MSCs have filled the flask surface; the normal morphology of proliferating MSCs is represented in Figures 1-5.
Figure 1. Initial Expansion of Human Mesenchymal Stem Cells (hMSCs (200x Magnification).
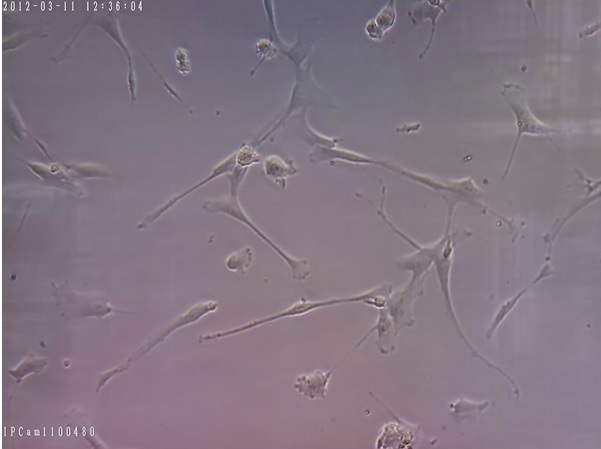
Figure 2. Cultured hMSCs in DMEM Medium with 3.5 ng/ml bFGF and Without Using EGF and Show the Division of Healthy hMSCs. (200x Magnification).
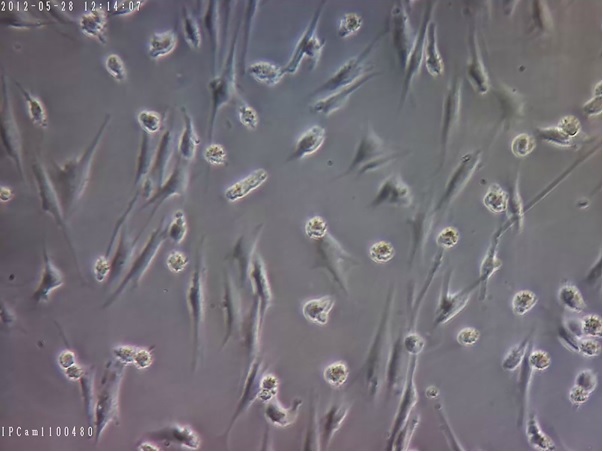
Figure 3. This Indicates Confluent Monolayer hMSCs with Normal Morphology (sample 1), which is Required to be sub-cultured. (about 80% confluence) (100x Magnification).
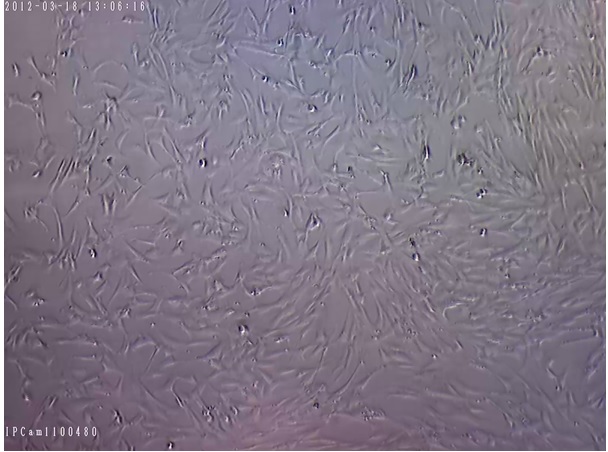
Figure 4. Human Mesenchymal Stem Cells after Sub-culturing (200x Magnification).
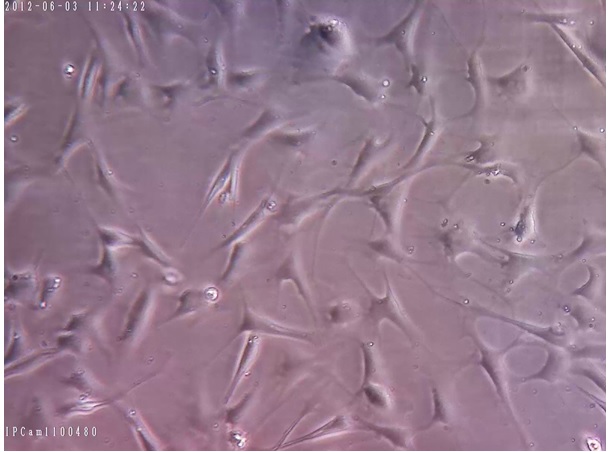
Figure 5. Monolayer hMSCs with 100% Confluence and Normal Morphology (sample 2), which Should be Sub-cultured (200x Magnification).
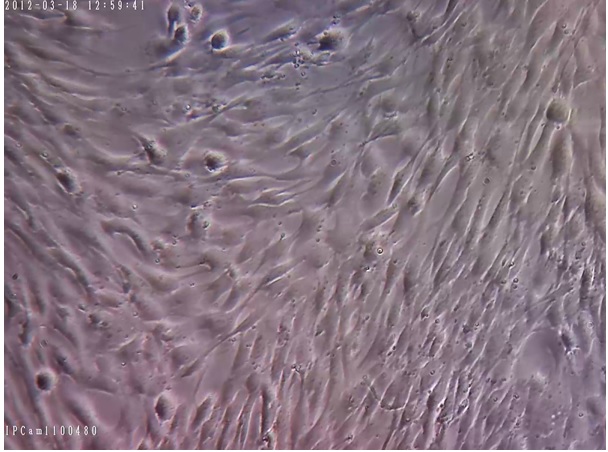
Assessment of MCSs using invert microscopy has demonstrated that the morphology of cells didn’t change in flasks while their medium was freshening every 3-5 days. Abnormal growth was not observed in all the culture flasks containing MSCs and all the cells maintained their monolayer expansion.
Karyotype analysis have shown that no numerical or structural chromosomal abnormalities including inversion, deletion, insertion and translocation or chromosomal breakage were demonstrated in all of the 100 studied MSCs metaphases.
Discussion
Very low percent of MSCs in total isolated bone marrow mononuclear cells (0.001-0.01%) makes their in vitro expansion inevitable to have adequate number of cells for transplantation [16]. Arising cancer hallmarks during long term passages of MSCs is a major limitation and has hindered their application in recent years. Moreover, method of MSCs preparation and growth factors used in culture medium may affect the potential of these cells to be cancerous following long term culture [14, 17]. There is still no generally accepted detailed protocol for number of MSCs culture passage and culture medium composition to avoid cell transformation and carcinogenesis. Herein, the morphology and size of MSCs didn’t change following 20 culture passage. Consistent with our results, in the long- term culture of MSCs derived from embryos’ limbs, the fibroblast like morphology of hMSCs didn’t change and no clonal chromosomal instability was also observed .
In contrast, in the other study, the size of bone marrow derived MSCs has been shown to be decreased after the passages 13 while their normal spindle shape and proliferation rate remained constant as expected. Similarly, hMSCs derived from adipose tissue expanded in DMEM medium containing 10% FBS were demonstrated to be small and compact cells with several chromosomal malformations [14]. Wang et al. and Ruan et al. have also shown smaller umbilical cord derived MSCs in long term culture, however, the overall status of chromosomal stability remained unchanged [18, 19]. In contrary, Redaelli et al. have demonstrated that with increasing the passage number, hMSCs become enlarged and enriched of extra- and intracellular debris especially after the 10th passage. In addition, in the harvested MSCs of 2 out of 8 candidate donors, clonal aberrations were detected [20]. In the other similar study, Izadpanah et al. reported that after the passage 15, the morphology of rhesus MSCs changed to be flat and large with increasing number of aneuploid cells after passage 20. Expansion of hMSCs in the other study demonstrated spherical and spindle cells with multiple cytogenetic defects including chromosomal aneuploidies and translocations. They have shown to be vigorously proliferated without any cell-cell contact inhibition [21]. In the other assay on MSCs derived from bone marrow of 62 normal candidates, clonal chromosomal abnormalities were observed after the second passage indicating the intermediate stage of carcinogenesis in long term culture. Ben David et al. by studying MSCs derived from 144 samples have found that some chromosomal aneuploidies as well as monosomy 13, which are the typical changes of mesenchymal tumors, were detected in the initial passages of expansions. Adamzyk et al. have described that the size of ovine MSCs harvested in DMEM medium containing 10% FBS was decreased in P0 passage. It seems, cell aggregation was more frequently seen in MSCs culture medium enriched with supplements and also supplements plus EGF while no cell-cell contact inhibition and large colonies were observed in the EGF and supplements free mediums. Although it is not clearly defined, these morphological changes may have influential effects on the function and differentiation capacity of MSCs.
It is important to note that all the chromosomal aberrations seen in karyotype analysis of MSCs culture are not required to be taken in to account. It was elucidated that some of chromosomal aneuploidies including trisomy 20, trisomy 7 and trisomy 5 frequently occur during leukomogenesis process within bone marrow and will be disappeared in later passages. Tarte et al. reported transient chromosomal aneuploidis in the MSCs cultures of some healthy donors and described that these cytogenetic aberrations were independent of cell senescence identified after long term expansion of MSCs [22]. Osipova et al. have demonstrated stable clonal chromosome aneuploidies initiated in the early passages of MSCs culture which was associated with normal fibroblast morphology even after long term culture.
Role of MSCs in cancer progression remained unclear; in one hand, they may promote cancer progression through transforming the immune microenvironment and immunosuppressive activity [23]. In the other hand, in an experiment on breast cancer cells it was revealed that MSCs suppressed growth of cancer cells through up- regulation of interferon β (IFN-β) and induction of STAT3 signaling pathway [24]. It was also shown that hMSCs increased rate of lymph node metastasis of esophageal cancer cells [25]. Migration of hMSCs toward cancer cells was determined to be dramatically higher than normal medium without any cancer cells. Immunosuppressive activity of MSCs is fulfilled through stimulation of T regulatory cells which may inhibit lymphocyte responses and autoimmunity and thereby may help cancer cells to escape and continue to grow [26].
In the present study, we used 3.5ng/ml recombinant human basic fibroblast growth factor (bFGF), which was about 30% lower than the standard protocol described by American Type Culture Collection (ATCC). According to ATCC guideline 20% FBS, 5 ng/mL rh FGF basic/ acidic and EGF are required for culturing of MSCs. In the present work, by using 3.5 ng/mL bFGF and without using any EGF, the hMSCs were successfully expanded and subcultured in 20 passages. Concentration of bFGF is the most challenging growth factor in MSCs culture and transplantation assays. The bFGF is essential for self-renewal and differentiation characteristics of stem cells [27]. The critical role of epidermal growth factors and fibroblast growth factors has been demonstrated in progression and invasion of different types of cancer [28]. Ritter et al. have shown that MSCs have receptors for bFGF and vascular epithelial growth factor (VEGF) that their activation induces MSCs migration capability to develop breast cancer [27]. Suppression of bFGF and FLK1 receptors using their specific antibodies has reduced the immigration of breast cancer cells. It was also demonstrated that >5 ng/ml exogenous bFGF was associated with outgrowth of hESCs colonies and increased proliferation rate of undifferentiated cells [29-31]. Higher expression of VEGF, FGF and SDF1-α genes in bone marrow-MSCs (BM-MSCs) have been described to be responsible for tumor growth through driving angiogenesis [32].
In conclusion, to the best of our knowledge, we primarily showed that MSCs could successfully expand in medium containing bFGF concentration lower than ATCC guideline. Further studies required to exactly determine the threshold of bFGF concentration in culture medium of MSCs in order to have adequate number of them without any morphological and chromosomal aberrations.
Acknowledgements
This study was supported by research grant (89-01- 54-10008) provided at Tehran University of Medical Sciences.
Author Disclosures
There is no conflict of interest among authors.
References
- Mesenchymal stromal cells and fibroblasts: a case of mistaken identity? Hematti P. Cytotherapy.2012;14(5). CrossRef
- Replicative senescence of mesenchymal stem cells: a continuous and organized process Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, et al . PloS One.2008;3(5). CrossRef
- Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion Baxter MA , Wynn RF , Jowitt SN , Wraith JE , Fairbairn LJ , Bellantuono I. Stem cells (Dayton, Ohio).2004;22(5):675-682. CrossRef
- Expansion of human mesenchymal stem cells on microcarriers Hewitt CJ , Lee K, Nienow AW , Thomas RJ , Smith M, Thomas CR . Biotechnology Letters.2011;33(11). CrossRef
- The role of growth factors in maintenance of stemness in bone marrow-derived mesenchymal stem cells Eom YW , Oh J, Lee J, Baik SK , Rhee K, Shin HC , Kim YM , et al . Biochemical and Biophysical Research Communications.2014;445(1). CrossRef
- Mesenchymal stem cells in health and disease Uccelli A, Moretta L, Pistoia V. Nature Reviews. Immunology.2008;8(9). CrossRef
- Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes Abdi R, Fiorina P, Adra CN , Atkinson M, Sayegh MH . Diabetes.2008;57(7). CrossRef
- Why are MSCs therapeutic? New data: new insight Caplan A. I.. The Journal of Pathology.2009;217(2). CrossRef
- Establishment and therapeutic use of human embryonic stem cell lines Suemori H. Human Cell.2006;19(2). CrossRef
- Mesenchymal stem cells enhance allogeneic islet engraftment in nonhuman primates Berman DM , Willman MA , Han D, Kleiner G, Kenyon NM , Cabrera O, Karl JA , et al . Diabetes.2010;59(10). CrossRef
- Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms Bernardo ME , Zaffaroni N, Novara F, Cometa AM , Avanzini MA , Moretta A, Montagna D, et al . Cancer Research.2007;67(19). CrossRef
- Loss of p53 induces tumorigenesis in p21-deficient mesenchymal stem cells Rodriguez R, Rubio R, Masip M, Catalina P, Nieto A, Cueva T, Arriero M, et al . Neoplasia (New York, N.Y.).2009;11(4). CrossRef
- Spontaneous expression of embryonic factors and p53 point mutations in aged mesenchymal stem cells: a model of age-related tumorigenesis in mice Li H, Fan X, Kovi RC , Jo Y, Moquin B, Konz R, Stoicov C, et al . Cancer Research.2007;67(22). CrossRef
- Spontaneous human adult stem cell transformation Rubio D, Garcia-Castro J, Martin MC , de la Fuente R, Cigudosa JC , Lloyd AC , et al . Cancer research.2005;65(8):3035-3039. CrossRef
- Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation Røsland GV , Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, Mysliwietz J, et al . Cancer Research.2009;69(13). CrossRef
- Multilineage potential of adult human mesenchymal stem cells Pittenger M. F., Mackay A. M., Beck S. C., Jaiswal R. K., Douglas R., Mosca J. D., Moorman M. A., Simonetti D. W., Craig S., Marshak D. R.. Science (New York, N.Y.).1999;284(5411). CrossRef
- Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis Tai M, Chang C, Kiupel M, Webster JD , Olson LK , Trosko JE . Carcinogenesis.2005;26(2). CrossRef
- Karyotype stability of human umbilical cord-derived mesenchymal stem cells during in vitro culture Ruan Z, Zhu LI , Yin Y, Chen G. Experimental and Therapeutic Medicine.2014;8(5). CrossRef
- Long-term cultured mesenchymal stem cells frequently develop genomic mutations but do not undergo malignant transformation Wang Y., Zhang Z., Chi Y., Zhang Q., Xu F., Yang Z., Meng L., et al . Cell Death & Disease.2013;4(12). CrossRef
- From cytogenomic to epigenomic profiles: monitoring the biologic behavior of in vitro cultured human bone marrow mesenchymal stem cells Redaelli S, Bentivegna A, Foudah D, Miloso M, Redondo J, Riva G, Baronchelli S, et al . Stem Cell Research & Therapy.2012;3(6). CrossRef
- Outgrowth of a transformed cell population derived from normal human BM mesenchymal stem cell culture Wang Y., Huso D. L., Harrington J., Kellner J., Jeong D. K., Turney J., McNiece I. K.. Cytotherapy.2005;7(6). CrossRef
- Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation Tarte K, Gaillard J, Lataillade J, Fouillard L, Becker M, Mossafa H, Tchirkov A, et al . Blood.2010;115(8). CrossRef
- Human mesenchymal stem cells creating an immunosuppressive environment and promote breast cancer in mice Ljujic B, Milovanovic M, Volarevic V, Murray B, Bugarski D, Przyborski S, Arsenijevic N, et al . Scientific Reports.2013;3. CrossRef
- Mesenchymal stem cells in the tumor microenvironment Guan J, Chen J. Biomedical Reports.2013;1(4). CrossRef
- Human umbilical cord mesenchymal stem cells promote carcinoma growth and lymph node metastasis when co-injected with esophageal carcinoma cells in nude mice Yang X, Li Z, Ma Y, Gao J, Liu S, Gao Y, Wang G. Cancer Cell International.2014;14(1). CrossRef
- Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction Prevosto C, Zancolli M, Canevali Paolo, Zocchi MR , Poggi A. Haematologica.2007;92(7). CrossRef
- Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture Amit M, Carpenter MK , Inokuma Ms , Chiu Cp , Harris Cp , Waknitz Ma , Itskovitz-Eldor J, Thomson Ja . Developmental biology.2000;227(2). CrossRef
- Colorectal cancer cells activate adjacent fibroblasts resulting in FGF1/FGFR3 signaling and increased invasion Henriksson ML , Edin S, Dahlin AM , Oldenborg P, Öberg Å, Van Guelpen B, Rutegård J, Stenling R, Palmqvist R. The American Journal of Pathology.2011;178(3). CrossRef
- Feeder-free growth of undifferentiated human embryonic stem cells Xu C., Inokuma M. S., Denham J., Golds K., Kundu P., Gold J. D., Carpenter M. K.. Nature Biotechnology.2001;19(10). CrossRef
- Properties of four human embryonic stem cell lines maintained in a feeder-free culture system Carpenter MK , Rosler ES , Fisk GJ , Brandenberger R, Ares X, Miura T, Lucero M, Rao MS . Developmental Dynamics: An Official Publication of the American Association of Anatomists.2004;229(2). CrossRef
- Long-term culture of human embryonic stem cells in feeder-free conditions Rosler ES , Fisk GJ , Ares X, Irving J, Miura T, Rao MS , Carpenter MK . Developmental Dynamics: An Official Publication of the American Association of Anatomists.2004;229(2). CrossRef
- Omental adipose tissue-derived stromal cells promote vascularization and growth of endometrial tumors Klopp AH, Zhang Y, Solley T, Amaya-Manzanares F, Marini F, Andreeff M, Debeb B, et al . Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2012;18(3). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2022
Author Details