Desmoplastic Small Round Cell Tumors: A Case Series of a Rare Tumor and Literature Review
Download
Abstract
Background: Desmoplastic small round cell tumor (DSRCT) is a rare and aggressive malignancy that predominantly affects males. It typically arises in the abdominal cavity and is typically diagnosed through histopathological examination. Most cases of DSRCT exhibit a characteristic EWSR1-WT1 gene fusion. In this report, we present a series of 3 cases of DSRCT encountered at our institution.
Case Presentation: All cases were diagnosed at an advanced stage, consistent with findings in previously available literature.
Conclusion: The optimal treatment approach for DSRCT involves a multidisciplinary team. The standard chemotherapy regimen used is typically the Ewing's sarcoma protocol of multiagent chemotherapy. Surgical interventions, such as cytoreduction and intraperitoneal hyperthermic chemotherapy, are the preferred procedures, although their feasibility is limited. Ongoing clinical trials investigating targeted therapies offer promising avenues for improving treatment outcomes and prognosis in DSRCT. DSRCT is a tumor associated with a poor prognosis. The development of novel treatment approaches is essential to enhance survival rates for patients with this disease.
Introduction
Desmoplastic Small Round Cell Tumors (DSRCT) is a rare and aggressive soft-tissue sarcoma that most frequently affects adolescent and young adult males. DSRCT was initially described by Gerald and Rosai in 1989 [1]. It is of mesenchymal cell origin and typically presents with multiple intra-abdominal tumors. It often presents as an advanced abdominal disease with multifocal deposits, peritoneal carcinomatosis, and omental involvement [2]. Here we report a series of 3 cases of DSRCT that we encountered and treated at our institute.
Case 1
A 42-year-old male was evaluated for mass per abdomen and abdominal pain at an outside center. Imaging with Positron Emission Tomography-Computed Tomography (PET CT) scan showed infra diaphragmatic adenopathy with hepatic, splenic, mesenteric, and marrow involvement. The initial clinical and pathological diagnosis was that of a Non-Hodgkin’s Lymphoma. Slide review at another center was reported as neuroendocrine carcinoma and received one cycle of single-agent carboplatin. He came to our institution for further treatment. On presentation, he had deranged liver functions. Given contradictory histopathology reports, it was decided to perform a repeat biopsy. The biopsy report was consistent with a DSRCT (Figure 1 (a) and Figure 1 (b)).
Figure 1a. Small Round Cells in Sheets Amid Desmoplastic Stroma (low power).
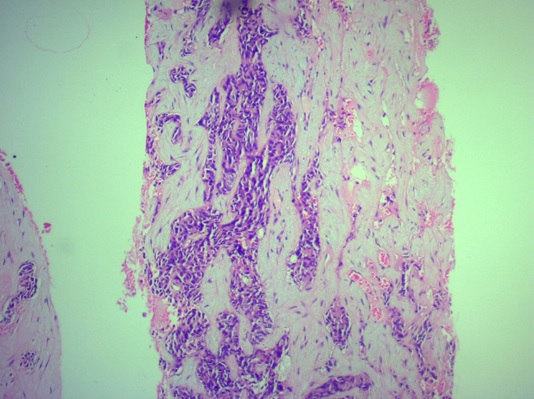
Figure 1b. DSRCT (High power)- Cells with High NC Ratio, the Tumor Cells Look Blue in the Desmoplastic Stroma.
Given deranged liver functions, it was decided to start on low-dose biweekly cyclophosphamide as per the tumor board’s decision. There was no improvement with the above protocol and the chemotherapy protocol was changed to Vincristine-Adriamycin-Cyclophosphamide (VAC) regimen which was delivered at a 50% dose. There was a subjective improvement with 2 cycles of VAC. It was decided to introduce Ifosfamide-Etoposide (IE) regimen. He received 3 cycles of the same and the CT scan showed a good response. He received 3 more cycles of the IE regime. But he started developing jaundice again and clinically started to deteriorate. A rechallenge with biweekly cyclophosphamide was contemplated but his performance status deteriorated rapidly. Hence, he was planned for further palliative care alone.
Case 2
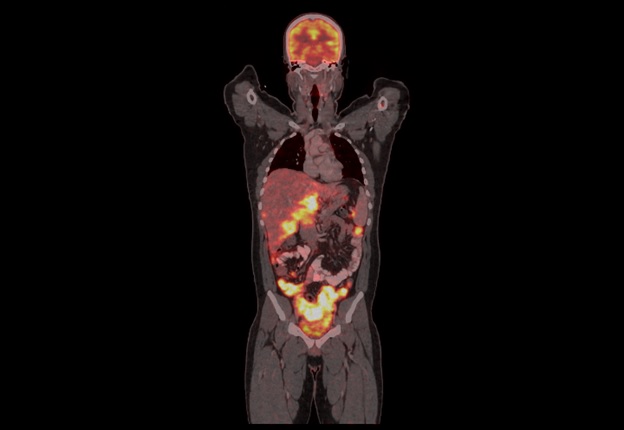
A 21-year-old male was evaluated elsewhere for increased frequency of stools. His imaging outside showed a pelvic mass in the rectovesical pouch encasing the colon. His histopathology report outside was suggestive of a urothelial-origin carcinoma. His imaging with a PET CT scan revealed a supradiaphragmatic and infra diaphragmatic disease with multiple mesenteric and peritoneal deposits with lung and marrow involvement (Figure 2 (a)).
Figure 2a. PET CT Image Showing Extensive Stage in Abdomen.
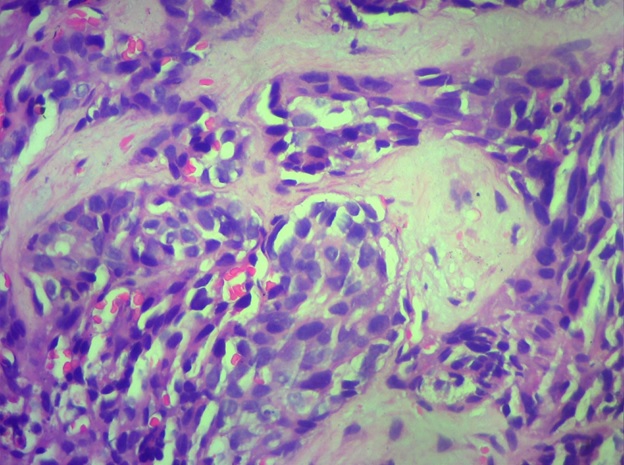
It was decided to conduct a repeat biopsy (as the previous biopsy had insufficient tissue) and he was started on chemotherapy with gemcitabine and carboplatin. His repeat biopsy was reported as DSRCT (Figure 2 (b) and Figure 2 (c).
Figure 2b. DSRCT High Power View.
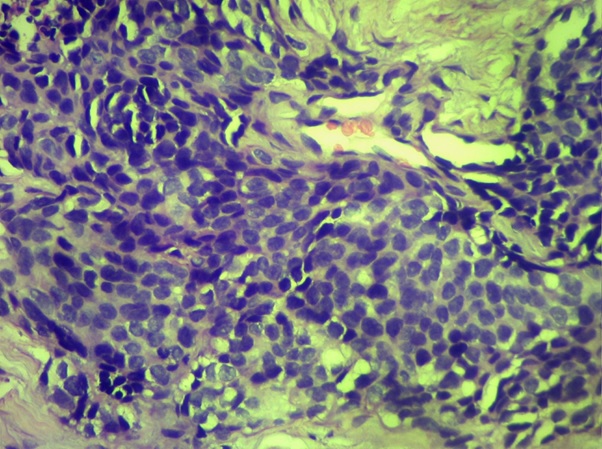
Figure 2c. DSRCT Showing Pan CK Positivity.
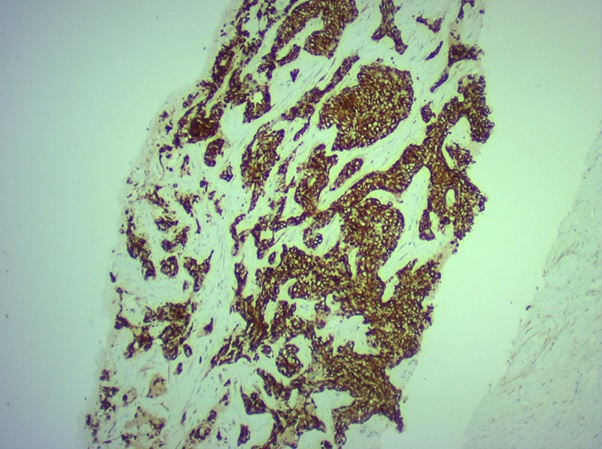
His NGS revealed Ewing’s sarcoma (EWSR 1) rearrangements. His case was discussed and was initiated on a VAC regimen of chemotherapy. He had a good clinical and radiological response. He has completed 6 cycles of the same and had good tolerance to chemotherapy. His PET CT scan post 6 cycles revealed a partial response to therapy. His case was discussed in the tumor board and is currently on etoposide and cyclophosphamide maintenance.
Case 3
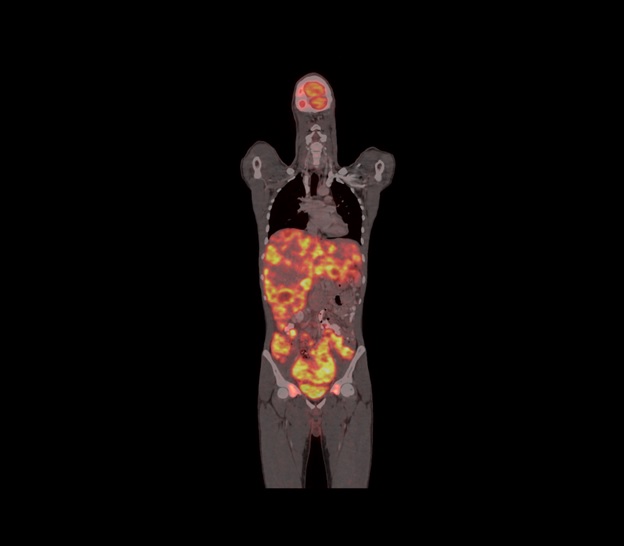
A 20-year-old male presented with low backache and altered bowel habits. His evaluation outside showed a rectosigmoid mass with peritoneal and liver metastasis. He was evaluated at our center with a PET CT scan which showed multiple nodal, liver, peritoneal, and skeletal lesions (Figure 3 (a).
Figure 3a. PET CT Imaging Showing Extensive Abdominal Disease.
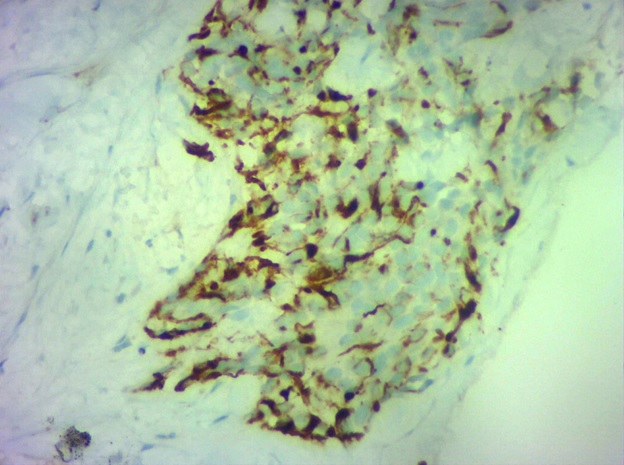
The initial working diagnosis was that of metastatic carcinoma rectum. A trucut biopsy of the liver lesion was done which was reported as DSRCT (Figure 3 (b).
Figure 3b. DSRCT Showing Desmin Positivity.
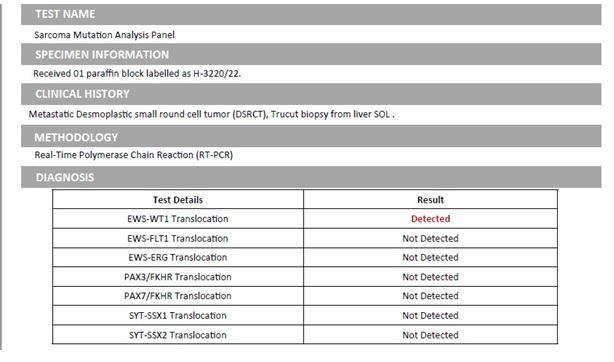
The sarcoma molecular analysis panel revealed EWS- Wilms’ tumor (WT1) translocation (Figure 3 (c).
Figure 3c. Molecular Test Showing the Classical Translocation.
He was started on an alternating VAC/IE regimen (similar to Ewing’s sarcoma protocol). CT scan revealed a partial response after 4 cycles and he is currently in his sixth cycle.
Discussion
Until recently, before its current description, DSRCT was a relatively unknown tumor that was considered by most clinicians to be a rare and lethal sarcoma. DSRCT mainly develops in adolescents and young adults; the mean age at diagnosis is approximately 22 years, and the male-to-female ratio is 4: 1 [3]. In our series also, the age group was fitting to the age range and all of the patients were males.
DSRCT is presumed to arise from progenitor cells with multi-phenotypic potential. It belongs to the family of “small round blue cell tumors”; nevertheless, analysis using molecular biology has proven DSRCT as a unique tumor. The cells co-express epithelial, mesenchymal, myogenic, and neural markers, but are distinguished by the presence of chromosomal translocation t (11;22) (p13;q12) resulting in the fusion of the EWSR1 and the WT1 genes, leading to a molecular cascade of which the up-regulation of PDGFRα is a hallmark event [4]. In our series, one of the patient’s molecular tests could not be done, but the tests of the last two patients were consistent with a diagnosis of DSRCT.
CT or Magnetic Resonance Imaging (MRI) showing multiple peritoneal implants increases the suspicion of DSRCT. The most common site of initial organ metastasis is the liver. The lungs, pleura, and mediastinum are the next most common locations. Bellah et al. analyzed the CT characteristics of 11 patients with DSRCT and found that the most characteristic features include bulky intra-abdominal soft-tissue masses involving omental and serosal surfaces, without a distinct organ of origin, and widespread tumor implants [5]. The usual clinical presentation is with nonspecific but persistent abdominal discomfort, altered bowel habits or bowel obstruction, significant weight loss, ascites, palpable masses, or infiltration of urinary organs causing hydronephrosis and urinary symptoms. Our patients also presented with mainly gut symptoms and imaging showed extensive peritoneal, nodal, and liver disease (advanced stage). Moreover, they can mimic various malignancies based on the clinical presentation and hence histological diagnosis is essential.
Proper consensus about treatment has not yet been established. DSRCT still remains a clinical challenge for oncologists because of the lack of standard guidelines. Surgical resection is appropriate wherever feasible.
Although complete cytoreductive surgery (CRS) is the standard therapy, the microscopic residual disease is often present. Hence, Hyperthermic Intraperitoneal Chemotherapy(HIPEC) has been examined in some studies as an adjunct strategy but with conflicting results. A recent prospective cohort study of 20 patients showed that CRS+HIPEC prolongs Relapse Free Survival (RFS) (14.9 vs. 4.5 months, p = 0.0012) and Overall Survival (OS) (44.3 vs. 12.5 months, p = 0.0013) when compared to other tumors [6]. But the conflicting reports have also emerged previously.
Looking at the real-world picture, most patients with DSRCT present in the advanced stage, and hence local therapies are rarely possible. DSRCT is known to be at least somewhat chemosensitive and radiosensitive. For advanced disease, symptom palliation is paramount. The reported Median Survival of DSRCT ranges from 17 to 25 months. Two retrospective studies have shown improvement in survival with maximal surgical debulking. They also concluded that a multimodality treatment is especially helpful in palliation and improvement in survival [7,8]. Subbiah et al. presented the largest series of patients diagnosed with DSRCT (187 patients) in a retrospective review where they showed that radiotherapy, surgery, intraperitoneal chemotherapy, removal of primary mass and metastases, age <30 years improved survival, cure rates were dismal [9]. Another study from the United Kingdom stated that the age, gender, or size of the presenting tumor had no prognostic significance and those with extra abdominal disease survived longer [10]. Surgical options could not be contemplated for any of our patients in view of the stage at the presentation.
Chemotherapeutic regimens used in various studies resemble the standard Ewing’s sarcoma protocol, especially because of the similarity in molecular studies. Kushner et al used the P6 protocol which consisted of 7 courses of chemotherapy with cyclophosphamide, doxorubicin, vincristine (HD-CAV), etoposide, and ifosfamide followed by surgery, radiotherapy, and myeloablative chemotherapy with autologous stem cell transplant (ASCT) in select cases and they had shown good results [11]. Bertuzzi et al. published a trial that included 7 patients with DSRCT treated with induction chemotherapy followed by high-dose chemotherapy and ASCT in those who responded, but it was a negative trial [12]. Scheer et al. in their recent trial concluded that patients treated with the VAIA regimen (ifosfamide, vincristine, doxorubicin, actinomycin D) presented longer event-free survival (29.4 months) compared to other protocols, including the P6 protocol [13]. As can be deduced, the apt chemotherapy protocol will still remain a matter of debate as prospective trials will never happen owing to the rarity of the disease. We used Ewing’s sarcoma protocol for all 3 patients and it has shown some response. The first patient did not have a lasting response, and the last two require a longer follow-up to assess the benefit. Nevertheless, they have shown significant improvement in quality of life with chemotherapy.
Novel treatment methods using precision oncology are essential in managing this disease. There is emerging data indicating the possible involvement of the mTOR pathway in DSRCT. In some case reports, utilizing mTOR inhibitors like temsirolimus and sirolimus showed mild improvements in time progression, but these have not yet been validated [14,15]. Targeting androgen receptors is yet another avenue that has been experimented with in view of the disease more commonly affecting young males, exploring a possible role of testosterone in the disease process [16]. One trial with antiandrogens reported a 3 to 4-month benefit among 3 patients with DSRCT, but again these are not corroborated further. Other potential targets like the c met pathway, and DeoxyRibonucleic acid (DNA) damage repair pathway are being evaluated [15]. Immune checkpoint inhibitors and NTRK inhibitors have also shown some efficacy in preclinical studies and have evoked interest in the possibility of future clinical trials with these agents.
In conclusion, DSRCT is a rare and aggressive disease and fatal for the majority of patients at this point in time. A definite treatment protocol has not been devised owing to the uncommonness of the disease. The current data and treatment protocols have not shown improved outcomes, thereby invoking the need for future clinical trials. Newer treatment approaches are the need of the hour in this disease with a bleak prognosis.
References
- Case 2. Desmoplastic small cell tumor with divergent differentiation Gerald WL , Rosai J. Pediatric Pathology.1989;9(2). CrossRef
- Management of desmoplastic small round cell tumor Hayes-Jordan A, LaQuaglia MP , Modak S. Seminars in Pediatric Surgery.2016;25(5). CrossRef
- Clinical, pathologic, and molecular spectrum of tumors associated with t(11;22)(p13;q12): desmoplastic small round-cell tumor and its variants Gerald WL , Ladanyi M, Alava E, Cuatrecasas M, Kushner BH , LaQuaglia MP , Rosai J. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.1998;16(9). CrossRef
- Fusion of the EWS and WT1 genes in the desmoplastic small round cell tumor Ladanyi M, Gerald W. Cancer Research.1994;54(11).
- Desmoplastic small round cell tumor in the abdomen and pelvis: report of CT findings in 11 affected children and young adults Bellah R, Suzuki-Bordalo L, Brecher E, Ginsberg JP , Maris J, Pawel BR . AJR. American journal of roentgenology.2005;184(6). CrossRef
- Desmoplastic Small Round Cell Tumor Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Results of a Phase 2 Trial Hayes-Jordan AA , Coakley BA , Green HL , Xiao L, Fournier KF , Herzog CE , Ludwig JA , McAleer MF , Anderson PM , Huh WW . Annals of Surgical Oncology.2018;25(4). CrossRef
- Desmoplastic small round cell tumors: prognostic indicators and results of surgical management Schwarz RE , Gerald WL , Kushner BH , Coit DG , Brennan MF , La Quaglia MP . Annals of Surgical Oncology.1998;5(5). CrossRef
- Results of multimodal treatment for desmoplastic small round cell tumors Lal DR , Su WT , Wolden SL , Loh KC , Modak S, La Quaglia MP . Journal of Pediatric Surgery.2005;40(1). CrossRef
- Multimodality Treatment of Desmoplastic Small Round Cell Tumor: Chemotherapy and Complete Cytoreductive Surgery Improve Patient Survival Subbiah V, Lamhamedi-Cherradi S, Cuglievan B, Menegaz BA , Camacho P, Huh W, Ramamoorthy V, et al . Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2018;24(19). CrossRef
- Desmoplastic small round cell tumour: characteristics and prognostic factors of 41 patients and review of the literature Wong HH , Hatcher HM , Benson C, Al-Muderis O, Horan G, Fisher C, Earl HM , Judson I. Clinical Sarcoma Research.2013;3(1). CrossRef
- Desmoplastic small round-cell tumor: prolonged progression-free survival with aggressive multimodality therapy Kushner BH , LaQuaglia MP , Wollner N, Meyers PA , Lindsley KL , Ghavimi F, Merchant TE , et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.1996;14(5). CrossRef
- High-dose chemotherapy in poor-prognosis adult small round-cell tumors: clinical and molecular results from a prospective study Bertuzzi A, Castagna L, Nozza A, Quagliuolo V, Siracusano L, Balzarotti M, Compasso S, Alloisio M, Soto Parra H, Santoro A. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2002;20(8). CrossRef
- Desmoplastic small round cell tumors: Multimodality treatment and new risk factors Scheer M, Vokuhl C, Blank B, Hallmen E, Kalle T, Münter M, Wessalowski R, et al . Cancer Medicine.2019;8(2). CrossRef
- Recurrent desmoplastic small round cell tumor responding to an mTOR inhibitor containing regimen Tarek N, Hayes-Jordan A, Salvador L, McAleer MF , Herzog CE , Huh WW . Pediatric Blood & Cancer.2018;65(1). CrossRef
- Desmoplastic Small Round Cell Tumor: A Review of Main Molecular Abnormalities and Emerging Therapy Mello CA , Campos FAB , Santos TC , Silva MLG , Torrezan GT , Costa FD , Formiga MN , et al . Cancers.2021;13(3). CrossRef
- Androgen and c-Kit receptors in desmoplastic small round cell tumors resistant to chemotherapy: novel targets for therapy Fine RL , Shah SS , Moulton TA , Yu I, Fogelman DR , Richardson M, Burris HA , et al . Cancer Chemotherapy and Pharmacology.2007;59(4). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2023
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times