Contribution of Mammography in the Screening of Suspicious Breast Cancer Lesions in Congolese Women in Kinshasa
Download
Abstract
Background: Mammography is a particular mammary imaging technique, an important tool for screening, diagnosis and monitoring of breast disease in general and breast cancer in particular, for which incidence and mortality are well established. Several studies have shown a reduction in breast cancer mortality reaching 40% in women screened by mammography. Thus, the prognosis after treatment is better because of early detection. In the DRC, in developing countries, the mortality rate is high because the diagnosis is late and therefore responsible for a bad prognosis. However, no studies on early detection by mammography have been performed to our knowledge. Our goal was to determine the mammography contribution in suspicious lesions detection of breast cancer in Kinshasa.
Method: A retrospective and descriptive study of 1738 mammograms at the Gombe Imaging Center from January 2012 to December 2015. The reports were prepared by radiologists. We re-read approximately 230 images under supervision to complete the missing data related to interpretation. Age, clinical information, breast density (BIRADS type), elemental or associated lesions, and ACR radiological diagnosis were the key parameters collected on an epidemiological data questionnaire and analyzed on the SPSS 20 software.
Results: 347 screening mammograms or 20% were performed with an average age of 48.6 years ; 25 of these (7.2%) had mammographic lesions suspected of malignancy, among which 23 were considered in situ. In addition, our study showed that high breast density is a radiologically visible risk factor.
Conclusion: This study has shown that mammography is a tool to integrate into cancer screening in our country. Because it allowed to detect suspicious lesions in asymptomatic women.
Introduction
Mammography is a medical imaging technique dedicated exclusively to breast exploration. It is used to detect, diagnose and monitor breast pathologies in general, and breast cancer in particular [1-3].
It is a technique that has been shown to reduce breast cancer mortality by 40% in screened women aged 50-69 years, as evidenced by 20 cohort studies and 20 case-control studies in high-income countries [1-7]. Screening mammography therefore aims to reduce breast cancer mortality through early detection, allowing for the most conservative early treatment possible, thereby increasing the chances of cure.
Worldwide, breast cancer is the most common cause of cancer death in women [8,9]. It is the most diagnosed cancer in women in high-income countries (1 in 8 women). It is the leading cause of cancer death in low- and middle-income countries, which is explained by the fact that a high proportion of women present with advanced disease, and therefore, it is associated with a poor prognosis [1,10]. In Central Africa, it remains the 2nd cause of cancer mortality after cervical cancer as confirmed by Mbala et al [11] in 2010 and Luyeye et al [12] in 2013 in our country, the Democratic Republic of Congo .
To reduce breast cancer mortality worldwide, it is crucial to provide access to early diagnosis and effective treatment, especially in developing countries.
Developed in the 1950s and 1960s following the publication of the first trials demonstrating its utility [13], mammographic breast cancer screening has become a standard of practice since the 1980s.
Mammographic breast cancer screening as part of a national or regional program has been tried in some African countries. Studies of breast cancer in our setting have focused on symptomatic women with documented breast cancer, most of them at an advanced stage [11,12]. Thus, we initiated this work to determine the contribution of mammography in the screening of suspected breast cancer lesions in our settings.
Materials and Methods
We therefore conducted a descriptive documentary study of the month of May 2016 - April 2017 within the Cabinet d’imagerie de la Gombe for the reason of archiving in terms of images and CRs over a period of at least 5 years, in addition to the availability of an operational mammography unit performing an average of 400 to 500 mammograms the year.
We included the files that were complete, i.e., that contained the report and the digital mammographic images made on the 2 basic incidences at least CC and MLO with strict respect of the success criteria, which images were obtained thanks to a Siemens senograph model Mammomat Select 2011.
The main information was collected using a pre-established form. It included: age, indication for mammography, breast density (BIRADS type), absence or presence of elementary lesions, absence or presence of associated lesions as well as their descriptions or characteristics and finally the radiological diagnosis according to the ACR recommendations. A review of 230 mammograms was performed to complete the main parameters not mentioned on the CRs.
For this study, we considered the following definitions
• High breast density: heterogeneous dense type breasts (c or 3), very dense (d or 4).The mass which corresponds to an opacity seen on at least 2 incidences, described according to its characteristics (shape, contours, density).
• A focus of microcalcifications is a grouping of more than 5 microcalcifications in an area of 2cm2, which may be benign, intermediate or suspicious according to morphology and distribution in the breast.
• Suspicious lesions in situ on mammography: images suggestive of breast cancer made essentially of foci of suspicious microcalcifications or mixed nodules of less than one centimeter without other associated elementary lesions.
• A mammogram is considered positive in this study when it presents lesions found in ACR categories 4, 5 or 6.
• The breast cancer detection rate corresponds to the proportion of women with positive mammograms.
Regarding the statistical analyses, the information collected on each form was precoded, entered in the form of an Epi data 3.1 questionnaire and then synthesized using Microsoft Office Excel 2016 software.
The data obtained from the variables of interest were summarized in the form of scientific Figures and Tables. The categorical or qualitative variables were presented as numbers (n=number) and percentages (%). The only quantitative variable (age) was summarized as mean and standard deviation. Pearson’s Chi-square test was used for comparison of proportions. The significance level (the p-value) was set at 5%. All statistical analyses were performed using IBM SPSS version 20.0 for Windows.
Results
1738 mammographic records were collected, representing 1738 women with a mean age of 43.7 ±12.3 years. Of the 1738 mammograms performed, 347 or 20.2% were indicated for screening with an average age of 48.6 years and 1391 or 79.8% for the diagnosis of any clinical breast abnormality with an average age of 42.5 years.
In the general characteristics of the screened population which constituted 20% of the total population studied. The majority of them, 335/347, had undergone mammography for the first time; there were as many premenopausal women as postmenopausal women.
Out of a total of 347: 13 had a family history of cancer, 11 had a personal history of breast pathology and 27 had a high breast density. Still in the screened population: 258 patients/347, i.e. 74%, were screened at the request of their attending physician, their gynecologist, or forced by their companies... 26% of the women screened had their mammograms done on the basis of individual detection. Out of the total population studied, which was 1738, only 5% of women who had no breast symptoms had their mammograms done individually.
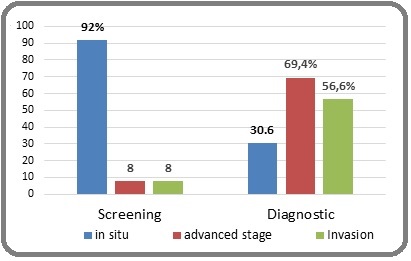
These 2 figures represent the detection rate of positive mammograms suspicious for cancer. Screening mammography detected 7.2% of images suspicious of malignancy in the asymptomatic group, i.e. 25 suspicious mammograms out of 347. The figure below represents the evolutionary stages of positive mammograms in two subgroups: For screening mammography, localized lesions (in situ) predominate while advanced stages are found in diagnostic mammography with lymph node involvement in 98 mammograms (Figure 1 and 2).
Figure 1. Screening (a) and diagnostic (b) mammographic findings.
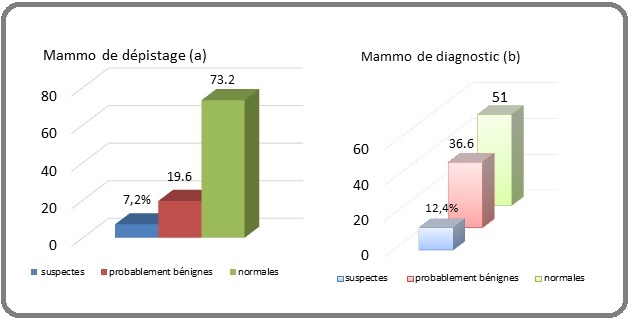
Figure 2. Distribution of Positive Mammograms According to Stage and Two Subgroups.
The elementary lesions seen on screening mammograms were only spiculated masses and microcalcifications of grouped distribution. The right breast was the preferential location of images suggestive of cancer on mammographic screening. The most incriminated quadrant was the superior-external quadrant.
This Table gives the characteristics of women with positive mammograms, thus all the brackets were concerned, the presence of risk factors and the intermediate breast density BI-RADS 2 are more found in these women (Table 1).
| Paramètre | Mammographie positive n (%) |
| Age | |
| ≤45 | 78 (39,4) |
| >45 à ≤50 | 28 (14,1) |
| >50 à ≤ 55 | 27 (13,6) |
| >55 à ≤60 | 24 (12,1) |
| >60 | 41 (20,7) |
| Risk factors | |
| Familial | 22 (68,6) |
| Personal history | 48 (61,5) |
| Breast density | |
| BIRADS 1 | 43 (21,7) |
| BIRADS 2 | 93 (47,0) |
| BIRADS 3 | 55 (27,8) |
| BIRADS 4 | 7 (3,5) |
| Post menopause | 92 (46,5) |
Table 2 shows that there is a significant difference between the 2 subgroups with respect to the different ACR categories.
| Paramètres | Mammo Dépistage n (%) | Mammo diag n (%) | P |
| ACR 0 | 11 (3,2) | 134 (9,6) | 0,0001 |
| ACR 1 | 254 (73,2) | 709 (51,0) | 0,0001 |
| ACR 2 | 44 (12,7) | 285 (20,5) | 0,0001 |
| ACR 3 | 13 (3,7) | 90 (6,5) | 0,0001 |
| ACR 4 | 14 (4,0) | 103 (7,4) | 0,0001 |
| ACR 5 | 11 (3,2) | 67 (4,8) | 0,0001 |
| ACR 6 | 0 (0,0) | 3 (0,2) | 0,0001 |
In addition to the malignant lesions targeted by the screening, the mammotest detected 44 benign ACR2 lesions (12%) and 11 indeterminate ACR 0 mammograms requiring further investigation in completely asymptomatic women.
Discussion
General considerations on screening mammography
Of 1738 mammograms performed, 347 or 20% of the women in the series had a screening mammogram, which is much lower than the rate in the West (over 75%) [13,14]. Antilla et al. in Finland in 2002 and Smigal et al. in the USA recorded rates of 81 and 78% respectively. Our results could be justified by the fact that there is no regional or national organized screening program. The same observation was made by Ouattara et al, who attributed the low participation in screening to a lack of awareness among women about breast cancer and the value of screening [15]. But also like Gueye in Senegal in 2014, we noted the low equipment of senographs in public hospitals and the poor territorial distribution of imaging centers that have them [16], the cost of this examination is high, between 60 and 100 US dollars, equivalent to the monthly income of the average Congolese. In addition, the quality of some images is poor, as they often do not meet the criteria of validity or interpretation required for screening tests.
Decentralization and exemption efforts should be undertaken in addition to the training that is already effective in this field of radiology in order to popularize mammography for screening purposes.
Two-thirds (67.7%) of the patients who came for screening in this series were over 45 years of age (Table 3).
| Variables | Total | Screening | Diagnostic |
| n = 1738 (100%) | n = 347 (20,2%) | n = 1391 (79,8%) | |
| Average age (years) | 43,7 | 48,6 | 42,5 |
| Type of mammo n (%) | |||
| Initial | 1686 (97,0) | 335 (19,9) | 1351 (80,1) |
| Subsequent | 52 (3,0) | 12 (23,1) | 40 (76,9) |
| Menopausal status n (%) | |||
| Pre-menopausal | 1114 (64,1) | 171 (15,4) | 943(84,6) |
| Postmenopausal | 624 (35,9) | 176 (28,2) | 448 (71,8) |
| RF n (%) | |||
| Family risk | 38 (2,2) | 13 (34,2) | 25 (65,8) |
| Previous breast disease | 81 (4,7) | 11 (13,6) | 70 (86,4) |
| High breast density | 398 (22,9) | 27 (6,8) | 371 (93,2) |
In France, for example, organized mass screening for women between 50 and 74 years of age is based on one mammogram every two years for women without major risk. Gueye et al. have noted that in low-income countries, which is the case for several African countries, breast cancer concerns mostly a younger population, and mammographic screening from the age of 40 years would be fully justified [16]. This was confirmed by two studies conducted on screening campaigns in Cameroon in 2010-2011 by Priso et al. in Douala and Guegang et al. in Yaoundé who recommended mammography screening from the age of 40 for Cameroonian women [17,18]. 96.5% of mammograms in this series were initial for both subgroups, as women were not sufficiently informed of the benefits of regular surveillance. In addition, the high cost of exploration, which is often not considered a priority (no immediate vital risk) by asymptomatic women, does not facilitate subsequent breast cancer screening by mammography. Socio-cultural factors such as modesty and guilt, particularly in the presence of male personnel, also seem to be a handicap in our environment. This was also observed by the teams of Bourée in Sudan and Gueye in Senegal [16,19].
Frequency and characteristics of suspected lesions detected
The detection rate of suspected cancer lesions was 7.2% with an advanced stage in 2 cases where family risk factors, history of breast pathology and high breast density were prevalent. These results are close to those of the Cameroonian study by Guegang et al. who found a detection rate of 11.6%, it should be noted that they included the ACR 3 category of lesions to watch out for in the group of suspicious lesions (which makes 10.9% in our series by including ACR3) [18], 10.8% as the detection rate in an Asian study by Wang et al. in Singapore in 2010 [7]. It should also be noted that in the group of symptomatic women, 12.4% or 173 mammograms suggestive of breast cancer were found.
Compared to the evolutionary stage, 92% of the lesions suspected of cancer were detected at an early stage, which demonstrates the value of screening mammography (early detection, more conservative treatment after anatomopathological confirmation and good prognosis). This contrasts with the results of symptomatic women,
in whom the diagnosis is made at a much more advanced stage. Of the 12.4% of suspicious mammograms in this group, approximately 70% were diagnosed at an advanced stage of the disease, with images of lymph node invasion in 57%. These results further reinforce the value of early detection by mammography.
Regarding the radiographic aspects of suspicious elementary lesions, the screening group was represented mainly by in situ mammographic lesions made of focal microcalcifications and masses less than 1cm in this series, Guegang et al also found more calcifications than masses [18].
Microcalcifications being only an indirect sign of pathological process are present in about 30% of malignant lesions of the breast in general, but in more than half of the subclinical malignant lesions of the breast and are the mode of revelation of 85-95% of the cases of carcinoma in situ in screening campaigns, found by Henrot et al, in France in 2014 [20].
For masses, the contour is the most discriminating morphological criterion between benign and malignant masses. An uncircumscribed contour on mammography is a criterion of suspicion requiring histological sampling. Berment et al, in a French study published in 2014 demonstrated that the PPV of malignancy varies according to the morphology of the contour, lower for micro lobulated contours, it increases for masked, then indistinct, until reaching 96% for spiculated contours [21].
Apart from microcalcifications and masses, architectural distortions are the 3rd form of cancer presentation, with a predictive value of 30% whose most frequent malignant etiology is invasive carcinoma, in this series distortions were not found on screening mammograms.
High breast density as a risk factor for breast cancer
Mammographic BMD is a well-established and strong predictor of breast cancer risk, which becomes 4 times stronger when associated with other risk factors (family history) [19,22-25]. Whatever the methodology used to assess breast density, all published studies concerning the general population have found an increased risk of breast cancer in women with dense breasts, and this can be generalized to different populations (postmenopausal or not, races or ethnicities) [23]. In this series, high breast density is the most common risk factor despite basic assessment methods. A meta-analysis of 42 studies that used qualitative methods of density assessment found an RR of about 2 and up to 4 for extremely dense breasts [22- 25]. Full-field digital mammography has been shown to be superior in detecting breast cancer in women under 50 with dense breasts. Some studies in older asymptomatic women with dense breasts have shown a better sensitivity of digital but not significant. Similarly, there was no significant difference between the two techniques regarding diagnostic accuracy and cancer PPV, both in screening and diagnostic settings (symptomatic patients). New methods of breast density quantification are being published, using digital mammography (automatic registration of calibration parameters) [23,26].
Non-irradiating techniques such as ultrasound and MRI are much more interesting for young populations and can also be used to overcome the difficulty of detection in dense breasts [26]. The decision to perform routine ultrasound (with strict diagnostic criteria for malignancy) should be de rigueur in the interpretation of a screening mammogram to avoid a high rate of close follow-up or interventional procedures for benign lesions [27].
Dense breasts are responsible for a higher rate of interval cancers than light breasts, the screening rate for surveillance should be increased [28].
Limitations and strengths
A retrospective study that did not allow us to collect as many risk factors as possible (breastfeeding, parity, age at menarche, body mass index, educational and socioeconomic level, previous radiation treatment, etc.) in the women who underwent mammography in this series, both in the screening population and in the population of symptomatic women. Lack of comparison of suspicious mammographic lesions detected with anatomopathological results. Despite these shortcomings, this study is to our knowledge the first to evaluate mammography screening in our country. As such, it has conceptual merit.
In conclusion, Screening mammography is effective in our setting and has made a significant contribution to breast cancer screening insofar as it has allowed the detection of suspicious lesions suggestive of breast cancer, almost all of these suspicious lesions being detected at an early stage, theoretically curable and with a good prognosis after anatomopathological confirmation of course.
The most common mammographic findings in screened women were microcalcifications and sub- centimeter nodules that were clinically unnoticeable in the absence of associated lesions. High breast density was the only mammographically detectable risk factor and was found in almost a quarter of the study population. However, its true value as a risk factor can only be applied after excluding or combining the factors influencing its modification.
Abreviations
ACR, american college of radiology; DM, dépistage mammographique; OR, odds ratio; IC, intervalle de confiance; CC, cradio-caudal; MLO, médiolatéral oblique; CR, compte-rendu; BIRADS, breast imaging reporting and data system
Author contributions
CM, principal investigator; AB, disposition of research materials and proofreading; JM, study direction proofreading; AM, proofreading; GL, proofreading; AM, proofreading; ML, direction and proofreading; NM, proofreading; SY, proofreading; SM, proofreading; NK, proofreading; KK, proofreading; TM, proofreading, formatting, and statistical editing.
References
- Cancer survival in Africa, Asia, and Central America: a population-based study Sankaranarayanan R, Swaminathan R, Brenner H, Chen K, Chia KS , Chen JG , Law SCK , et al . The Lancet. Oncology.2010;11(2). CrossRef
- Reduction in mortality from breast cancer after mass screening with mammography. Randomised trial from the Breast Cancer Screening Working Group of the Swedish National Board of Health and Welfare Tabár L., Fagerberg CJ , Gad A., Baldetorp L., Holmberg LH , Gröntoft O., Ljungquist U., Lundström B., Månson JC , Eklund G.. Lancet (London, England).1985;1(8433). CrossRef
- Decreased breast cancer mortality through mammographic screening: results of clinical trials Feig SA . Radiology.1988;167(3). CrossRef
- Report of the International Workshop on Screening for Breast Cancer Fletcher SW , Black W., Harris R., Rimer BK , Shapiro S.. Journal of the National Cancer Institute.1993;85(20). CrossRef
- A comparison of the performance and impact of breast cancer screening programmes in East Anglia, U.K. and Bouches du Rhône, France McCann J., Wait S., Séradour B., Day N.. European Journal of Cancer (Oxford, England: 1990).1997;33(3). CrossRef
- The SwedishOrganised Service Screening Evaluation Group. Reduction in mortalityfromorganized service screening withmammography Cancer EpidemiolBiomarkers Prev.2006;15(1).
- The impact of mammographic breast cancer screening in Singapore: a comparison between screen-detected and symptomatic women Wang WV , Tan SM , Chow WL . Asian Pacific journal of cancer prevention: APJCP.2011;12(10).
- Globocan 2012 v1.0, Estimated cancer incidence, mortality and prevalenceworldwidein 2012 : IARC Cancer Base 11 Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Matherset C, et al . 2013.
- IARC 2014.Cancer incidence in five continents, vol. I-X .
- Facteurs liés au diagnostic tardif du cancer du sein : expérience du CHU Mohammed VI Marrakech Aloulou S, Mahfoudi A, Khouchani M, et al . The pan AfrMedical Journal.2015;21:162.
- Le profil clinique, histopathologique et moléculaire du cancer mammaire chez la femme congolaise. Annales afr de méd 2011 ; vol(n°)pages Mbala , Nkwembe , et al . 2010.
- La place de l’imagerie dans la gestion pré et post mastectomie. J AfrImagMéd 2014 ; 2011 ; vol(n°)pages Luyeye , et al . 2013.
- Sensibilité du programme et efficacité du dépistage du service de Mammographie à Helsinki, en Finlande.J of Medical Screening Anttila A, Koskela J, Hakama M. Journal of medical screening.2002;9(4).
- Trends in breast cancer by race and ethnicity: update 2006 Smigal C, Jemal A, Ward E, Cokkinides V, Smith R, Howe HL , Thun M. CA: a cancer journal for clinicians.2006;56(3). CrossRef
- Problématique du dépistage du cancer du sein en milieu africain tropical : étude préliminaire.J Le Sein 2002 Ouattara DN , Diabate AS , Nzi KP . ;12(4):291-294.
- Problématique de la prise en charge des cancers du sein au Sénégal: une approche transversale Gueye SMK , Gueye M, Coulbary SA , Diouf A, Moreau JC . The Pan African Medical Journal.2016;25. CrossRef
- Profil épidémiologique et clinique de la pathologie mammaire à l’hôpital général de Douala. HealthSci.Dis 2012 Priso BE , Nguemgne C , Njamen NT . ;11(2).
- Apport de la mammographie et de l’anatomopathologie dans la recherche des lésions tumorales mammaires au cours d’une campagne de dépistage et de diagnostic de masse à Yaoundé. J Afr Imag Méd 2011; (4), 7: 345-354. Guegang E, Moifo B, Belley E, Sando Z, Sandjong I, Tebeu PM Pierre Marie, Mahamat M, et al . 2011;7.
- Dépistage du cancer du sein au Soudan.Médecine et santé tropicales Bourée P. 24.2014;1:28-29.
- Les microcalcifications mammaires : quelles lésions en anatomie pathologique ? Henrot P., Leroux A., Barlier C., Génin P.. Journal de Radiologie Diagnostique et Interventionnelle.2014;95(2). CrossRef
- Les masses en mammographie : quelles lésions anatomopathologiques sous-jacentes ? Berment H., Becette V., Mohallem M., Ferreira F., Chérel P.. Journal de Radiologie Diagnostique et Interventionnelle.2014;95. CrossRef
- Breast density and outcome of mammography screening: a cohort study Olsen Ah , Bihrmann K, Jensen Mb , Vejborg I, Lynge E. British journal of cancer.2009;100(7). CrossRef
- Quels risques, pour quellesfemmes ? Densité mammaire et cancer du sein. 30es journées de la SFSPM, La Baule 2008. Tardivon A. .
- Mammographic densities and breast cancer risk Boyd N. F., Lockwood G. A., Byng J. W., Tritchler D. L., Yaffe M. J.. Cancer Epidemiol Biomarkers Prev.1998;7(12).
- Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis McCormack VA , Santos Silva I. Cancer Epidemiol Biomarkers Prev.2006;15(6). CrossRef
- Computing mammographic density from a multiple regression model constructed with image-acquisition parameters from a full-field digital mammographic unit Lu LW , Nishino TK , Khamapirad T, Grady JJ , Leonard MH , Brunder DG . Physics in Medicine and Biology.2007;52(16). CrossRef
- Breast screening with ultrasound in women with mammography-negative dense breasts: evidence on incremental cancer detection and false positives, and associated cost Corsetti V, Houssami N, Ferrari A, Ghirardi M, Bellarosa S, Angelini O, Bani C, Sardo P, Remida G, Galligioni E, Ciatto S. European Journal of Cancer (Oxford, England: 1990).2008;44(4). CrossRef
- Recommandations de l'american college of radiology pour le dépistage du cancer du sein Feigh SA , d’Orsi C , Hendrick RE , et al . Recommandations de l'american college of radiology pour le dépistage du cancer du sein.1999;9(2).
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2023
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times