A Comparison of the Subjective Responses to Two Hypofractionated Palliative Radiotherapy Regimens in Patients with Locally Advanced Inoperable Head and Neck Cancer
Download
Abstract
Background: Patients with advanced head and neck cancers typically succumb to uncontrolled local disease. Even with aggressive therapy, median survival is about 12 months, and the five-year overall survival <20%. Radiation treatment used in palliative setting is an option for some patients who are not eligible for curative therapy. Palliative treatment aims to reduce cancer-related symptoms while causing the fewest side effects and toxicities as possible.
Materials and Methods: After approval from the institutional ethical committee, a prospective randomised study was conducted on squamous cell carcinomas of locally advanced head and neck cancer patients treated with palliative radiotherapy. A total of 62 patients were chosen and randomly assigned (1:1) to one of two treatment groups. Patients in Arm-A received 40 Gy in 16 fractions of radiation therapy, while those in Arm-B received 30 Gy in 10 fractions. The primary goal was to evaluate the subjective responses in both arms, including pain, dysphagia, and bleeding.
Results: Mean pain score after completion of radiotherapy was 4.69 and 3.65 in Arm-A and Arm-B respectively, with significant p-value of <0.001. The mean pain score 3 months after completion of treatment was 2.39 and 3.61 in Arm-A and Arm-B respectively, with significant p-value of <0.001. Comparing both the arms dysphagia relief at completion of treatment was higher in Arm-B compared to Arm- A with mean scores of 1.95 and 2.03 which was not significant. After 3 months, it was higher in Arm-A compared to Arm-B, which was also insignificant, while Arm A patients received significant relieve from dysphagia at completion of treatment and during follow-ups. Bleeding was not a significant factor in both the arms.
Conclusion: Our study found for immediate pain relief, 30Gy/10 fractions was better, but for long-term relief, 40Gy/16 fractions was better. Alleviation of swallowing difficulties was seen in both regimens.
Introduction
Patients with advanced Squamous cell carcinomas of the head and neck (SCCHN) have a terrible prognosis and typically pass away from uncontrolled localised illness. Even with vigorous therapy, the median survival is just about 12 months, and the five-year survival is less than 20% [1]. Because the disease affects many vital tissues, including the spinal cord, salivary glands, mandible, nerves, major blood arteries, and organs of speech, swallowing, hearing, and respiration, head and neck malignancies pose a considerable treatment challenge. Pain, dysphagia, odynophagia, otalgia, hoarseness, coughing, and respiratory distress are typical symptoms. Treatment toxicities and side effects frequently overlap significantly. With data from sizable prospective randomised controlled trials and meta-analyses, curative intent care of loco-regionally progressed SCCHN has become increasingly evidence-based [2-3] yielding a substantial body of high-quality evidence for developing consensus guidelines and recommendations [4,5]. Palliative radiation is well known for its ability to effectively relieve symptoms and enhance the quality of life (QOL) in patients with advanced, incurable cancers, and it makes up a sizeable component of cancer treatment worldwide [6-8]. The presence of distant metastatic disease at initial presentation, very advanced locoregional disease, extensive surgery or radiation therapy to the head and neck, comorbidities and poor performance status precluding tolerance of curative-intent therapy, and patient choice are all factors in the delivery of palliative-intent treatment. Patients in this situation receiving definitive, curative-intent cancer therapy are thought to endure significant treatment-related toxicity due to the burden of treatments required daily over several weeks. These patients’ health and quality of life are significantly impacted by such toxicity, which offsets the advantages of possible curative treatments [9].
Two studies found significant results in symptom relief using hyperfractionated split-course radiotherapy regimens [10,11]. Studies utilising conformal hypofractionated radiotherapy techniques has shown significant palliation [12,13].
Radiation treatment used in palliative cancer-directed therapy may still be an option for some patients who are ineligible for radical curative intent therapy. Palliative treatment aims to reduce cancer-related symptoms while causing the fewest side effects and toxicities possible [14].
Rationale and knowledge gap: There isn’t much written about palliative treatments for controlling symptoms in advanced, incurable HNSCC, which makes it hard to come up with guidelines and recommendations that everyone agrees on. So, it seems like a good idea to look into the role of palliative radiotherapy for long-term relief of symptoms with a low risk of side effects [15].
To strike a balance between speedy and effective palliation and limiting treatment-related toxicity, hypofractionated radiotherapy (high dose per fraction) may be pursued in this group of patients.
Objective
The main objective of this study was to assess and compare the subjective response to palliative radiotherapy between two arms.
Materials and Methods
Study Design
A prospective randomised study was conducted on the histologically proven squamous cell carcinomas of locally advanced head and neck cancer patients attending the radiation oncology department at Dr B. Borooah Cancer Institute, Guwahati, for palliative radiotherapy after approval from the institutional ethical committee and permission of Srimanta Sankaradeva University of Health Science, Assam. The main objective of this study was to assess and compare the subjective response to palliative radiotherapy between two arms.
The study period was 1 year (1st July 2021 to 30th June 2022), inclusive of both patient enrolment and data analysis. Patients with very advanced head and neck cancers who were planned for Palliative Radiotherapy as per the multidisciplinary tumour board decision of Dr. B. Borooah Cancer Institute and attend the department of radiation oncology were eligible for inclusion in the study. Patients who were found eligible for inclusion in the study were explained in detail about the study protocol. Patients who signed a consent form knowing what the study entails were randomly divided 1:1 into two study arms to receive palliative radiotherapy. In arm A, patients received radiotherapy to a dose of 40 Gy in 16 fractions with 2.5 Gy per fraction, one fraction per day, delivered five days a week over a total duration of three weeks and one day. In arm B, patients received radiotherapy to a dose of 30 Gy in 10 fractions with 3 Gy per fraction, one fraction per day, delivered five days a week over a total duration of two weeks. The patients were assessed after completion of treatment and every 2 weeks up to 3 months (Figure 1).
Figure 1. Design of the Study and Patient Selection Criteria.
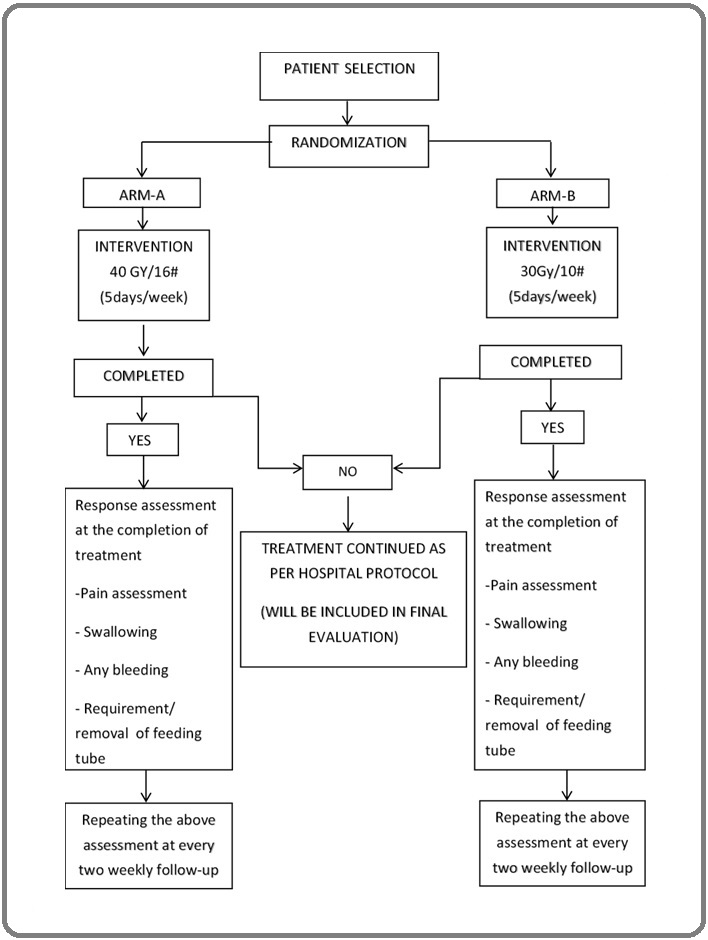
Inclusion and Exclusion criteria
Inclusion criteria were
Clinically and histologically proven head and neck carcinoma, AJCC 8th Edition stage III and above head and neck cancers (oral cavity, oropharynx, larynx, hypopharynx) who are not fit for radical radiotherapy, patient’s age between 18 to 70 years, those patients who are willing to participate in the trial and have given their written informed consent. Exclusion criteria were: cancer of the nasopharynx, paranasal Sinuses and thyroid gland; the patient who did not give written informed consent for study participation. Patients with ECOG (Eastern Cooperative Oncology Group) performance score of 3 or below, patient who has received any prior radiotherapy for his/her disease- either radical or palliative, patient with a previous history of head and neck malignancy, patients with pregnancy or other known contraindications to radiotherapy.
Pre-treatment workup
It consists of the complete history and physical examination, laryngopharyngoscopy, biopsy of primary tumour for histopathological confirmation, CECT Scan (Head and Neck), chest X-ray or thoracic CT scan, abdominal USG, ECG, cardiology evaluation and fitness, dental evaluation with management, nasogastric tube insertion if indicated, tracheostomy. Laboratory investigations include- CBC, renal Function Tests, liver Function Tests, viral markers, blood grouping and typing. Patient preparations include- all the patients were counselled to quit smoking and alcohol, advised to take a soft diet to prevent mucosal injury, frequent mouth gargling with soda bicarbonate water/Benzydamine, dental care, and good oral hygiene to be maintained.
Treatment technique
Thermoplastic moulds are used as a planning immobilization device. Patients were treated in a Cobalt-60 teletherapy unit using Gamma rays or a linear accelerator with 6 MV photons. Simple conventional X-ray-based planning was used. Target volume consisted of the primary or gross volume disease with margin and with or without the draining lymph nodes based on the clinical situation. Patient’s nutritional status, weight and feeding pattern (requirement of a feeding tube or parenteral nutrition) before treatment, during radiotherapy and at every follow-up after treatment will also be recorded and assessed.
Subjective Response Assessment
The Universal Pain Assessment Tool (UPAT) combines the Visual Analog Scale (VAS) with a Numeric Rating Scale (NRS) for pain intensity ranging from 0 to 10 is used to determine the severity of pain [16]. Modified Takita’s grading and World Health Organization (WHO) scale were used for assessment for grading of Dysphagia and Bleeding, respectively [17,18].
Statistical Requirements
The data obtained from the study were tabulated using Microsoft Excel and analysed statistically using GraphPad/ SPSS software. The chi-square test and Wilcox on the signed-rank test were used to evaluate the association between categorical variables. Data were checked for normality using Kolmogorov-Smirnova and Shapiro-Wilk tests. All data were then analysed using SPSS version 21. A p-value of less than 0.05 is considered statistically significant at a 5% significance level.
Results
A total of 124 patients who met the eligibility criteria were recruited for the protocol. They were randomised 1:1 by simple randomisation method as Arm A- 62 patients and Arm B- 62 Patients. One patient in Arm-Died during treatment, and one in Arm- B defaulted to treatment. A total of 61 patients were analysed in each arm, respectively. Most patients were in the age group of 50–70 years, with 53 and 50 patients being male; stage IVA was 33.2% and 40.5% in Arm A and Arm B, respectively. The rest of the baseline characteristics have been depicted in Table 1.
| Parameters | Arm A (n=62) (%) | Arm B (n=62) (%) | |
| Age(years) | 40-50 | 12 (19.3) | 14 (22.9) |
| 50-60 | 25 (40.3) | 21 (32.9) | |
| 60-70 | 25 (40.3) | 27 (44.5) | |
| Sex | Male | 53 (85.5) | 50 (80.6) |
| Female | 9 (14.5) | 12 (19.3) | |
| ECOG | 0 | 36 (58) | 20 (32.3) |
| 1 | 17 (27.4) | 24 (38.7) | |
| 2 | 9 (14.5) | 18 (29) | |
| Subsites | Oral cavity | 9 (14.5) | 6 (9.6) |
| Oropharynx | 25 (40.3) | 17 (27.4) | |
| Larynx | 18 (29) | 31 (50) | |
| Hypopharynx | 10 (16.1) | 8 (13) | |
| Stage | III | 2 (3.2) | 3 (4.9) |
| IVA | 33 (53.2) | 40 (64.5) | |
| IVB | 27 (43.5) | 19 (30.6) |
Table 2 compares the response of the patients within the same arm where there was significant pain relief after treatment and during follow-ups up to 3 months with a significant p-value of <0.001.
| Response | Duration | P-value | |
| Arm A | Arm B | ||
| Pain | After Completion of RT vs Pre-RT Pain Score | <0.001 | <0.001 |
| 1 st Two weekly follow-ups vs Pre-RT Pain Score | <0.001 | <0.001 | |
| 2 nd Two weekly follow-ups vs Pre-RT Pain Score | <0.001 | <0.001 | |
| 3 rd Two weekly follow-ups vs Pre-RT Pain Score | <0.001 | <0.001 | |
| 4 th Two weekly follow-ups vs Pre-RT Pain Score | <0.001 | <0.001 | |
| 5 th Two weekly follow-ups vs Pre-RT Pain Score | <0.001 | <0.001 | |
| At the end of the third month vs Pre-RT Pain Score | <0.001 | <0.001 | |
| Dysphagia | After Completion of RT vs Pre-RT Dysphagia | 0.001 | 0.443 |
| 1 st Two weekly follow-ups vs Pre-RT Dysphagia | <0.001 | 0.255 | |
| 2 nd Two weekly follow-ups vs Pre-RT Dysphagia | <0.001 | 0.255 | |
| 3 rd Two weekly follow-ups vs Pre-RT Dysphagia | <0.001 | 0.19 | |
| 4 th Two weekly follow-ups vs Pre-RT Dysphagia | <0.001 | 0.148 | |
| 5 th Two weekly follow-ups vs Pre-RT Dysphagia | <0.001 | 0.148 | |
| At the end of the third month vs Pre-RT Dysphagia | <0.001 | 0.799 | |
| After Completion of RT vs Pre-RT Bleeding | 0.907 | 0.111 | |
| 1 st Two weekly follow-ups vs Pre-RT Bleeding | 0.334 | 0.399 | |
| 2 nd Two weekly follow-ups vs Pre-RT Bleeding | 0.094 | 0.084 | |
| 3 rd Two weekly follow-ups vs Pre-RT Bleeding | 0.111 | 0.084 | |
| 4 th Two weekly follow-ups vs Pre-RT Bleeding | 0.077 | 0.04 | |
| 5 th Two weekly follow-ups vs Pre-RT Bleeding | 0.08 | 0.045 | |
| At the end of the third month vs Pre-RT Bleeding | 0.936 | 0.047 |
Dysphagia was significantly reduced in Arm A with a p-value of 0.001 after treatment and <0.001 during follow-ups up to 3 months, while it was not statistically significant in Arm B. Table-3 compares the mean scores between the two arms, where it was found that mean pain scores after RT completion were 4.69 and 3.65 in Arm-A and Arm-B, respectively, with a significant p-value of <0.001.
| Response | Duration | Arm-A | Arm-B | P-value |
| (Mean score) | (Mean score) | |||
| Pain | Pre-RT Pain Score | 6.95±1.541 | 6.52±1.446 | 0.087 |
| Pain after Completion of RT | 4.69±1.104 | 3.65±1.319 | <0.001 | |
| Pain- 1 st Two weekly follow-up | 3.25±1.105 | 3.50±1.251 | 0.207 | |
| Pain- 2 nd Two weekly follow-up | 3.44±1.009 | 3.58±1.300 | 0.373 | |
| Pain- 3 rd Two weekly follow-up | 3.64±1.126 | 3.53±1.264 | 0.834 | |
| Pain- 4 th Two weekly follow-up | 3.54±1.104 | 3.61±1.192 | 0.622 | |
| Pain- 5 th Two weekly follow-up | 3.56±1.148 | 3.65±1.269 | 0.636 | |
| Pain- At the end of the third month | 2.39±1.333 | 3.61±1.259 | <0.001 | |
| Dysphagia | Pre-RT Dysphagia Grade | 2.76±1.363 | 1.81±1.199 | <0.001 |
| Dysphagia after completion of RT | 2.03±0.999 | 1.95±0.982 | 0.538 | |
| Dysphagia-1 st Two weekly follow-up | 1.69±0.564 | 1.58±0.737 | 0.139 | |
| Dysphagia-2 nd Two weekly follow-up | 1.57±0.499 | 1.58±0.737 | 0.565 | |
| Dysphagia-3 rd Two weekly follow-up | 1.66±0.544 | 1.55±0.619 | 0.215 | |
| Dysphagia-4 th Two weekly follow-up | 1.56±0.563 | 1.53±0.593 | 0.735 | |
| Dysphagia-5 th Two weekly follow-up | 1.62±0.553 | 1.53±0.593 | 0.31 | |
| Dysphagia- At the end of the third month | 1.74±0.964 | 1.79±1.189 | 0.682 | |
| Bleeding | Pre-RT Bleeding Grade | 0.27±0.772 | 0.23±0.663 | 0.783 |
| Bleeding after completion of RT | 0.2±0.542 | 0.37±0.707 | 0.086 | |
| Bleeding- 1 st Two weekly follow-up | 0.11±0.412 | 0.15±0.438 | 0.579 | |
| Bleeding- 2 nd Two weekly follow-up | 0.07±0.309 | 0.08±0.275 | 0.5 | |
| Bleeding- 3 rd Two weekly follow-up | 0.07±0.309 | 0.08±0.275 | 0.5 | |
| Bleeding- 4 th Two weekly follow-up | 0.05±0.284 | 0.05±0.216 | 0.679 | |
| Bleeding- At the end of the third month | 0.21±0.581 | 0.06±0.248 | 0.181 |
The mean pain score 3 months after completion of RT was 2.39 and 3.61 in Arm-A and Arm-B, respectively, with a significant p-value of <0.001. Comparing both arms, dysphagia relief at completion of treatment was higher in Arm-B as compared to Arm- A with mean scores of 1.95 and 2.03, which was not significant. After 3 months of follow-up, it was higher in Arm-A compared to Arm-B, which was also insignificant. Bleeding was not a significant factor in the arms.
Discussion
This study is a prospective randomised trial comparing two different palliative radiation therapy regimens for the treatment of locally advanced head and neck cancers. The study was carried out on patients diagnosed with head and neck cancer (LAHNC). Despite the developments that have been made in treatment strategies for the management of LAHNC, the advanced stage of the disease that is present at the time of presentation continues to contribute to the high local failure rates. These rates might fluctuate anywhere from 55% to 70% at any particular time. No fixed timetable has been carved in stone concerning the dose fractionation of radiation utilised in the palliative management of LAHNC. When it comes to palliation in LAHNC, different writers have used a variety of different schedules of total dose as well as varying amounts of radiation fractions.
Palliative care is necessary to manage the locoregional disease and alleviate symptoms for patients who are not surgical candidates or cannot tolerate the toxicities of definitive chemotherapy or radiation therapy.
When considering radiobiological, financial, and logistical factors, a hypofractionated schedule is the one that comes out on top. This treatment uses radiotherapy, but each fraction contains a significantly higher dose than normal. Generally speaking, duration in its entirety is shorter. When applied in a curative setting, these regimes produce late effects that are more severe than those produced by conventional fractionation. Acute reactions can be controlled when the doses are kept at a low level, and the treatment’s tolerability can be improved by shortening the amount of time it is administered. These schedules are ideal for patients with poor performance status, the goal of treatment being to alleviate symptoms while minimising harm. The prognosis for these patients is generally bad, and the outcome is poor. Many phases I and phase II studies on advanced SCC of the head and neck have looked at hypofractionated palliative radiotherapy as a part of palliation.
The QUAD SHOT was created to deliver single, high-intensity radiation doses well below the threshold at which mucositis could develop [10]. The total dose of 14 Gy is split into four smaller doses and given over two days. A total of 42-65 Gy in 12 fractions can be given to responders using this strategy. Five-thirds of patients with very advanced disease and poor performance status saw objective responses, and nearly half saw an improved quality of life. Other palliative schedules include that of Paris et al. who used 3.7 Gy twice a day for two days and then repeated this schedule once a month for three months [11]. Even though 40% of the participants did not complete the course, 77% of the cases had responses, and 85% of patients agreed that their presenting symptoms had improved subjectively. Fortin et al [12] delivered 2500 cGy in 5 fractions using IMRT, and most of them reported improvement in their overall quality of life, physical functioning, swallowing, and discomfort compared to their initial conditions. The study conducted by Mohanti et al. [19] a higher symptom response rate of 50% compared to Fortin et al. Kancherlaet al. [13] gave patients 2000 cGy over 5 days, then they rested for 2 weeks, and finally, they gave them another 2000 cGy in just 1 week. There was a 79% symptom response rate 4 to 6 weeks after therapy had ended. The heterogeneity of advanced SCC of the head and neck and the challenges of measuring the quality of life rather than just survival make it hard to compare these protocols with one another.
In our study, we found that for immediate pain relief, the 30 Gy/10 fractions schedule was better, but for long-term relief, the 40 Gy/16 fractions arm was better. Alleviation of swallowing difficulties was seen in both schedules. Acute toxicities were seen more in the 40Gy/16 fraction arm, possibly due to longer treatment duration than 30Gy/10 fractions.
Comparing both the schedules for the treatment of patients with LAHNC in our study, palliation of symptoms can be achieved in both treatment schedules but higher in the 40Gy/16 fraction schedule with minimal acute and late side effects in both treatment schedules. Limitations of our study is a shorter follow-up of only 3 months.
In conclusion, Therefore, it is possible to conclude that palliation was achieved in both groups significantly better in the 40Gy/16 fraction schedule. Because our hospital sees more patients than we have radiation therapy equipment, this radiobiological superiority is especially useful. This radiobiological benefit comes from a hypofractionated radiotherapy regimen that requires less time overall. Confirmation of this protocol’s long-term effects and survival benefits will require additional research with a longer duration of follow-up.
Acknowledements
The authors would like to acknowledge Dr. B. Borooah Cancer Institute for conducting research work. No funding was required for this study.
The authors declare that they have nothing to disclose and no conflicts of interest.
The study was conducted after the approval from the ethics committee of the institution.
References
- Radiotherapy with or without mitomycin c in the treatment of locally advanced head and neck cancer: results of the IAEA multicentre randomised trial Grau C, Prakash Agarwal J, Jabeen K, Rab Khan A, Abeyakoon S, Hadjieva T, Wahid I, et al . Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology.2003;67(1). CrossRef
- Conventional versus modified fractionated radiotherapy: a meta-analysis based on individual data of patients with head and neck squamous cell carcinoma (HNSCC) Bourhis J, Syz N, Overgaard J, Ang KK , Dische S, Horiot JC . Radiother Oncol.2002;64(suppl 1):S23.
- Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer Pignon JP , Bourhis J, Domenge C, Designé L. Lancet (London, England).2000;355(9208).
- Guidelines & Advice. 2022 Cancer Care Ontario . Available from: https://www.cancercareontario.ca/en/guidelines-advice. Accessed September 27, 2022..
- European Society for Medical Oncology. Guidelines 2022 Available from: https://www.esmo.org/guidelines. Accessed September 20, 2022...
- International consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases Chow E, Wu JSY , Hoskin P, Coia LR , Bentzen SM , Blitzer PH . Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology.2002;64(3). CrossRef
- Whole-brain radiotherapy in the management of brain metastasis Khuntia D, Brown P, Li J, Mehta MP . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2006;24(8). CrossRef
- A Medical Research Council (MRC) randomised trial of palliative radiotherapy with two fractions or a single fraction in patients with inoperable non-small-cell lung cancer (NSCLC) and poor performance status. Medical Research Council Lung Cancer Working Party Bleehen NM , Girling DJ , Machin D, Stephens RJ . British Journal of Cancer.1992;65(6). CrossRef
- Palliative surgery for head and neck cancer with extensive skin involvement Jang DW , Teng MS , Ojo B, Genden EM . The Laryngoscope.2013;123(5). CrossRef
- Phase II study of multiple daily fractionations in the palliation of advanced pelvic malignancies: preliminary report of RTOG 8502 Spanos W, Guse C, Perez C, Grigsby P, Doggett RL , Poulter C. International Journal of Radiation Oncology, Biology, Physics.1989;17(3). CrossRef
- Phase I-II study of multiple daily fractions for palliation of advanced head and neck malignancies Paris KJ , Spanos WJ , Lindberg RD , Jose B, Albrink F. International Journal of Radiation Oncology, Biology, Physics.1993;25(4). CrossRef
- Palliative Radiation Therapy for Advanced Head and Neck Carcinomas: A Phase 2 Study Fortin B, Khaouam N, Filion E, Nguyen-Tan PF , Bujold A, Lambert L. International Journal of Radiation Oncology, Biology, Physics.2016;95(2). CrossRef
- The role of split-course hypofractionated palliative radiotherapy in head and neck cancer Kancherla KN , Oksuz DC , Prestwich RJD , Fosker C, Dyker KE , Coyle CC , Sen M. Clinical Oncology (Royal College of Radiologists (Great Britain)).2011;23(2). CrossRef
- Palliative radiation therapy for head and neck cancer: toward an optimal fractionation scheme Chen AM , Vaughan A, Narayan S, Vijayakumar S. Head & Neck.2008;30(12). CrossRef
- Palliative care in patients with cancer of the head and neck Forbes K. Clinical Otolaryngology and Allied Sciences.1997;22(2). CrossRef
- Use of the universal pain assessment tool for evaluating pain associated with TMD in youngsters with an intellectual disability Dugashvili G, Van den Berghe L, Menabde G, Janelidze M, Marks L. Medicina Oral, Patologia Oral Y Cirugia Bucal.2017;22(1). CrossRef
- Palliative radiotherapy in esophageal cancer Prasad NRV , Karthigeyan M, Vikram K, Parthasarathy R, Reddy KS . The Indian Journal of Surgery.2015;77(1). CrossRef
- The risk of bleeding in thrombocytopenic patients with acute myeloid leukemia Webert K, Cook RJ , Sigouin CS , Rebulla P, Heddle NM . Haematologica.2006;91(11).
- Short course palliative radiotherapy of 20 Gy in 5 fractions for advanced and incurable head and neck cancer: AIIMS study Mohanti BK , Umapathy H, Bahadur S, Thakar A, Pathy S. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology.2004;71(3). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2023
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times