Radiation Induced Thyroid Gland Changes in Nasopharyngeal Carcinoma Patient after Chemoradiotherapy in DR Sardjito Public Hospital
Download
Abstract
Objective: The objective of this study was to determine the volumetric change in thyroid gland in nasopharyngeal carcinoma patients after undergoing chemoradiotherapy that could alter thyroid gland function. Furthermore, this study correlates the mean dose effect towards the decrease in thyroid volume to predict the outcoming clinical signs of radiation induced hypothyroidism (RHT).
Methods: This study consists of a cross sectional retrospective evaluation of multiplanar image that comprised three planes of axial, coronal, and sagittal of all NPC patients who underwent chemoradiotherapy and received a computed tomography (CT) imaging before and three months after completing treatment to the evaluate the alteration of thyroid volume.
Result: A significant decreased in thyroid volume between pre and 3 months post chemoradiotherapy was obtained in the result with p value < 0.001, along with the mean dose of 58 Gray effect towards the decreased thyroid volume with p value = 0.005.
Conclusion: Our study demonstrated that reduction in thyroid volume may be seen as early as 3 months after chemoradiotherapy in nasopharyngeal carcinoma that correlates with mean dose effect towards the thyroid volume. This volume changes also accompanied by the alleviation of free T3 and free T4 before and after chemoradiotherapy. Therefore, efforts should be made to do an early evaluation of radiation induced hypothyroidism (RHT). Early treatment and additional evaluation might be considered to enhanced the quality of life of the nasopharyngeal carcinoma patient. Highlights/Key points: Significant decreased in thyroid volume and its function among NPC patients evaluated by CT imaging and thyroid panel. Correlation of the mean dose volume effect towards the decrease in thyroid volume. Prediction of the outcoming clinical signs of radiation-induced hypothyroidism in NPC patients after chemoradiotherapy.
Introduction
Nasopharyngeal carcinoma (NPC) is a highly prevalent malignancy causing death worldwide. NPC is a frequent cancer in Indonesia, posing as fourth most common cancer after cervical cancer, breast cancer, and skin cancer, also the most common malignancy in the head and neck region. An ethnically diverse country like Indonesia that consists population of around 225 million where NPC is prevalent among different native people and causing major socioeconomic problem with an overall incidence estimated at 6.2/100000 or around 12000 new cases per year [1]. Most NPC initiated in the fossa of Rosenmuller in the nasopharynx which is a transitional area where the cuboidal epithelium transformed into squamous epithelium [1]. NPC developed at the submucosal level, spreading outside the anatomical border of the nasopharynx. The most common clinical features are associated with hearing problem, serous otitis media, tinnitus, nasal obstruction, anosmia, unilateral/bilateral neck mass, even eye symptoms usually as a diplopia or blurred vision. The initial diagnosis was difficult to make due to the non-specific sign and symptoms of NPC. The treatment of NPC was complex, not cost effective, and places a significant socioeconomic burden to the patients and their families [1, 2]. Radiotherapy with intensity- modulated radiotherapy (IMRT) technique with or without chemotherapy was considered as the standard treatment for NPC. Despite this more precise technique of radiation, normal tissue in the head and neck region are inevitably exposed to radiation [2, 3]. One of the most common late complication in NPC treatment is radiation-induced hypothyroidism (RHT) that causes various severity of clinical symptoms such as fatigue, mood swings, daytime sleepiness, weight gain, cold intolerance, constipation, dyspnea, soft tissues edema, dry skin, and increased risk of cardiovascular disease. The incidence of RHT ranged between 22,4 – 53,9 %. In head and neck cancer patients and also associated with thyroid exposure dose, thyroid volume, chemotherapy, pituitary exposure dose, and pretreatment thyroid stimulating hormone (TSH). Previous study [1, 3] revealed that among 69 NPC patients who underwent radiation therapy using IMRT technique demonstrated significant risk factor for the thyroid volume and its relation to the mean radiotherapy dosage. This study confirmed that thyroid exposure dose was clearly associated with the risk of RHT [3]. The volume of thyroid that spared from high dose was a recent indicator of the RHT incidence. It was postulated that the small thyroid gland might have less functional subunit to produce thyroid hormone and send negative feedback by increasing TSH level from the pituitary gland. RHT is associated with the damage to the thyroid cells and small blood vessels of the gland as well as capsular fibrosis. These damages cause cell degeneration which in turn leads to necrosis and fibrosis that results in decreased thyroid gland volume [3]. Pil et al. (2016) explained another theory regarding the pathophysiology of radiation induced thyroid damage which is believed to be multifactorial [4]. Radiation inhibits follicular epithelial function and progressively alters the endothelium, resulting in cell degeneration and necrosis, follicular disruption and vascular degeneration, inflammation, and partial epithelial degeneration that further contribute to the decreased thyroid volume and its physiological function. This is an irreversible condition requiring lifelong treatment [3, 4]. Considering the nonspecific symptoms of RHT are associated with the NPC itself and its treatment, RHT can often left undiagnosed and untreated [5]. The aim of this study was to determine the volumetric change in thyroid gland in nasopharyngeal carcinoma patient after undergoing chemoradiotherapy that could alter thyroid gland function. Furthermore, we wanted to correlate the mean dose effect towards the decrease in thyroid volume to predict the outcoming clinical signs of RHT.
Materials and Methods
This study consists of a cross sectional retrospective evaluation of multiplanar image that comprised three planes of axial, coronal, and sagittal of all NPC patients who underwent chemoradiotherapy and received a computed tomography (CT) imaging before and three months after completing treatment to the evaluate the alteration of thyroid volume. Approval of this study was obtained from the ethical committee from Medical and Health Research Ethic Committee (MHREC) Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada with registration number of KE/FK/0078/EC/2021.
Patient Selection and Treatment
There are thirty patient who was diagnosed with locally advanced NPC based on the Eighth Edition American Joint Committee on Cancer (AJCC) head and neck cancer staging system were consecutively included at the DR Sardjito Public Hospital, Yogyakarta, Indonesia over January to December 2021. All patients were given informed consent for the participation in the study. The radiotherapy technique of IMRT was applied with a dose of approximately 70 Gray to the. Primary tumor and involved lymph nodes divided into 5 fractions per week, 1 fraction per day. Chemotherapy was administered concurrently with Cisplatin but the chemotherapy regimen was not analyzed in this study.
Measurement
Each patient underwent routine CT imaging before treatment and three months after treatment. Thyroid volume measurements were calculated based on this CT examination. All CT images shared the same coordinate system for volume analysis. Patients’ records, including inpatient and outpatient notes and lab results were also reviewed to assess the development of clinical RHT that consist of elevated TSH in the presence of low free T3 and T4 concentration.
Statistical Analysis
Descriptive analysis of patients’ characteristics was presented as the mead + standard deviation (SD). The difference of mean thyroid volume between pre-treatment and three months post-treatment was analyzed using paired t-test. Fisher exact test was applied to see the effect of each variable. A p < 0.05 was considered statistically significant in two-tailed statistical test. Analysis was conducted using Statistical Package for Social Sciences (SPSS) version 16.0 for Windows/Mac.
Results
Patients’ Characteristic
Thirty patients with nasopharyngeal cancer who underwent chemoradiotherapy were included in the analysis. The patient and tumor characteristic are shown in Table 1.
| Number of Patients (Percentage) | |
| Age (year) | |
| < 30 years old | 2 (6.7) |
| 30 – 60 years old | 25 (83.3) |
| > 60 years old | 3 (10) |
| Gender | |
| Male | 21 (70) |
| Female | 9 (30) |
| T stage | |
| T1 – T2 | 18 (60) |
| T3 – T4 | 12 (40) |
| N stage | |
| N0 – N1 | 17 (56.7) |
| N2 | 9 (30) |
| N3 | 4 (13.3) |
| Stage | |
| I – II | 10 (33.3) |
| III – IV | 20 (66.7) |
| Thyroid Volume | |
| Decrease | 27 (90) |
| No Decrease | 3 (10) |
| fT3 | |
| Decrease | 23 (76.7) |
| No Decrease | 7 (23.3) |
| fT4 | |
| Decrease | 20 (66.7) |
| No Decrease | 10 (33.3) |
| TSH | |
| Increase | 22 (73.3) |
| No Increase | 8 (26.7) |
The age majority of the research subjects was in the range of 30 – 60 years old for the percentage of 83.3% followed by > 60 years old (10%) and < 30 years old (6.7%). The tumor staging majority was T1 – T2 (60%) and T3 – T4 (40%).
The characteristic of each variable was shown as minimum, maximum, mean, and standard deviation (SD). The mean age of the subjects was 48.3 years old (SD=11.18) with minimum age of 17 years old and maximum age of 69 years old. The mean total radiation dose was 70.65 (SD = 0.51) with the lowest dose of 68.64 Gray and the highest dose was 72.93 Gray. The mean radiation dose was 58 Gray with the lowest 49.9 Gray and highest of 65.60 Gray. This mean radiation dose of 58 Gray was appointed as cut off point for the effect of mean dose analyses that will be provided further in the result section. The results also showed thyroid gland respond to radiation by an alighting mean level of fT3 from pre-treatment of 3.20 pg/mL to 2.63 pg/mL post treatment, accompanied by alighting in fT4 from pretreatment to post treatment with 1.25 pg/mL to 1.12 pg/mL respectively, also there was an elevation in TSH from 1.61 µIU/mL to 2.41 µIU/ mL (Table 2).
| Variable | Minimum | Maximum | Mean ± SD |
| Age (year) | 17 | 69 | 48.3 ± 11.18 |
| Total Radiation Dose (Gy) | 68.64 | 72.93 | 70.65 ± 0.96 |
| Mean Radiation Dose (Gy) | 49.9 | 65.6 | 58.00 ± 0.51 |
| Pre FT3 (pg/mL) | 2.24 | 5.1 | 3.20 ± 0.73 |
| Post FT3 (pg/mL) | 1.53 | 5 | 2.63 ± 0.62 |
| Pre FT4 (ng/dL) | 0.55 | 2.01 | 1.25 ± 0.30 |
| Post FT4 (ng/dL) | 0.81 | 1.48 | 1.12 ± 0.18 |
| Pre TSH (µIU/mL) | 0.04 | 5.66 | 1.61 ± 1.20 |
| Post TSH (µIU/mL) | 0.06 | 11.45 | 2.41 ± 2.35 |
| Pre thyroid volume (mL) | 6.2 | 24.1 | 11.93 ± 5.57 |
| Post thyroid volume (mL) | 3.2 | 17.6 | 8.53 ± 4.24 |
Changes of thyroid volume
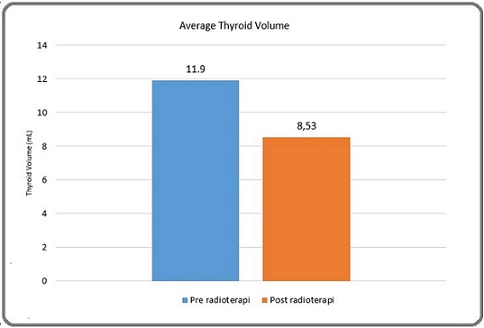
Analyses of the shifting of the mean thyroid volume showed an alleviation from 11.93 to 8.53 pretreatment and post treatment respectively. A paired t-test was done to assess whether this difference was significant. Table 3 showed p value of 0.001 that indicates the significant difference of mean thyroid volume between pretreatment and post treatment.
| Variable | Pre | Post | p value |
| Thyroid Volume (mL) | 11.93 ± 5.57 | 8.53 ± 4.24 | 0.001* |
*paired t-test p < 0.05
Figure 1 demonstrated a decline in thyroid volume before and three months after chemoradiotherapy.
Figure 1. Average Thyroid Volume Pre and Post Treatment.
Mean dose effect towards thyroid volume
We also analyzed the mean dose effect towards the thyroid volume using Fisher exact test, the cut off point for the radiation dose was 58 Gray, therefore we divided the subjects based on the mean radiation dose exposure into < 58 Gray and > 58 Gray and compared the proportion of the subjects with both decreased and non-decreased thyroid volume.
Table 4 demonstrated the effect of mean dose radiation on the decreased thyroid volume that showed significant result with p = 0.005, odds ratio of 0.048.
| Decreased thyroid volume | OR | 95% CI | p value | |||
| Yes | No | Min | Max | |||
| Mean Dose | 0.048 | 0.005 | 0.464 | 0.005* | ||
| > 58 Gy | 6 | 14 | ||||
| < 58 Gy | 9 | 1 |
*Fisher exact p<0.05
Discussion
The thyroid gland is one of the largest endocrine glands around the neck area, the hormones regulate body metabolism that control the vital function such as nerve reflexes, heart rate, and body temperature. The thyroid gland could affect a wide range of physiological function of all organs in the body. The particular susceptibility of the thyroid gland to irradiation is well known [5]. Teimuri et al (2022) [6] mentioned that when the thyroid gland is exposed to radiation, 40-67% of the patients develop thyroid problems, the most common one is hypothyroidism as well as thyroid cancer. The primary goal in radiation therapy is to deliver adequate amount of radiation dosage to the tumor while delivering the lowest radiation damage to the adjacent healthy tissues [6, 7].
Radiation induced hypothyroidism (RHT) is one of the most common late complications in nasopharyngeal carcinoma (NPC) patients who underwent definitive radiotherapy (RT) with or without chemotherapy. RHT usually occurs as a late adverse effect peaking its incidence in 2 years after treatment and affecting quality of life and risking a life-threatening condition [7].
NPC is a peculiar type of head and neck cancer, it differs from other cancer in head and neck region in three aspects, its primary treatment is definite radiotherapy (RT) with or without chemotherapy, intensity-modulated radiotherapy (IMRT) is the standard treatment technique, pituitary gland is partially included in the radiation field, even though the majority symptom in NPC is neck mass, surgical neck dissection that involved thyroid gland is not performed, and long survival outcome is expected [5, 8]. Although there is an advance RT technique nowadays, irradiation to normal organ is still inevitable. RHT is the most common late effect after RT exposure to the neck. Incidence of RHT ranged from 23-53% in head and neck cancer, and 14-54% in NPC in particular [9].
Diagnosis of RHT entails laboratory tests of TSH, free T3 and T4, with or without clinical signs/ symptoms. Clinical hypothyroidism is mostly defined as high serum TSH level with low T4 and presence of clinical presentations of hypothyroidism including generalized symptoms such as fatigue, weakness, cold intolerance, weight gain, dry skin, edema, constipation, and menorrhagia, neuromuscular symptoms like myalgia, joint pain, cognitive dysfunction, impaired consciousness, and also psychiatric symptom like depression. On the other hand, a condition of increased TSH level and low/ normal T4 without symptoms is defined as subclinical/ biochemical hypothyroidism [9].
Study done by Wenyong et al (2021) [10] revealed that during NPC treatment, a few subsequent changes may occur in the primary tumor and its surrounding tissues, inflicting a challenge for efficient healthy organ sparing. The study result showed that salivary and thyroid gland underwent substantial geometric changes during chemoradiotherapy for NPC including the volume, position, and shape.
Our findings were consistent with the previous study where we found significant reduction in thyroid volume. We found out that overall patients experienced decreased mean thyroid volume as much as 28.5% over baseline, even though this decrease was statistically significant, whether this change was clinically relevant was still unclear.
Here we also evaluated the difference in thyroid volume decrease based on different local thyroid dose regions through assessing changes in thyroid volume between > 58 Gray and < 58 gray on CT images. Our result as demonstrated in Table 4 indicated a significantly larger decreased in thyroid tissue that received < 58 Gray compared to those who received > 58 Gray, starting 3 months after radiotherapy (p = 0.005).
Our study confirmed a significant inverse relationship between thyroid volume and risk of RHT as thyroid volume is a surrogate for the functional reserve of thyroxine production, it is understandable that patient with smaller thyroid volume are more vulnerable to RHT. Our study finding indicates that a single radiation dose volume constraint for the thyroid gland is not universally applicable to all patients. Instead, individualized constraints tailored for different thyroid volumes should be developed, with more stringent threshold for patients with small thyroid volume. A systematic review by Chow et al (2022) [11] mentioned that certain factors such as young age, female, and history of neck surgery, or chemoradiotherapy have been shown to confer a higher risk of RHT in patients with head and neck cancer. Many of the reported associations were unadjusted for differences in thyroid dosimetry and thyroid volume [11, 12].
The choice for 58 Gray as cut-off point used in this study was based on previous study by Lin et al (2018) [8], for conventional radiotherapy, anterior cervical field was used to treat the whole neck down to the suprasternal notch, while for IMRT the planned target volume of the neck node usually includes the level IV right around the adjacent thyroid gland. Since currently no dose constraint is applied to the thyroid gland during planning, both techniques may deliver substantial dose to the thyroid gland ranging from 54-66 Gray [8, 13]. Due to the shrinkage of the thyroid gland and reduction of the level after the completion of radiotherapy, it was expected that the cases of RHT were increased [8].
There was an increased incidence of radiation- induced hypothyroidism until 24 months post radiation and associated with radiation dose to thyroid gland in 56 nasopharyngeal carcinoma patients undergoing IMRT. According to Miller and Agrawal radiation dose of more than 2.25 Gray will induce thyroid gland follicular cell death through apoptotic mechanism. The incidence of hypothyroidism increased when the thyroid gland received >45 Gray radiation dose [13]. A similar study stated that giving a mean dose of less than 50 Gray to the thyroid gland when treating nasopharyngeal carcinoma can prevent complications of hypothyroidism post radiotherapy [14]. Moreover, another study also revealed that the incidence of post radiotherapy hypothyroid was caused by the dose and area of radiation directly received by thyroid gland [15].
The mechanism of decreased thyroid function is associated with cells damage and thyroid gland small blood vessels and thyroid gland capsule fibrosis. These small blood vessels damage will cause cell degeneration which in turn will lead to necrosis and fibrosis [15]. Observation made using an electron microscope on post radiotherapy blood vessels showed fibrosis on the blood vessel walls causing narrowing of the lumen. The narrowing of the lumen will cause hypoxemia and thyroid gland cells will be deprived of nutrients, thereby reducing the ability to synthesize. This will cause thyroid gland to shrink. Shrinkage of the thyroid gland can be seen on 6 months post radiotherapy and stabilizes over the next 12 months. This shrinkage is related to the average radiation dose to the thyroid gland. In this study, there was a decrease in thyroid volume at 3 months post radiotherapy with an average radiation dose of 58 Gray [15, 16].
Furthermore, there was also a decrease in thyroid function in FT3, FT4 and TSH levels 3 months post radiotherapy. Another hypothesis states that in nasopharyngeal carcinoma patients undergoing radiotherapy, there is an abnormal increase in thyroid antibodies, thyro-peroxidase antibodies (anti TPO) and thyroglobulin antibodies (anti TG), which are indicators of thyroiditis from an autoimmune response. After high doses of radiation, the thyroid gland will experience inflammatory and trigger the production of anti-TPO. Anti-TPO will destroy the TPO enzyme so that it blocks thyroid hormone production, which in turn will cause hypothyroidism. Under normal circumstances, TPO and TG are enzymes and glycoproteins that play a role in thyroid hormone synthesis [17, 18].
In conclusion, in radiation therapy for NPC, exposure to non-target organ such as thyroid was inevitable. This study showed that there was a 28.5% decrease in thyroid volume in patients with nasopharyngeal carcinoma who received chemoradiotherapy, followed by a slight decreased in free T3 and T4, also increased TSH level indicating early sign of thyroid malfunction. Therefore, it is important to consider this risk when planning radiation therapy also it is mandatory to evaluate thyroid function and condition before and after NPC treatment.
Acknowledgments
Statement of Transparency and Principals
Funding Statement
No funding resources
Approval
If it was approved by the Research Committee of Faculty of Medicine, Public Health, and Nursing/DR Sardjito Public Hospital, Yogyakarta, Indonesia.
Conflict of Interest
None to declare
Ethical Declaration
This study was approved by the ethical committee from Medical and Health Research Ethic Committee (MHREC) Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada with registration number of KE/FK/0078/EC/2021.
References
- Nasopharyngeal carcinoma in Indonesia: epidemiology, incidence, signs, and symptoms at presentation Adham M, Kurniawan AM , Muhtadi AI , Roezin A, Hermani B, Gondhowiardjo S, Tan IB , Middeldorp JM . Chinese Journal of Cancer.2012;31(4). CrossRef
- Successful Identification of Nasopharyngeal Carcinoma in Nasopharyngeal Biopsies Using Deep Learning Chuang WY , Chang SH , Yu WH , Yang CK , Yeh CJ , Ueng SH , Liu YJ , et al . Cancers.2020;12(2). CrossRef
- A multivariable normal tissue complication probability model for predicting radiation-induced hypothyroidism in nasopharyngeal carcinoma patients in the modern radiotherapy era Wongwattananard S, Prayongrat A, Srimaneek N, Hayter A, Sophonphan J, Kiatsupaibul S, Veerabulyarith P, et al . 2022. CrossRef
- The incidence of hypothyroidism after radiotherapy for head and neck cancer Pil J, Nevens D, Van der Vorst A, Gadan C, Nuyts S. B-ENT.2016;12(4).
- Acute Thyroid Profile Changes During External Beam Irradiation of Neck Madhani N, Saini SK , Srivastava S, Agarwal SK , Odedara P. Indian Journal of Otolaryngology and Head and Neck Surgery: Official Publication of the Association of Otolaryngologists of India.2019;71(Suppl 1). CrossRef
- Examining the relationship between non-alcoholic fatty liver disease and hypothyroidism Teimouri K, Pakravan S, Azadbakht K. Journal of Parathyroid Disease.2022;10(1). CrossRef
- Radiation-induced thyroid changes: a retrospective and a prospective view Massimino M, Gandola L, Mattavelli F, Pizzi N, Seregni E, Pallotti F, Spreafico F, et al . European Journal of Cancer (Oxford, England: 1990).2009;45(14). CrossRef
- Pattern of radiation-induced thyroid gland changes in nasopharyngeal carcinoma patients in 48 months after radiotherapy Lin Z, Yang Z, He B, Wang D, Gao X, Tam SY , Wu VWC . PloS One.2018;13(7). CrossRef
- Hypothyroidism after radiotherapy for nasopharyngeal carcinoma Prayongrat A, Lertbutsayanukul C. Annals of Nasopharynx Cancer.2020;4(0). CrossRef
- Geometric Changes in the Parotid, Submandibular, and Thyroid Glands during Intensity Modulated Radiotherapy for Nasopharyngeal Carcinoma: A Cohort Study Tan W. Journal of Analytical Oncology.2020;9. CrossRef
- Dose-volume predictors of post-radiation primary hypothyroidism in head and neck cancer: A systematic review Chow JCH , Cheung KM , Cheung GTC , Tam AHP , Lui JCF , Lee FKH , Au KH , et al . Clinical and Translational Radiation Oncology.2022;33. CrossRef
- Thyroid volume changes following adjuvant radiation therapy for breast cancer Roberson J, Huang H, Noldner C, Hou W, Mani K, Valentine E, Ryu S, Stessin A. Clinical and Translational Radiation Oncology.2023;39. CrossRef
- A dosimetric study on radiation-induced hypothyroidism following intensity-modulated radiotherapy in patients with nasopharyngeal carcinoma Xu Y, Shao Z, Tang T, Liu G, Yao Y, Wang J, Zhang L. Oncology Letters.2018;16(5). CrossRef
- Predictors of radiation-induced hypothyroidism in nasopharyngeal carcinoma survivors after intensity-modulated radiotherapy Zhai R, Lyu Y, Ni M, Kong F, Du C, Hu C, Ying H. Radiation Oncology (London, England).2022;17(1). CrossRef
- Dose-volumetric parameters for predicting hypothyroidism after radiotherapy for head and neck cancer Kim MY , Yu T, Wu HG . Japanese Journal of Clinical Oncology.2014;44(4). CrossRef
- The threshold of hypothyroidism after radiation therapy for head and neck cancer: a retrospective analysis of 116 cases Fujiwara M, Kamikonya N, Odawara S, Suzuki H, Niwa Y, Takada Y, Doi H, et al . Journal of Radiation Research.2015;56(3). CrossRef
- Subsequent thyroid disorders associated with treatment strategy in head and neck cancer patients: a nationwide cohort study Lin CL , Wu SY , Huang WT , Feng YH , Yiu CY , Chiang WF , Ho SY , Lin SH . BMC cancer.2019;19(1). CrossRef
- Research progress of radiation-induced hypothyroidism in head and neck cancer Zhou L, Chen J, Tao CJ , Chen M, Yu ZH , Chen YY . Journal of Cancer.2021;12(2). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2024
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times