Beyond Limits: A Case of Progressive Leiomyosarcoma of the Mandible
Download
Abstract
Leiomyosarcoma (LMS) is a rare malignancy which develops from the smooth muscles. It constitutes about 3 to 10% of all sarcomas in the head and neck area. Furthermore, it is extremely rare in the area of the mouth and jaws due to the rarity of smooth muscle in these areas. Spindle cells with visible smooth muscle differentiation, distinct features of atypia and presence of actin and desmin in Immunohistochemical (IHC) stains are characteristic of LMS. The common pattern of spread is similar with other sarcomas, involving the lung and rarely the lymphatics. Surgery, Radiotherapy and Chemotherapy have been described as standard forms of management. The prognosis is usually poor as rate of local, regional and distant metastasis is high. In this paper, we present a case of a 23 year-old female who presented with Leiomyosarcoma of the mandible. She remained to have good performance status and was able to undergo multiple lines of treatment for progressive disease prior to her demise.
Introduction
Sarcomas are rare malignancies accounting only for about 1% of cases in adults. Even rarely encountered is leiomyosarcoma (LMS) which develops from smooth muscles in the gastrointestinal tract, bladder and retroperitoneal space. They may also be seen in the head and neck area but is extremely rare due to the rarity of smooth muscle in this area. Over the past 60 decades, only 65 cases of leiomyosarcoma (LMS) located in the mandible were described in the English literature [1].
Leiomyosarcomas have an indolent growth but may grow up to 10 cm with nonspecific symptoms. Locations in the head and neck are tongue, mandible, palate, cheeks, maxillary sinuses, upper and lower gingiva. Leiomyosarcomas are composed of spindle cells with visible smooth muscle differentiation and distinct features of atypia with immunohistochemical (IHC) stains show the presence of actin and desmin [1]. Differential diagnosis are more common tumors including spindle cell carcinoma, spindle cell myoepithelioma and melanoma [2]. The nature of spread is the same with other sarcomas, metastasizing more commonly to the lungs and rarely through the lymphatic system.
Multimodality treatment with surgery, radiotherapy and chemotherapy is the management of choice and 5-year survival rate is high with free surgical margins being the most important predictor. Local recurrence, regional lymph nodes and distant metastasis to the lungs occur is about 34%, 15% and 35% of cases respectively, hence long term follow-up is necessary.
Case Presentation
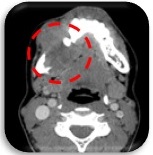
This is a case of a 23 year old female, with no known comorbid illness, who presented with an eight-month history of gradually enlarging right mandibular mass which was noted after a tooth extraction. A prior consult at another institution was done where biopsy showed fibrodysplastic changes. She was advised surgery during this time but she was lost to follow up. Due to the persistence of the mass (Figure 1) with eventual loss of appetite and pain, she sought second opinion at our institution. Slide review showed Spindle Cell Neoplasm.
Figure 1. Mandibular Mass (Axial, CT-scan).
Immunohistochemical stains raise the possibility of high grade leiomyosarcoma cannot totally exclude fibroblastic osteosarcoma (Table 1).
| Stain | Results |
| CK | Negative |
| P63 | Negative |
| Myf 4 (Myogenin) | Negative |
| S100 | Negative |
| SMA | Positive, Strong, Patchy |
| Desmin | Positive, Weak, Patchy |
| Caldesmon | Negative |
Initial work up showed no other metastatic foci. She was initially admitted for gastrostomy tube insertion and nutritional build up prior to treatment. Patient underwent 6 cycles of Neoadjuvant AIM protocol (Doxorubicin, Ifosfamide, Mesna) from July to November 2016 with tolerable adverse events. A month after the last cycle of AIM, she underwent Segmental Mandibulectomy, Right; Excision of Right Submandibular gland; Reconstruction using Vascularized Fibula graft. Intraoperatively, a 4 x 3 x 2cm, fungating, irregularly shaped mass is seen involving the right body of the mandible, extending to the right mandibular parasymphysis. Patient tolerated the procedure well with no intraoperative complications. She remained stable throughout the hospital stay and was deemed fit for discharge. Histopathology of the mandibular mass showed Leiomyosarcoma, intermediate grade, tumor size 5.6cm in widest dimension, with involvement of the mandibular bone and surrounding tissues, no definite lymphovascular invasion, lesion is within 1 mm of lateral soft tissue margin. Patient’s disease was downstaged to Stage IIB (ypT2N0M0) from Stage III. She was then referred to radiation oncology and received 35 fractions of radiation therapy.
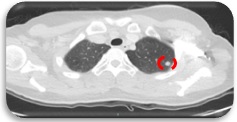
Upon reevaluation, Chest CT scan showed multiple subcentimeter non-calcified pulmonary, subpleural, and fissural nodules in both lungs, largest was seen in the left upper lobe, measuring 0.7 cm (Figure 2).
Figure 2. Subcentimeter Nodule, Lung (Axial, CT Scan).
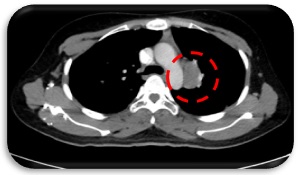
Patient was seen by TCVS and lesions were deemed unresectable at this time. She was advised to undergo systemic chemotherapy with Eribulin. From June 2017 to August 2017, she underwent 3 cycles of Eribulin which she tolerated well with no treatment delays. Repeat Chest CT scan showed further increase in size and number of the pulmonary nodules, the largest in the left upper lobe this time measures 1.7 x 1.5 cm. There was also a note of interval appearance of an enlarged lymph node in the left paraaortic region measuring approximately 3.3 x 2.3 cm, soft tissue fullness in the prevascular space suggestive of small-sized confluent lymph nodes (Figure 3).
Figure 3. Paraaortic Lymph node (Axial, CT-Scan).
Patient was started on 2nd line metastatic treatment with Pazopanib 400 mg once daily. Patient was compliant with the medication and follow-up. On March 2018, she underwent evaluation CT scans which showed interval decrease in sizes of the bilateral pulmonary nodules, the largest one still detected in the left upper lobe measuring 0.9 x 1.0 cm. A cavitary lesion is noted in the apicoposterior segment of the left upper lobe measuring 0.7 cm. The enlarged paraaortic lymph node measures 4.6 x 1.8 cm. A prevascular lymph node measures 1.2 x 1 cm from previous 1 x 0.9 cm. Soft tissue fullness in the prevascular space is stable in appearance. There is a focus of decreased bone density with sclerotic rim at the T11 vertebral body, possibly a lytic lesion. Small-sized cervical lymph nodes are noted bilaterally during this evaluation. Anti-Koch’s medication was started and patient was comanaged with Pulmonology service. Since there are limited treatment options, Pazopanib was continued but increased to 800 mg/day. Patient tried to take the medication regularly despite the financial hurdles. She tolerated the treatment well.
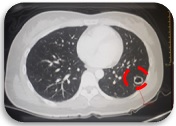
However on September 2018, 6 months after increasing Pazopanib dose, patient again progressed on the lung. The non-calcified nodule in the lateral basal segment of the left lower lobe shows interval increase in size measuring 1.3 x 1.8 cm and exhibits cavitation (Figure 4).
Figure 4. Lung Nodule with Cavitation (Axial, CT Scan).
Subcentimeter non-calcified fissural fissure were appreciated. Left para-aortic region lymph node showed interval progression measuring 4.1 x 2.1 cm and 1.4 x 1 cm which both showed central hypodensity suggestive of necrosis or cystic transformation.
There are l imited options for Progressive Leiomyosarcoma. Consensus during this time was either to continue Pazopanib at current dose and monitor closely or chemotherapy with Gemcitabine-Docetaxel. At this time, patient has good performance score, no subjective complaints and unremarkable physical examination. Treatment options were discussed to the patient and family and they opted to continue treatment with Pazopanib and decide later on for chemotherapy if still with progression on subsequent CT scan. Cavitation noted on the latest CT scan was discussed with Pulmonology service. The cavitation may indicate treatment response to Anti-Koch’s treatment given for latent Tuberculosis (TB). There is no need to start patient on Active TB treatment at this time. She was compliant with the medication and was clinically stable. On the next evaluation scans, the left lower lobe mass measured 2.9 x 3.6 cm from the previous of 1.3 x 1.8cm, and the cavitation is no longer present (Figure 5).
Figure 5. Left Lung mass (Axial, CT Scan).

Enlarged lymph nodes in the left para-aortic region showed confluence with an aggregate measurement of 4.3 x 2.1 cm, the lytic lesion on T11 appears unchanged but there is apparent progression in the lytic lesion in the left iliac bone.
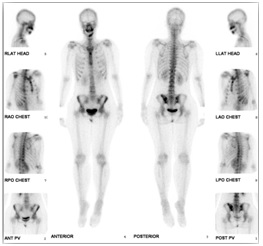
Bone scan was done to evaluate the extent of the bone metastasis which showed osteoblastic lesion in the left iliac bone, increase in radionuclide activity in the middle and left aspect of the mandible and stable right mandibular ramus which may be due to inflammatory changes or metastasis (Figure 6).
Figure 6. Osteoblastic Lesion in the Left Iliac Bone, Increase Radionuclide Activity in the Middle and Left Aspect of the Mandible (Bone Scan).
The possibility of resection of the lung lesions was again entertained. Patient and family are amenable for reexamination by TCVS. The case was discussed with TCVS service. Left lower lobe lesion is resectable however the patient is not an ideal candidate for surgery due to noted lesions in the right lung. The proposed surgery is VATS which is also financially worrisome for the patient’s family and may delay contemplated systemic treatment. During this time, the mandibular plate was also seen eroding into the skin. Patient was assessed by Otolaryngology service and planned to observe and prioritize systemic treatment. Third line metastatic treatment with Gemcitabine-Docetaxel was started. She had episodes of afebrile neutropenia and GCSF support was given, she was also given antibiotics for non-healing wound on the mandible. After 3 cycles of chemotherapy, patient was scheduled for removal of titanium plate as this was already protruding out of the skin.
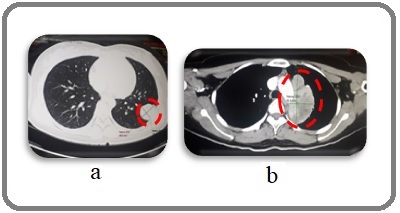
On repeat evaluation scan, non-calcified mass in the left lower lobe showed progression in size to 3.1 x 4.1 cm from 2.9 x 3.6 cm (Figure 7a). There was also a significant increase in size in the previously noted necrotic lymph node in the left paraaortic region measuring 8.2 x 4.2 cm (Figure 7b).
Figure 7. a, Left Lobe Mass; b, Paraaortic Lymph Node (Axial, CT Scan).
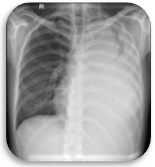
Oral Vinorelbine was started after clearance from Otolaryngology service post-operatively. Role of Radiation Therapy to the lungs was also contemplated however after discussion with the Radiation oncology service, the risk for universal pneumonitis is high in such cases with a large tumor volume. During the treatment with Vinorelbine, patient started complaining of left upper back pain which was tolerable and relieved with Ibuprofen. Patient tolerated the first cycle of chemotherapy. On the supposed 2nd cycle of treatment, patient presented with post-tussive vomiting, increased left upper back pain, generalized weakness and epigastric pain. Neurologic examination was unremarkable. Physical examination showed decreased breath sounds on the left. Chest X-ray PA-Lateral view was requested and results showed progression of the left upper lobe mass, opacified left lung which may relate to pleural effusion (Figure 8).
Figure 8. Opacified Left Lung (PA View, Chest X-ray).
Interventional radiology service was called in and patient was advised admission. Chest Ultrasound showed 2135 cc of non-loculated pleural effusion in the left chest. Pigtail catheter insertion on the left chest was done with an initial drainage of 500 ml serosanguinous fluid. Pleural fluid culture and sensitivity showed no growth after 3 days, MTB Culture and KOH were also negative. Cytology showed Atypical Cells. Patient was referred to Clinical Nutrition and Pain Management services for supportive management. Patient was discharged stable with the pigtail catheter maintained.
On follow up, the options for the next plan of management were discussed extensively with the patient and family. Should they opt for further treatment, medications will be based on case reports and small Phase II trials as an off-label management. Option for best supportive care and advanced care planning was also discussed. Parents decided to still go through with the treatment with Sunitinib but patient requested to go home to the province first to rest. Patient eventually succumbed to death.
Discussion
Leiomyosarcoma is a rare disease and data on treatment beyond progression is scarce. This case is a good learning precedent for patients who can still tolerate further management. The standard of care mandibulectomy with adjuvant radiotherapy was given to the patient.
During the first progression, the treatment options were Eribulin, Pazopanib, Dacarbazine, Gemcitabine and combination treatment with Gemcitabine-Docetaxel. Eribulin was studied in a phase II trial assessing the activity and safety of Eribulin in different types of soft tissue sarcoma. Progression-free survival was evaluable for leiomyosarcoma and adipocytic sarcoma at 12 weeks. Side effects were generally tolerable with grade 3-4 events of Neutropenia, leucopenia and anemia [3]. Adapting the data from PALETTE trial, Pazopanib showed 4.6 months and 12.5 months progression-free and over-all survival advantage over placebo in metastatic non-adipocytic soft-tissue sarcoma after previous chemotherapy [4].
A multi-center, phase III trial evaluated Trabectedin and Dacarbazine in patients with advanced liposarcoma and leiomyosarcoma after prior treatment with anthracycline. Trabectedine showed superior disease control [5]. Gemcitabine-Docetaxel yielded superior progression-free (6.2 months vs. 3 months) and overall survival (17.9 months vs. 11.5 months) to gemcitabine alone but with increase toxicity [6]. Eribulin, being more histology specific, based on these studies with better toxicity profile and convenience of administration was presented to the patient and family. The patient consented to treatment with eribulin.
Patient progressed after only 3 cycles of Eribulin. She previously received Anthracycline-based neoadjuvant chemotherapy, however progressed only after 6 months post-surgery. Taking these into consideration, progression-free survival for this patient seems to be short with chemotherapy. Pazopanib, a multi-targeted kinase inhibitor was started as a second line metastatic treatment. She was initially started at 400 mg/day and eventually increased to 800 mg/day after 7 months due to progression in the lungs. She continued with the treatment for another 7 months for a total of 14 months.
On further progression, the option to give combination treatment with Gemcitabine-Docetaxel was revisited [6]. A case series evaluating addition of Temozolomide to Pazopanib post treatment failure in patient was advanced sarcoma was also considered [7]. In this case series with a total of nine patients, two patients have Non-uterine Leiomyosarcoma. One patient showed stable disease for 7 months and the other has progressive disease after only a month. Gemcitabine-Docetaxel was offered to the patient however, opted to defer chemotherapy and continue Pazopanib until the next evaluation.
The fourth line of treatment was 3 cycles of Gemcitabine-Docetaxel. Prior to this treatment, surgical resection of the lung lesion was again considered. This patient on repeated evaluation showed stable primary site and bone metastasis was controlled with bone-modifying agents. However, the lung lesions showed short intervals of progression. The feasibility of resection, risks and benefits and impact on treatment were carefully discussed with the TCVS service. Eventually, systemic chemotherapy was continued.
When the patient progressed again after chemotherapy, the options were limited. Intravenous Vinorelbine, which was studied in a Phase II trial involving pediatric patients, showed activity in soft tissue sarcoma with a response rate of 36% [8]. A retrospective analysis of Intravenous Vinorelbine Chemotherapy given at 15-30 mg/m2 weekly for 2 to 3 weeks with 1 week rest for previously treated soft-tissue sarcomas showed stable disease in 26%, with median OS and PFS as 6.4 months and 1.8 months respectively [9]. This study included 20 patients with Leiomyosarcoma out of a total population of 58. By dose correspondence [10], our patient was given 80 mg/m2 oral Vinorelbine on Days 1 and 8 with one week rest.
Despite subsequent treatments, patient continued to progress. Best supportive care was already contemplated after progression with fifth line of treatment. However, in a patient that is young, with previously good performance score, asymptomatic, and is willing to undergo further treatment, there is somehow an obligation to look for data beyond the limits of current guidelines as relatives would still like to know treatment options. Discussion with the family is crucial at this point together with the help of the multi-disciplinary team.
Other treatment regimens studied for Soft tissue sarcoma are based on case reports and small phase II studies. Sunitinib was reported in a case report of renal leiomyosarcoma with lung metastasis. It was given at a dose of 37.5 mg/day and patient had stable disease for 26 months with mild fatigue, hand-foot syndrome and stomatitis [11]. Everolimus was studied in a phase II trial including patients with metastatic or recurrent bone and soft tissue sarcomas after failure with anthracycline and ifosfamide. Median PFS was 1.9 months and Median OS was 5.8 months [12]. Sorafenib given at 400 mg twice daily showed a partial response for 1 patient, stable disease for 18 patients and progressive disease in 18 patients in a phase II trial evaluating soft tissue sarcoma [13]. Pembrolizumab has also been investigated in soft-tissue sarcoma, however, the SARC028 Phase II trial showed poor response in Leiomyosarcoma [14].
Multi-disciplinary approach has always been the best mode of management for malignancy. Thorough planning and coordination with all the services involved including the supportive oncology team and the caregivers is imperative in cancer care. In a patient with multiple lines of treatment, pushing the boundaries of standard of care, all treatment options, side effects, expected results should be presented to the patient. In these cases, informed consent cannot be overemphasized.
Acknowledgements
The authors would like to acknowledge the invaluable input of the multidisciplinary team involved in this case – Pathology, TCVS, ENT-HNS, Pulmonology, Radiation Oncology, Internal Medicine, Infectious Disease, Clinical Nutrition, Pain Management, Interventional Radiology, Radiology, Nuclear Medicine and all the allied health care professionals; and the family who remained cooperative throughout the course of the disease.
References
- Leiomyosarcoma in the mandible: A rare case report Lewandowski B, Brodowski R, Pakla P, Stopyra W, Gawron I. Medicine.2016;95(27). CrossRef
- Primary leiomyosarcoma of the mandible Moghadam SA , Khodayari A, Mokhtari S. Journal of oral and maxillofacial pathology: JOMFP.2014;18(2). CrossRef
- Activity of eribulin mesylate in patients with soft-tissue sarcoma: a phase 2 study in four independent histological subtypes Schöffski P, Ray-Coquard IL , Cioffi A, Bui NB , Bauer S, Hartmann JT , Krarup-Hansen A, et al . The Lancet. Oncology.2011;12(11). CrossRef
- Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial Graaf WTA , Blay JY , Chawla SP , Kim DW , Bui-Nguyen B, Casali PG , Schöffski P, et al . Lancet (London, England).2012;379(9829). CrossRef
- Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Multicenter Clinical Trial Demetri , et al . Journal of Clinical Oncology.2015.
- Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected] Maki RG , Wathen JK , Patel SR , Priebat DA , Okuno SH , Samuels B, Fanucchi M, et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2007;25(19). CrossRef
- Temozolomide post pazopanib treatment failure in patients with advanced sarcoma: A case series Bupathi M, Hays JL , Chen JL . PLoS ONE.2017;12(11). CrossRef
- Phase II evaluation of intravenous vinorelbine (Navelbine) in recurrent or refractory pediatric malignancies: a Children's Oncology Group study Kuttesch JF , Krailo MD , Madden T, Johansen M, Bleyer A. Pediatric Blood & Cancer.2009;53(4). CrossRef
- A retrospective analysis of vinorelbine chemotherapy for patients with previously treated soft-tissue sarcomas Anderson SE , Keohan ML , D'Adamo DR , Maki RG . Sarcoma.2006;2006. CrossRef
- Oral versus intravenous vinorelbine: clinical safety profile Gebbia V, Puozzo C. Expert Opinion on Drug Safety.2005;4(5). CrossRef
- Oral administration of sunitinib malate for long-term survival of a patient with multiple lung metastases from renal leiomyosarcoma Li X, Gao H, Tang C, Liu X. OncoTargets and Therapy.2016;9. CrossRef
- Multicenter phase II study of everolimus in patients with metastatic or recurrent bone and soft-tissue sarcomas after failure of anthracycline and ifosfamide Yoo C, Lee J, Rha SY , Park KH , Kim TM , Kim YJ , Lee HJ , et al . Investigational New Drugs.2013;31(6). CrossRef
- Phase II study of sorafenib in patients with metastatic or recurrent sarcomas Maki RG , D'Adamo DR , Keohan ML , Saulle M, Schuetze SM , Undevia SD , Livingston MB , et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2009;27(19). CrossRef
- Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial Tawbi HA , Burgess M, Bolejack V, Van Tine BA , Schuetze SM , Hu J, D'Angelo S, et al . The Lancet. Oncology.2017;18(11). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2023
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times