Correlation between Germinal Center Differentiation and Double/Triple Hit Score in the Classification of Diffuse Large B Cell Non-Hodgkin Lymphoma: A Clinicopathological Approach
Download
Abstract
Background: Diffuse large B cell lymphoma (DLBCL) is considered the most common type of non-Hodgkin lymphoma. The global incidence of DLBCL has been doubled in the past decades, highlighting the need for more effective treatment regimens. The curability of DLBCL is heavily influenced by a number of factors, such as the age, the international prognostic index (IPI) score, the molecular cell of origin (COO) subtype, and presence/ absence of specific chromosomal rearrangements or protein expression. Recent gene profiling studies have classified DLBCL into two main categories, germinal center B-cell type (GCB) and activated B-cell type (ABC). Validation of these subtypes has become more applicable after the immunohistochemical algorithm suggested by Hans, which included three commercially available markers, CD10, BCL6, and MUM-1/IRF4. In addition to this classification, DLBCL prognostic models based on different sets of genes or immunohistochemical markers have been proposed. More recently, DLBCLs with translocations of MYC, along with a B-cell lymphoma 2 (BCL2) and/or B-cell lymphoma 6 (BCL6) rearrangement, are now called double-hit lymphoma (DHL) or triple-hit lymphoma (THL), respectively. Furthermore, the co-expre sion of MYC and BCL2 proteins without underlying rearrangements is considered a new adverse prognostic indicator termed double-expressor lymphoma (DEL). The present study is aiming to combine morphological, immunophenotypical, and genetic features of DLBCLs to reach a more reproducible subtyping.
Aim: To identify the relation between clinicopathological features as patient age, sex, site of tumor, clinical presentation and tumor stage and the germinal center differentiation in DLBCL (germinal center B-cell (GCB) or activated B-cell (ABC)) and the double/triple genetic hits status.
Material and Methods: The material of this study comprised 52 formalin-fixed paraffin- embedded blocks representative of diffuse large B cell lymphoma cases retrieved from the archival material available at the Pathology Department, Faculty of Medicine, Alexandria University. Subclassification of cases into (germinal center / and post-germinal center types) using a panel of immunohistochemical markers including CD10, BCL6 and MUM1 was done and scoring of double/triple hit expression using a panel of immunohistochemical markers including c-MYC, BCL6 and BCL2 was also done and correlation with their subtypes and clinical data was performed.
Results: there was significant association between germinal center differentiation and tumor stage (MCp= 0.018).
Conclusion: Germinal center differentiation status is a valid method to evaluate the prognosis of DLBCL.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma (NHL), accounting for 30% to 40% of all newly diagnosed lymphomas worldwide. DLBCL represents a spectrum of malignancies morphologically and biologically heterogeneous which is reflected in its highly variable clinical course and markedly diversified outcomes [1]. This heterogeneity encouraged numerous investigators to study the biology of DLBCL, with focus on whether it could be further subdivided into different entities [2].
Historically, lymphoma classification has been based on either the morphology of tumor cells (into centroblastic, immunoblastic, anaplastic and other rare morphologies like plasmablastic), that was adopted by the old Kiel classification, or on the suggested normal counterpart (cell of origin) that was adopted by The REAL (Revised European American Lymphoma) /WHO classification of NHL, 3rd edition 2001 [3, 4]. However, adopting those methods as the sole mean of classification is inadequate or incomplete in defining certain subtypes of lymphoma [5].
The 2008 WHO classification listed a large number of DLBCL sub-groups based not only on the putative cell of origin but also on the morphologic, biologic, immunophenotypic and clinical parameters [6].
Although this classification was more reproducible than the old ones, still many cases of DLBCL did not fit into any of the specific disease subgroups described and were referred to as DLBCL non-otherwise specified (NOS) which remained as a diagnosis of exclusion and were considered as the most common category of DLBCL [7].
Long-term remission was achieved since rituximab (R) was added to cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) approximately a decade ago, and R-CHOP has now become the standard treatment [8].
Nevertheless, approximately 40% of patients with DLBCL suffer relapses within a short time frame and eventually die as a result of the disease [9].
In the year 2000, Alizadeh et al [10] studied two distinct molecular entities of DLBCL using gene expression profile (GEP). Based on these studies, DLBCLs have been divided into two main groups based on the putative cell of origin. The first group is the germinal center B cell-like (GCB)- DLBCL which exhibits a transcriptional profile that resembles that of a GCB cell with the expression of CD10 and the transcriptional repressor BCL6 and harbouring highly mutated immunoglobulin genes with ongoing somatic hypermutations (SHM) [11]. The activated B cell- like (ABC)-DLBCL group which shows several features of B cell receptor (BCR) activated B-cells with up-regulation of genes required for plasma cell differentiation namely the multiple myeloma 1 / interferon regulatory factor 4 protein (MUM1/IRF4) [11, 12].
Hans et al [13] in 2004 proposed a small set of immunohistochemical markers including CD10, BCL6, and MUM1 that demonstrated an 80% concordance to GEP. However, this classification was not adopted in the more recent 2016 WHO classification, and still it needs more specifications [14].
Translocation of the MYC gene, which is a gene involved in many cellular functions including proliferation, has been shown to be prognostically unfavorable [15, 16]. In addition, translocation of BCL2, which is known to be a central anti-apoptotic gene, and found to be a marker of poor prognosis in some studies but not all [17, 18].
DLBCL with translocation of both MYC and BCL2, termed double-hit lymphoma (DHL), is characterized by poor response to standard therapy and an aggressive clinical course. This fact reveals the synergistic clinical effect of combined activation of these both genes; MYC promotes cellular proliferation and BCL2 blocks cellular death [19, 20]. A new entity was introduced focusing on BCL6 translocation, which also has a prognostic implication, and when combined with both MYC and BCL2 will give rise to a triple-hit lymphoma [21].
Studies of MYC, BCL2 and BCL6 in DLBCL have focused on molecular methods, mainly fluorescence in situ hybridization (FISH). However, activation of those genes leads to increased expression of the protein products that could be traced by immunohistochemical studies [22]. Later on, a new term was introduced which is the double expressors and triple expressors lymphoma. However, it may not be related to the underlying chromosomal aberrations and the relation between genetic hit and protein expression is still a dilemma [23].
Classifications of diffuse large B-cell lymphoma, NOS, is a great diagnostic and prognostic dilemma, not only facing pathologists but also confronting clinicians and researches. A good classification of lymphoma is surely of great help in risk stratification of patients and in directing therapeutic modalities especially when targeted therapy was introduced in the field of lymphoma. Combined morphological, immunophenotypical, and genetic features should be incorporated in any classification of DLBCL if a reproducible subtyping is aimed.
Aim of the work
To correlate between the germinal center differentiation in DLBCL (germinal center B-cell (GCB) or activated B-cell (ABC)) and the double/triple genetic hits status. Also to identify the relation between these classifications and other clinicopathological features as patient age, sex, site of tumor, clinical presentation and tumor stage.
Materials and Methods
The material of this study comprised 52 formalin-fixed paraffin- embedded blocks representative of diffuse large B cell lymphoma, NOS cases retrieved from the archival material available at the Pathology Department, Faculty of Medicine, Alexandria University.
Inclusion Criteria
Cases of diffuse large B cell lymphoma, NOS, primary diagnosed non recurrent cases with available complete clinical data and enough tissue for immunohistochemistry panel.
Immunohistochemical staining
Four micron thick sections were prepared from the paraffin blocks of each case and mounted on positively charged slides. The avidin –biotin immunoperoxidase method was applied.
-Assessment of germinal center differentiation was carried out by two different pathologists for subclassification of cases into (germinal center / and post-germinal center types) using a panel of immunohistochemical markers including CD10, BCL6 and MUM1.
- Scoring of Double/Triple Hit expression: using a panel of immunohistochemical markers including c-MYC, BCL6 and BCL2.
- Immunohistochemistry was be performed following manufacturer’s protocols [24].
Finally, both classifications were be statistically analysed using SPSS statistics software version 23.
Quantitative data were tested for normality using Kolmogorov-Smirnov test. Monte-Carlo Exact test was used to test the association between qualitative variables. In all applied statistical tests of significance, P value (<0.05) was considered significant.
Results
This study included 52 cases of DLBCL. Their ages ranged from (14-92 years) with a mean (52.2±17.1years). Twenty eight patients (53.8%) were males and twenty four (46.2%) were females with male to female ratio of 1.16:1. As regards site of the tumor, twenty nine cases (55.8%) were nodal with the first presenting nodal site cervical in 11 cases, abdominal in 5 cases, axillary in 6 cases and inguinal in 7 cases. Further twenty three cases (44.2%) were extranodal (Tonsils in 6 cases, stomach in one case, intestinal mass in 3 cases, nasopharengeal mass in 4 cases, skin in 2 cases, thyroid in one case, and retroperitoneal or intramuscular mass in 6 cases). The studied cases were staged according to Modified Ann Arbor staging system of lymphoma [25] into, stage I (9 cases, 17.3%), stage II (21 cases, 40.4%), stage III (13 cases, 25%) and stage IV (9 cases, 17.3%).
Immunohistochemical assessment
In the current study, 28 cases (54%) were positive for CD10 and 24 cases (46%) were negative. Regarding BCL6, 30 cases (58%) were showing positive nuclear staining in ≥ 30% of tumor cells and 22 cases (42%) were showing negative staining or staining less than 30% of tumor cells. MUM-1 staining was positive in more than 30% of tumor cells in 11 cases (21%) and was negative or less than 30% in 41 cases (79%). According to Hans classification [13] 30 cases (57.7%) were classified as germinal center B-cell like DLBCL (GCB-DLBCL) and 22 cases (42.3%) were classified as non-germinal center activated B-cells DLBCL (NGC/ABC- DLBCL) (Figure 1).
Figure 1. DLBCL, GCB, A, Showing Positive Membranous Staining of CD10 Immunostain, (CD10, X400); B, Showing Positive Nuclear Staining of BCL6 Immunostain, (BCL6, X400); C, Showing Negative Staining of MUM-1 Immunostain. (MUM-1, X400); Relation between clinical data and germinal center differentiation.
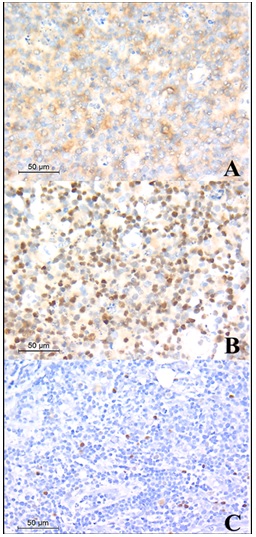
Cases were further classified according to expression of BCL6, BCL2 and c-MYC into three groups, cases were expressing non or single expressor (Non/SE) which shows negative results of three markers or only one positive marker and those were 36 (69.2%) while 12 cases expressed a combination of C-MYC and BCL-2 or BCL-6 (DE) (23%) and only four cases (7.8%) were expressing the three markers (TE). Out of 30 cases of GCB-DLBCL, 19 cases (63.3%) were only expressing one marker or negative for the three markers, 9 cases (30%) were double expressors and 2 cases (6.7%) were triple expressors. Regarding the 22 cases of ABC-DLBCL, 17 cases (77.3%) were (Non/SE), 3 cases (13.6%) were double expressors and 2 cases (9.1%) were expressing the three markers.
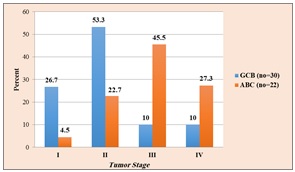
As regards sex; percentage of male patients was a little higher in GCB-DLBCL than ABC-DLBCL (54.5% Vs 53%), but this was statistically not significant (χ2=.008, p= 0.931, Chi-Square test) and concerning the tumor site, although nodal site was higher in both GCB and ABC-DLBCL than extranodal sites, there was no statistically significant association (χ2= 0.023, p= 0.879, Chi-Square test). As regard tumor stage, it was found that most of GCB-DLBCL cases were stage I and II (24 cases out of 30, 80%) while most of ABC-DLBCL cases were higher stage, III and IV, (16 out of 22 cases, 72.8%) and this finding was statistically significant (χ2= 15.1,p= 0.002, Chi-Square test) (Figure 2).
Figure 2. Relation between Germinal Center Differentiation and Tumor Stage.
There was no statistically significant association between age of patients and germinal center differentiation, (t=.294, P=.77, Independent sample t test).
Relation between clinical data and double/triple hit expression
Concerning age, the median was nearly the same between the three groups (Non/SE, DE and TE) as (53, 50 and 50 years respectively) with no statistical significance in this distribution, (H= 1.167, p= 0.558, Kruskal-Wallis test). Regarding gender, it was found that (Non/SE) group and (TE) group are showing equal distribution of male and female (1:1), while in case of (DE) group males were double the number of females (2:1), but this was statistically not significant (MCp= 0.739, Monte Carlo exact probability). The (Non/SE) group and (DE) group were detected more in nodal site but in the (TE) group, they were detected in nodal and extra nodal sites equally. Statistically, there was no significance for this distribution (MCp= 1.0, Monte Carlo exact probability) (Table1).
| Expression Classification | Test of Significance (P) | ||||||
| Non/SE (n=36) | DE (n=12) | TE (n=4) | |||||
| No. | % | No | % | No | % | ||
| Sex | |||||||
| Male | 18 | 50 | 8 | 66.7 | 2 | 50 | MCp=0.739 |
| Female | 18 | 50 | 4 | 33.3 | 2 | 50 | |
| Site | |||||||
| Nodal | 20 | 55.6 | 7 | 58.3 | 2 | 50 | MCp=10 |
| Extranodal | 16 | 44.4 | 5 | 41.7 | 2 | 50 | |
| Stage | |||||||
| I | 7 | 19.4 | 2 | 16.7 | 0 | 0 | MCp=0.289 |
| II | 12 | 33.3 | 7 | 58.3 | 2 | 50 | |
| III | 8 | 22.2 | 3 | 25 | 2 | 50 | |
| IV | 9 | 25 | 0 | 0 | 0 | 50 | |
| Age (Years) | |||||||
| Mean ± SD | 53.19±15.51 | 50.08±20.64 | 50.0±24.11 | H=1.167, p=0.558 | |||
| Median (Min. -Max.) | 53.5 (14-78) | 48 (25-92) | 44 (28-84) |
MCp, Monte Carlo exact probability; H, Kruskal-Wallis test; SD, Standard deviation
In the matter of tumor stage, it was found that most of DE and TE cases were stage II and III (83.3% and 100% respectively) while in (Non/SE) group, cases were arranged near equally between stages (I and II) and (III and IV) (52.7% and 47.3% respectively) This finding was statistically not significant (MCp= 0.289, Monte Carlo exact probability).
Discussion
Diffuse large B cell lymphoma (DLBCL) is considered the most common type of non-Hodgkin lymphoma, representing 35% to 40% of new cases annually worldwide. The global incidence of DLBCL has been doubled in the past decades, highlighting the need for more effective treatment regimens [26].
The curability of DLBCL is heavily influenced by a number of factors, such as the age, the international prognostic index (IPI) score, the molecular cell of origin (COO) subtype, and presence/absence of specific chromosomal rearrangements or protein expression. According to the World Health Organization (WHO) guidelines, it becomes standard to determine molecular subsets of DLBCL cases; which highlights the impact of genomic alterations on disease biology and prognosis [12,27].
In this paper, we report that the germinal center differentiation is a useful algorithm to predict the clinical outcome of DLBCL in the rituximab era, with the GCB subtype associated with a favourable prognosis. Out of 52 cases, 30 cases (57.7%) were classified as germinal center B-cell like DLBCL (GCB-DLBCL) and 22 cases (42.3%) were classified as non-germinal center activated B-cells DLBCL (NGC/ABC- DLBCL) and there was statistically significant relation with stage which as most of higher stage cases were ABC. This agreed with the results of Zizhen et al. [28], who found that the non-GCB DLBCL was significantly associated with higher stage.
Alizadeh and colleagues used GEP to classify DLBCL cases into GCB and non-GCB type. Although they used hundreds of genes to stratify the cases, they concluded that there was residual clinical heterogeneity that cannot be explained by their classification. They could not identify which of the genes that distinguish GCB from non-GCB cases were the most important determinants of response to therapy [10].
Although FISH is a gold standard for detecting DHL. However, FISH is difficult to perform and takes long time to operate. Besides, the reagents are expensive. In contrast, it is convenient, rapid and low cost to detect the protein expression of corresponding genes by IHC. Therefore, IHC is commonly used in clinics to detect C-MYC, BCL-2, and BCL-6 protein expression in DLBCL.
In this study, regarding tumor stage, it was found that most of double expressors and triple expressors cases were stage II and III (83.3% and 100% respectively) while in (Non/SE) group, cases were arranged near equally between stages (I and II) and (III and IV) (52.7% and 47.3% respectively) This finding was statistically not significant and this was against literature as Green et al.[29] reported that in DLBCL, “double expression” is also associated with poor prognosis of lymphoma. Swerdlow et al.[30] believe that although 70–80% of DHL or THL also shows “double expression,” DEL is not equivalent to and is more common than DHL, accounting for 18–33% of DLBCL (Green et al., 2012b; Valera et al., 2013), and only <20% of patients in DEL showed DHL [29,30]. This finding in our study could be attributed to small sample size and that most of our cases (36 out of 52) was negative for the three markers or expressing only one of them.
In conclusion, DLBCL should be classified into GCB and non-GCB subtypes because they prognostically differ. Hans’ algorthim is a simple and reliable method for the classification of DLBCL, but it should be applied on a large number of cases to be used as a routine method for DLBCL evaluation.
Acknowledgements
General
The authors would like to thank Ehsan Eldeghidy for consultation on statistical analysis.
Funding Statement
The research is funded by the research team.
Approval
The research is approved by the faculty of medicine, at Alexandria University.
Conflict of Interest
None declared.
Ethical Declaration
Clinical data were collected and evaluated according to the approval of the Faculty of Medicine Ethics Committee, Alexandria University (IRB no. 00007555, FWA NO. 00015712).). The study was conducted by the international standards of good clinical practice and with the 1964 Helsinki declaration and its later amendments.
Authors Contribution
Israa sobhy Abdel razek okap, Hanan Yehia Ahmed Tayel, Amany Abd El-Bary Abd El-Latif, Dalia Ahmed Mohamed Nafea, and Nagwa Abdel Razek Mashali participated in the study design and coordination. Israa sobhy Abdel razek okap, Hanan Yehia Ahmed Tayel, Amany Abd El-Bary Abd El-Latif, and Nagwa Abdel Razek Mashali performed the immunohistochemical analysis. Israa sobhy Abdel razek okap, Hanan Yehia Ahmed Tayel and Amany Abd El-Bary Abd El-Latif wrote the main manuscript text and prepared Figure 1 and 2. The authors have read and approved the final manuscript.
Data Availability
Raw data sharing does not apply to Immunohistochemistry as no datasets were generated.
References
- Aggressive B-cell lymphomas—from morphology to molecular pathogenesis Chen BJ , Fend F, Campo E, Quintanilla-Martinez L. Annals of Lymphoma.2019;3(0). CrossRef
- Comparative molecular cell-of-origin classification of diffuse large B-cell lymphoma based on liquid and tissue biopsies Hunter E, McCord R, Ramadass AS , Green J, Westra JW , Mundt K, Akoulitchev A. Translational Medicine Communications.2020;5(1). CrossRef
- Diffuse large B-cell lymphoma Hunt KE , Reichard KK . Archives of Pathology & Laboratory Medicine.2008;132(1). CrossRef
- The histological and biological spectrum of diffuse large B-cell lymphoma in the World Health Organization classification Menon MP , Pittaluga S, Jaffe ES . Cancer Journal (Sudbury, Mass.).2012;18(5). CrossRef
- editor Diffuse large B-cell lymphoma. Seminars in radiation oncology Ng AK . Elsevier.2007.
- The 2008 WHO classification of lymphomas: implications for clinical practice and translational research Jaffe ES . Hematology. American Society of Hematology. Education Program.2009. CrossRef
- Understanding the New WHO Classification of Lymphoid Malignancies: Why It's Important and How It Will Affect Practice Jaffe ES , Barr PM , Smith SM . American Society of Clinical Oncology Educational Book.2017;(37). CrossRef
- Addition of rituximab to standard chemotherapy improves the survival of both the germinal center B-cell-like and non-germinal center B-cell-like subtypes of diffuse large B-cell lymphoma Fu K, Weisenburger DD , Choi WWL , Perry KD , Smith LM , Shi X, Hans CP , et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2008;26(28). CrossRef
- Cancer statistics, 2012 Siegel R, Naishadham D, Jemal A. CA: a cancer journal for clinicians.2012;62(1). CrossRef
- Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling Alizadeh AA , Eisen MB , Davis RE , Ma C, Lossos IS , Rosenwald A, Boldrick JC , et al . Nature.2000;403(6769). CrossRef
- Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma Davis RE , Ngo VN , Lenz G, Tolar P, Young RM , Romesser PB , Kohlhammer H, et al . Nature.2010;463(7277). CrossRef
- Aggressive lymphomas Lenz G, Staudt LM . The New England Journal of Medicine.2010;362(15). CrossRef
- Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray Hans CP , Weisenburger DD , Greiner TC , Gascoyne RD , Delabie J, Ott G, Müller-Hermelink HK , et al . Blood.2004;103(1). CrossRef
- The 2016 revision of the World Health Organization classification of lymphoid neoplasms Swerdlow SH , Campo E, Pileri SA , Harris NL , Stein H, Siebert R, Advani R, Ghielmini M, et al . Blood.2016;127(20). CrossRef
- Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab Barrans S, Crouch S, Smith A, Turner K, Owen R, Patmore R, Roman E, Jack A. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2010;28(20). CrossRef
- MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy Savage KJ , Johnson NA , Ben-Neriah S, Connors JM , Sehn LH , Farinha P, Horsman DE , Gascoyne RD . Blood.2009;114(17). CrossRef
- The t(14;18) is associated with germinal center-derived diffuse large B-cell lymphoma and is a strong predictor of outcome Barrans SL , Evans PAS , O'Connor SJM , Kendall SJ , Owen RG , Haynes AP , Morgan GJ , Jack AS . Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2003;9(6).
- Immuno-fluorescence in situ hybridization index predicts survival in patients with diffuse large B-cell lymphoma treated with R-CHOP: a GELA study Copie-Bergman C, Gaulard P, Leroy K, Briere J, Baia M, Jais JP , Salles GA , et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2009;27(33). CrossRef
- B-cell lymphomas with concurrent IGH-BCL2 and MYC rearrangements are aggressive neoplasms with clinical and pathologic features distinct from Burkitt lymphoma and diffuse large B-cell lymphoma Snuderl M, Kolman OK , Chen YB , Hsu JJ , Ackerman AM , Dal Cin P, Ferry JA , et al . The American Journal of Surgical Pathology.2010;34(3). CrossRef
- B-cell lymphomas with MYC/8q24 rearrangements and IGH@BCL2/t(14;18)(q32;q21): an aggressive disease with heterogeneous histology, germinal center B-cell immunophenotype and poor outcome Li S, Lin P, Fayad LE , Lennon PA , Miranda RN , Yin CC , Lin E, Medeiros LJ . Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc.2012;25(1). CrossRef
- Triple-hit B-cell Lymphoma With MYC, BCL2, and BCL6 Translocations/Rearrangements: Clinicopathologic Features of 11 Cases Wang W, Hu S, Lu X, Young KH , Medeiros LJ . The American Journal of Surgical Pathology.2015;39(8). CrossRef
- Comprehensive gene expression profiling and immunohistochemical studies support application of immunophenotypic algorithm for molecular subtype classification in diffuse large B-cell lymphoma: a report from the International DLBCL Rituximab-CHOP Consortium Program Study Visco C, Li Y, Xu-Monette ZY , Miranda RN , Green TM , Li Y, Tzankov A, et al . Leukemia.2012;26(9). CrossRef
- Double hit and double expressors in lymphoma: Definition and treatment Riedell PA , Smith SM . Cancer.2018;124(24). CrossRef
- Carleton's histological technique: Oxford University Press Carleton H, Drury R, Wallington E. USA.1980.
- Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting Lister TA , Crowther D, Sutcliffe SB , Glatstein E, Canellos GP , Young RC , Rosenberg SA , Coltman CA , Tubiana M. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.1989;7(11). CrossRef
- Diffuse large B-cell lymphoma: current strategies and future directions Cultrera JL , Dalia SM . Cancer Control: Journal of the Moffitt Cancer Center.2012;19(3). CrossRef
- Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways Lenz G, Wright GW , Emre NCT , Kohlhammer H, Dave SS , Davis RE , Carty S, et al . Proceedings of the National Academy of Sciences of the United States of America.2008;105(36). CrossRef
- Correlation between immunophenotype classification and clinicopathological features in chinese patients with primary gastric diffuse large B-cell lymphoma Zizhen Z, Hui C, Yanying S, Danping S, Jiahua L, Chao H, Xingzhi N. Pathology oncology research: POR.2013;19(2). CrossRef
- Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone Green TM , Young KE , Visco C, Xu-Monette ZY , Orazi A, Go RS , Nielsen O, et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2012;30(28). CrossRef
- Diagnosis of 'double hit' diffuse large B-cell lymphoma and B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and Burkitt lymphoma: when and how, FISH versus IHC Swerdlow SH . Hematology. American Society of Hematology. Education Program.2014;2014(1). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2023
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times