Clinico-Epidemiological Presentation and Treatment Outcome of Peripheral T-Cell Lymphoma, Not Otherwise Specified (PTCL-NOS): A Single-Institution Experience
Download
Abstract
Background and objective: Peripheral T-cell lymphomas (PTCLs) are aggressive malignant lymphoproliferative disorders, accounting for 10-15% of all non-Hodgkin's lymphomas (NHLs). PTCL, not otherwise specified (PTCL-NOS), comprises approximately 6% of all NHLs and does not meet the criteria for other mature peripheral T-cell lymphomas as defined by the World Health Organization (WHO) classification. With limited long-term data evaluating the outcome of PTCL-NOS in the Indian population, this study aimed to determine the clinico-epidemiological profile and treatment outcomes of patients with PTCL-NOS at a cancer care center in Northeast India.
Material and Methods: This retrospective observational analysis was performed on the medical records of patients diagnosed with PTCL-NOS between January 2015 and December 2018 at Dr. B. Borooah Cancer Institute.
Results: Our study evaluated 53 patients with PTCL-NOS, with a mean age at presentation of 45.3 years (SD ± 16.3 years). The majority of patients (77.3%) presented with advanced stage (III/IV) disease, with bone marrow involvement in 35.8% of patients. B-symptoms were present in 58.4% of patients. Extra-nodal sites of involvement were observed in 64.1% of patients. Anthracycline-based chemotherapy regimens were the most commonly used in the frontline setting (71.6% of patients). Radiation therapy (involved field radiotherapy [IFRT]) was received by 26.4% of patients. The overall response rate (ORR) to first-line chemotherapy was 94.3%, with complete response (CR) achieved by 62.3% of patients. Twenty-nine (58%) patients experienced relapsed or progressive disease. With a median follow-up duration of 38 months, the 3-year progression-free survival (PFS) rate and 3-year overall survival (OS) rate were 27.6% and 33.9%, respectively. The median PFS was 22 ± 3.3 months, and the median OS was 28 ± 7.1 months.
Conclusion: PTCL-NOS represents an aggressive subtype of NHL with limited therapeutic options and poor outcomes. Proper treatment adherence and the availability of newer therapeutic options are crucial for enhancing overall patient outcomes.
Introduction
Peripheral T-Cell Lymphoma (PTCL) is one of the rare and aggressive subtypes of non-Hodgkin’s lymphoma (NHL) arising from post-thymic lymphocytes. Peripheral T-cell lymphomas constitute about 10-20% of all NHL’s and are more aggressive than other B-cell lymphomas [1, 2]. According to the World Health Organisation 2016 classification, there are more than 20 different subtypes of PTCL and the common histological subtypes include PTCL not otherwise specified (PTCL-NOS), anaplastic large cell lymphoma (ALCL), angioimmunoblastic T-cell lymphoma (AITL) followed by rarer entities such as natural killer T-cell lymphoma (NK-T cell lymphoma), hepatosplenic T-cell lymphoma (HSTCL), subcutaneous panniculitis T-cell lymphoma, adult T-cell leukemia/ lymphoma (ATLL), and others [3]. Although PTCL accounts for approximately less than 10% of all NHL in the Western world, the incidence of T-cell lymphomas is higher in Asia, with an incidence of 20% noted in a retrospective study from India [4]. PTCL differs significantly from other NHL in terms of clinical behavior, prognosis, and response to therapy.
The most frequent subtype of PTCL is PTCL- not otherwise specified (PTCL-NOS). PTCL-NOS comprise 6% of all NHLs, and, are a heterogeneous group of predominantly nodal T-cell lymphoma that do not meet the criteria for other mature peripheral T-Cell lymphoma as defined in WHO classification [5]. With current immunophenotypic and molecular markers, about 30-50% of PTCL cases are not further classifiable and are categorized as PTCL-NOS [1]. Gene expression profiling has improved the diagnosis of PTCL-NOS, which allows a better classification within specified subgroups [6,7]. PTCL-NOS usually affects elderly males with frequent involvement of extra-nodal structures [8].
The prognosis of patients with PTCL-NOS is poor with a 5-year failure-free survival (FFS) and overall survival (OS) rates of approximately 20-30% and the optimal therapy remains challenging [1]. Chemotherapy remains a standard therapeutic option for PTCL-NOS. With standard anthracycline-based therapy, the complete response rate ranges from 40-60%, with overall survival (OS) of 30-40% [9,10].
With advances in the understanding of disease biology and the development of newer treatment options, the outcome of PTCL-NOS is changing [11]. Clinically, the IPI (International Prognostic Index) remains the most effective prognostic model to define risk groups within PTCL-NOS [12]. But, the prognostic index for PTCL unspecified’ (PIT) is also used to determine the risk groups which further predicts the long-term outcome [9].
Early relapse despite standard therapy leads to a poorer outcome of PTCL-NOS. Even though there are enough data about the clinical pattern and epidemiology of PTCL-NOS in the Western population, there are few reports from developing countries like India. Due to limited published data on PTCL from Indian patients, the exact magnitude and disease trend are not well understood. Hence, additional institutional data are required to assess the epidemiology, clinical course, and better treatment options for PTCL-NOS in India.
In the current study, we aimed to determine retrospectively the clinico-epidemiological pattern and treatment outcome (in terms of responses and three-year survival) of PTCL-NOS in a cancer care center in North-East India.
Materials and Methods
A retrospective observational analysis was performed on medical records of histologically proven cases of PTCL-NOS who were diagnosed and treated at the Department of Medical Oncology, Dr. B. Borooah Cancer Institute (BBCI) during the period from January 2015 to December 2018. Patients were followed up till December 2021. Data were collected retrospectively from individual medical case records.
The diagnosis was made by biopsy or histopathological examination of the tissue with appropriate immunohistochemistry (IHC) markers. The IHC markers used for diagnosis were CD3, CD4, CD8, ALK-1, CD56, CD30, EBV LMP-1, EMA, Ki-67, CD138, CD20, TDT, LCA, CD5, CD7, CD15, CD10, PAX-5, CD117, CD99 and CD21.
The medical records were reviewed for:
(i) Epidemiological data (name, gender, age, performance status)
(ii) Clinical stage of the disease
(iii) Site of involvement, and extranodal sites of disease
(iv) Histopathology and IHC
(v) Detail treatment protocol including chemotherapy and radiotherapy
(vi) Treatment outcome: response rate, survival, and toxicity.
Inclusion criteria: Adult patients (age ≥18 years) of PTCL-NOS attending Dr. B. Borooah Cancer Institute during the period from January 2015 to December 2018 who was diagnosed by biopsy/ histopathological examination and confirmed by IHC.
Exclusion criteria: Patients younger than 18 years and patients who had synchronous other malignancy or were previously diagnosed and/ or treated for other malignancy were excluded.
The bulky disease was defined as any site of disease ≥7.5 cm in adults. Contrast-enhanced computed tomography (CECT) scans of the neck, chest, abdomen, and pelvis or 18F-fluorodeoxyglucose positron emission tomography with computed tomography (PET-CT) were used for staging and response assessment. Staging included unilateral bone marrow trephine biopsy.
All patients started on treatment with chemotherapy were evaluated for response and outcome. Response to treatment was assessed by International Workshop Criteria (Cheson’s criteria) [13]. The response was defined as complete remission (CR: the disappearance of all evidence of disease), partial remission (PR: regression of measurable disease (≥50% decrease in sum of the product of diameter, and no new sites), stable disease (SD: failure to attain CR/ PR or PD) and progressive disease (PD: any new lesion or increase by 50% of previously involved sites from nadir). Progression-free survival (PFS) was calculated from the date of diagnosis to the date of tumor progression or relapse, death, or last follow-up. Overall survival (OS) was defined as the time interval between diagnosis to the last follow-up or time of death (to any cause).
Treatment-induced toxicities were recorded according to criteria of common toxicity criteria version 5.0 (CTCAE v5.0) [14]. Dose modifications if any were recorded for all patients. Response assessment and follow-up data were obtained from case files and the hospital’s electronic database.
Statistical Methods
Patient and demographic characteristics were analyzed using median/centiles and mean. The effects of variables on recurrence and death were evaluated by univariate and multivariate Cox regression model analysis. The survival curve was estimated using the Kaplan-Meier method. Analyses were performed in IBM SPSS software v19.0. Two-tailed p-values less than 0.05 were considered statistically significant at a 95% confidence interval.
This study received approval from the Institutional Ethics Committee. A waiver for informed consent was obtained since the patients had already received treatment before the initiation of this study.
Results
Demographic Characteristics
Between January 2015 and December 2018, a total of 466 patients were diagnosed with Non-Hodgkin’s lymphoma. Among them, 91 (19.5%) patients were diagnosed with peripheral T-cell lymphoma (PTCL). Out of these 91 patients, 62 (62/91; 68.1%) were diagnosed with PTCL-NOS by immunohistochemistry. Out of 62 patients, 9 patients refused treatment or defaulted during the initial period of therapy. Altogether 53 patients were finally included for analysis who fulfilled the inclusion criteria. Thirty-one patients (58.4%) were from a rural background, and 22 (41.6%) were from urban locality. The majority (83.1%) of the patients were male. The mean age of presentation was 45.3 years (standard deviation SD ± 16.3 years). Forty patients (75.5%) were below the age of 60 years (Table 1).
Patient and Disease Characteristics
The median duration of symptoms before diagnosis was 3.4 ± 0.4 months. Forty-one out of 53 patients (77.3%) presented with an advanced stage of disease (stage III/ IV). The majority (81.1%) of the patients presented with ECOG performance status (PS) of 0 or 1. Bone marrow was involved in 19 (35.8%) patients and B-symptoms were present in 31(58.4%) patients. Involvement of nodal region, extra-nodal region, and both at baseline were seen in 19 (35.8%), 9 (16.9%), and 25 (47.2%) patients respectively. The bulky disease was detected in 15 (28.3%) patients. Baseline thrombocytopenia was observed in seven (15.1%) patients (Table 1).
| Characteristics (N= 53) | No. of Patients (%) |
| Age at presentation (years) | |
| Mean ± SD, Range | 45.3 ± 16.3, 18 - 74 |
| < 60 | 40 (75.5) |
| ≥ 60 | 13 (24.5) |
| Gender | |
| Male | 44 (83.1) |
| Female | 9 (16.9) |
| ECOG PS* | |
| 0 and 1 | 43 (81.1) |
| ≥2 | 10 (18.9) |
| Residence | |
| Rural | 46 (60.1) |
| Urban | 30 (39.1) |
| Stage | |
| I | 3 (5.6) |
| II | 9 (16.9) |
| III | 19 (35.8) |
| IV | 22 (41.5) |
| Site of involvement | |
| Nodal | 19 (35.8) |
| Extra-nodal | 9 (16.9) |
| Both | 25 (47.2) |
| B-Symptoms | 31 (58.4) |
| Bone Marrow Involvement | 19 (35.8) |
| Bulky Disease | 15 (28.3) |
| Baseline Thrombocytopenia (Platelets<150×10 9 /L) | 7 (15.1) |
*ECOG, Eastern cooperative oncology group; PS, Performance status
The prognostic index for PTCL unspecified (PIT) was calculated in 47 patients as baseline LDH data were available for only those 47 patients (Table 2).
| Characteristics (N= 47) | No. of Patients (%) |
| PIT Score: | |
| Low Risk (0) | 9 (19.1) |
| Low-intermediate Risk (1) | 13 (27.6) |
| High-intermediate Risk (2) | 16 (34.1) |
| High Risk (3-4) | 9 (19.1) |
Treatment Characteristics
Chemotherapy was the main modality of treatment for all patients. Pre-phase chemotherapy with vincristine, cyclophosphamide, and prednisolone was received by 14 (26.4%) patients. The majority of the patients received anthracycline-based CHOP* chemotherapy in a first-line setting. Thirty-eight patients (71.6%) received the CHOP regimen, followed by the GDP regimen in 7 (13.2%) patients, and the CHEOP regimen in 2 (3.7%) patients (Figure 1).
Figure 1. Chemotherapy Regimens, *CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone), GDP (gemcitabine, dexamethasone, and cisplatin), CHEOP (cyclophosphamide, doxorubicin, etoposide, vincristine, and prednisolone), ICE (ifosfamide, carboplatin, and etoposide), GEMOX (gemcitabine and oxaliplatin), DHAP (dexamethasone, high-dose cytarabine, and cisplatin).

Patients who received at least two or more chemotherapy cycles were included in the analysis. The average number of chemotherapy cycles received in the front line was 5.6 (range 2 - 8).
In the relapsed setting, commonly used chemotherapy regimens were GDP, ICE, GEMOX, and DHAP*.
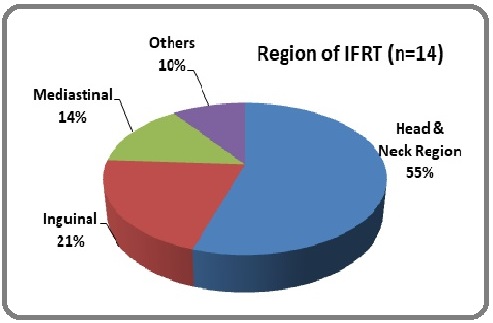
Radiation therapy in the form of involved field radiotherapy (IFRT) was received by 14 (26.4%) patients. The average IFRT dose used was 40-45 Gy in 20-25 fractions. The Head & neck region was the most commonly irradiated site (55%) followed by the inguinal region (21%) (Figure 2).
Figure 2. Region of IFRT.
Treatment outcome and survival
Response rate
Overall response rate (ORR) following first-line chemotherapy was 94.3%. Thirty-three patients (62.3%) attained complete response (CR) and 17 (32.1%) patients achieved partial response (PR). Three (5.6%) patients had progressive disease during first-line chemotherapy (Table 3).
| Response rate (N= 53) | No. of patients (%) |
| Overall response rate (ORR) = CR + PR | 50 (94.3) |
| Complete response (CR) | 33 (62.3) |
| Partial response (PR) | 17 (32.1) |
| Progressive disease (PD) | 3 (5.6) |
| PD from CR/PR | 29/50 (58) |
Among 50 patients who had responded to first-line chemotherapy, 29 (58%) patients relapsed/ progressed during the follow-up period. Altogether twenty-six patients received salvage chemotherapy (23 relapsed patients & three patients with primary progressive disease). Complete response was seen in 11 (42.3%) patients, partial response was observed in 9 (34.6%) patients and disease progressed in 6 (23.07%) patients on the salvage chemotherapy regimen.
Survival
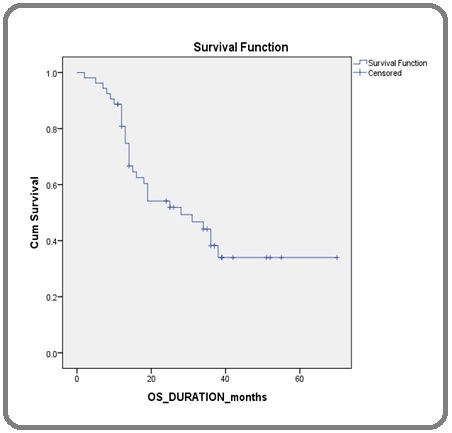
With a median follow-up period of 38 months (range 2-70 months), PFS rates at 1 year, 2 years, and 3 years are 64.1%, 39.6%, and 27.6% respectively. OS rates at 1-year, 2-years, and 3 years are 84.9%, 49.05%, and 33.9% respectively. The median PFS of the entire study population was 22 ± 3.3 months and the median OS was 28 ± 7.1 months (Table 4) (Figure 3, 4).
| Survival rates | PFS (%) | OS (%) |
| 1-Year | 64.1 | 84.9 |
| 2-Years | 39.6 | 49.05 |
| 3-Years | 27.6 | 33.9 |
Figure 3. Kaplan-Meier Curve Showing Median PFS .

Figure 4. Kaplan-Meier Curve Showing Median OS .
Treatment-related Toxicity
Of fifty-three evaluable patients, toxicity data were available in a limited number of patients from the case records. Both hematological and non-hematological toxicities were recorded in a total of 35 (66%) patients. Grade 3 and 4 neutropenia was observed in nine patients. One patient died due to febrile neutropenia. Grade 3 and 4 diarrhea was documented in seven patients. Long-term peripheral neuropathy was seen in two patients (Table 5).
| Toxicities (N=35) | No. of Patients (%) |
| Neutropenia | |
| Grade I/II | 13 (37.1) |
| Grade III/IV | 9 (25.7) |
| Oral mucositis | |
| Grade I/II | 22 (62.8) |
| Grade III/IV | 10 (28.5) |
| Diarrhoea | |
| Grade I/II | 11 (31.4) |
| Grade III/IV | 7 (20) |
| Urinary Tract infection | 3 (8.5) |
| Respiratory Tract Infection/ Pneumonia | 2 (5.7) |
| Neuropathy (all grades) | 2 (5.7) |
| Death due to treatment toxicities | 1 (2.8) |
Discussion
PTCL is one of the uncommon and clinically aggressive subtypes of NHL which originates from post-thymic peripheral T-cells or NK cells. Although PTCL accounts for approximately 10% of all NHL in Western countries, its incidence is more common in the Asian population which was noted in an Indian retrospective study [4]. PTCL-NOS is the most common histological subtype of PTCL found in other Indian studies [15]. We also observed PTCL-NOS as the most common subtype (68.1%) in our study. The median age of presentation in our study was 45.3 years which is comparable to other Indian studies but is one decade younger than that reported in Western literature [16, 17]. The majority of the patients (77.3%) in our study presented with advanced-stage (III/IV) disease which is comparable to another Indian study by Kesana et al [16]. Presences of B-symptoms were seen in 58.4% of the patients and extra-nodal sites of involvement were seen in 64.1% of the patients in our study, which is similar to other existing literature [18]. Bone marrow was involved in 35.8% of the patients in our study as reported in other studies [19, 20]. Various prognostic scores were used in different literature for risk categorization of patients with PTCL [21]. In our study, risk categorization was done using PIT criteria which revealed that the majority of the patients were in either the high-intermediate or high-risk group [9].
Approximately 15-20% of the PTCL-NOS presents in the early stage (stage I/II) [22]. In this study, we found that 22.5% of the patients presented with early-stage disease. As the early presentation of PTCL-NOS is rare, the standard therapy remains challenging. Combination therapy with systemic chemotherapy with involved field radiation therapy (IFRT) is effective in early-stage disease. Majority of the patients present with advanced disease which necessitates the use of systemic chemotherapy in almost all of them. The standard therapeutic option for patients with stage III–IV disease is conventional-dose systemic anthracycline-based chemotherapy. The most extensively used anthracycline-based chemotherapy regimen is the CHOP regimen which consists of cyclophosphamide, doxorubicin, vincristine, and prednisolone. With first-line anthracycline-based chemotherapy treatment, ORR was found to be approximately 60-70% but is associated with early relapse and dismal 5-years OS around 20-25% in previous literature [1,8]. The CHOP-based chemotherapy regimen was used in the majority of PTCL-NOS studies from Western countries as well as other Indian trials [15]. In our study, we found 71.6% of the patients received a CHOP-based chemotherapy regimen in the front-line setting, followed by a gemcitabine-based GDP regimen (13.2%) and very few patients received an etoposide-based regimen. It has been shown in a German study that the addition of etoposide to a standard anthracycline-based regimen may improve event-free survival in young good risk patients with T-cell lymphoma with consolidative autologous hematopoietic stem cell transplant (SCT) in advanced high-risk PTCL-NOS has been studied in a few studies [25, 26]. None of the patients in our study has received consolidative SCT due to logistic constraints. Gemcitabine-based GDP regimen showed good response rates (ORR 64%) in salvage or relapsed setting in various trials [27]. In our study, the gemcitabine-based GDP regimen was the most commonly used in the relapsed setting.
The role of radiation therapy in the management of PTCL-NOS is still debatable. Some studies have shown to haveimprovedoutcomeswithRTwhenusedasaconsolidative therapy in early localized disease or advanced disease after chemotherapy [28, 29]. In our study, 26.4% of the patients received IFRT with an average RT dose of 40-45 Gy.
The overall response rate to front-line chemotherapy in our study was found to be 94.3% with a complete response rate of 62.3% and a partial response rate of 32.1%. The response rates were comparable with other Asian studies which included all subtypes of PTCL and analyzed according to subtypes [30, 31]. After the treatment with standard anthracycline-based chemotherapy and radiotherapy, the relapse rate was 58% during the follow-up period which is similar to some Indian studies but differs from available Western literature which showed a relapse rate of around 21% with more patients with the primary refractory disease [21, 32]. The difference in relapse rate may be due to the limited use of consolidative SCT in resource constraint countries like India. The prognosis and outcome of PTCL are based on histological subtypes. Despite standard therapy including ASCT, the outcome of PTCL-NOS is inferior as compared to a few other subtypes of PTCL for both the frontline and relapsed setting. Median overall survival (OS) was 28 ± 7.1 months with 3-year OS being 33.9% which is relatively lower than a study done by Massimo et al. [33]. PFS rates at 2 years and 3 years were 39.6% and 27.6% respectively. Similar results were also observed in an Indian study by Nemani S et al.[15]. However, a Western study by Corradini P et al. reported a 12-year event-free survival rate of approximately 30% with the additional use of SCT [34]. Survival and treatment outcome largely depends on risk stratification according to various prognostic models. In our study survival analysis was not done according to each risk group because of the poor availability of LDH data from medical records. Recent advances in the treatment of relapsed PTCL have included the use of romidepsin and pralatrexate, but the addition of these agents to upfront therapy has not shown promising results [35-37].
Limitations of our study include retrospective with fewer patients and inadequate follow-up data for analysis. Being a retrospective one, unavoidable selection bias is a potential weakness of our study. Limited access to stem cell transplants and novel therapies makes the outcome poorer to some extent. Randomized prospective studies are needed to improve prognosis further and to optimize treatment in patients with PTCL-NOS.
In conclusion, PTCL-NOS is one of the rare and complex subtypes of mature T-cell lymphoma. Majority of the patients present in the advanced stage with adverse clinical parameters. Inadequate anthracycline sensitivity in PTCL-NOS and early relapse with CHOP-based chemotherapy warrants for development of novel and affordable agents for treatment. Treatment adherence and the availability of newer therapeutic options in resource- restraint countries are needed to improve the survival of PTCL-NOS.
Acknowledgments
We would like to thank all the staff responsible for the delivery of patients’ care.
Compliance with Ethical Standards
The study was approved by the Institutional Ethics Committee at the Dr. B Borooah Cancer Institute, Guwahati, India.
Conflict of Interest
Authors declare no conflict of interest.
References
- International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes Vose J, Armitage J, Weisenburger D. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2008;26(25). CrossRef
- Epidemiology of the non-Hodgkin's lymphomas: distributions of the major subtypes differ by geographic locations. Non-Hodgkin's Lymphoma Classification Project Anderson JR , Armitage JO , Weisenburger DD . Annals of Oncology: Official Journal of the European Society for Medical Oncology.1998;9(7). CrossRef
- Peripheral T-cell lymphoma Foss FM , Zinzani PL , Vose JM , Gascoyne RD , Rosen ST , Tobinai K. Blood.2011;117(25). CrossRef
- Frequency and distribution of lymphoma types in a tertiary care hospital in South India: analysis of 5115 cases using the World Health Organization 2008 classification and comparison with world literature Arora N, Manipadam MT , Nair S. Leukemia & Lymphoma.2013;54(5). CrossRef
- T-cell non-Hodgkin lymphoma Rizvi MA , Evens AM , Tallman MS , Nelson BP , Rosen ST . Blood.2006;107(4). CrossRef
- Molecular profiling improves classification and prognostication of nodal peripheral T-cell lymphomas: results of a phase III diagnostic accuracy study Piccaluga PP , Fuligni F, De Leo A, Bertuzzi C, Rossi M, Bacci F, Sabattini E, et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2013;31(24). CrossRef
- Gene expression signatures delineate biological and prognostic subgroups in peripheral T-cell lymphoma Iqbal J, Wright G, Wang C, Rosenwald A, Gascoyne RD , Weisenburger DD , Greiner TC , et al . Blood.2014;123(19). CrossRef
- Report of the European Task Force on Lymphomas: workshop on peripheral T-cell lymphomas Campo E, Gaulard P, Zucca E, Jaffe ES , Harris NL , Diebold J, Schlegelberger B, et al . Annals of Oncology: Official Journal of the European Society for Medical Oncology.1998;9(8). CrossRef
- Peripheral T-cell lymphoma unspecified (PTCL-U): a new prognostic model from a retrospective multicentric clinical study Gallamini A, Stelitano C, Calvi R, Bellei M, Mattei D, Vitolo U, Morabito F, et al . Blood.2004;103(7). CrossRef
- Peripheral T-cell lymphoma, not otherwise specified: a report of 340 cases from the International Peripheral T-cell Lymphoma Project Weisenburger DD , Savage KJ , Harris NL , Gascoyne RD , Jaffe ES , MacLennan KA , Rüdiger T. Blood.2011;117(12). CrossRef
- Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification Savage KJ , Chhanabhai M, Gascoyne RD , Connors JM . Annals of Oncology: Official Journal of the European Society for Medical Oncology.2004;15(10). CrossRef
- Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group Schmitz N, Trümper L, Ziepert M, Nickelsen M, Ho AD , Metzner B, Peter N, Loeffler M, Rosenwald A, Pfreundschuh M. Blood.2010;116(18). CrossRef
- Revised response criteria for malignant lymphoma Cheson BD , Pfistner B, Juweid ME , Gascoyne RD , Specht L, Horning SJ , Coiffier B, et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2007;25(5). CrossRef
- Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 5.0 (www.http://ctep.cancer.gov), Publish Date: Nov 27, 2017 .
- Peripheral T cell lymphoma: Clinico-pathological characteristics & outcome from a tertiary care centre in south India Nemani S, Korula A, Agrawal B, Kavitha ML , Manipadam MT , Sigamani E, George B, Srivastava A, Viswabandya A, Mathews V. The Indian Journal of Medical Research.2018;147(5). CrossRef
- Clinicopathological characteristics, prognostic factors,and outcomes in peripheral T‑cell lymphoma: Experience from a single center in India Kesana S, Ganeshan P, Sagar T, et al . Cancer Research, Statistics, and Treatment.2020;3:3-12.
- Prognostic significance of T-cell phenotype in aggressive non-Hodgkin's lymphomas. Groupe d'Etudes des Lymphomes de l'Adulte (GELA) Gisselbrecht C, Gaulard P, Lepage E, Coiffier B, Brière J, Haioun C, Cazals-Hatem D, et al .. Blood.1998;92(1).
- Clinicopathological profile and utility of prognostic tools in peripheral T‑cell lymphoma Lokanatha D, Namratha MS , Govind KB , Lakshmaiah KC , et al . J Appl Hematol.2016;7:102-107.
- Peripheral T-cell lymphomas, unspecified (or not otherwise specified): a review Rodriguez-Abreu D, Filho VB , Zucca E. Hematological Oncology.2008;26(1). CrossRef
- Clinical characteristics, prognostic factors, and treatment outcomes of 139 patients of peripheral T cell lymphomas from AIIMS, New Delhi, India Raina V, Singhal MK , Sharma A, et al . J Clin Oncol.2010;28(15):e18549.
- International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma N Engl J Med.1993;329(14):987-994. CrossRef
- Peripheral T-cell lymphomas: initial features, natural history, and prognostic factors in a series of 174 patients diagnosed according to the R.E.A.L. Classification López-Guillermo A, Cid J, Salar A, López A, Montalbán C, Castrillo JM , González M, et al . Annals of Oncology: Official Journal of the European Society for Medical Oncology.1998;9(8). CrossRef
- Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of young patients with good-prognosis (normal LDH) aggressive lymphomas: results of the NHL-B1 trial of the DSHNHL Pfreundschuh M, Trümper L, Kloess M, Schmits R, Feller AC , Rudolph C, Reiser M, et al . Blood.2004;104(3). CrossRef
- T-cell lymphomas in studies of the German high-grade NHL study group (DSHNHL). In: Presentation at the 10th International Conference on Malignant Lymphoma. 2008. Abstract 094 Schmitz N, Ziepert M, Nickelsen M, et al . .
- High-dose chemotherapy and autologous stem cell transplantation in peripheral T-cell lymphoma: the GEL-TAMO experience Rodríguez J, Caballero MD , Gutiérrez A, Marín J, Lahuerta JJ , Sureda A, Carreras E, et al . Annals of Oncology: Official Journal of the European Society for Medical Oncology.2003;14(12). CrossRef
- Long-term outcomes of autologous PBSCT for peripheral T-cell lymphoma: retrospective analysis of the experience of the Fukuoka BMT group Numata A, Miyamoto T, Ohno Y, Kamimura T, Kamezaki K, Tanimoto T, Takase K, et al . Bone Marrow Transplantation.2010;45(2). CrossRef
- Gemcitabine, dexamethasone, and cisplatin (GDP) as salvage chemotherapy for patients with relapsed or refractory peripheral T cell lymphoma-not otherwise specified Qi F, Dong M, He X, Li Y, Wang W, Liu P, Yang J, et al . Annals of Hematology.2017;96(2). CrossRef
- Survival advantage with the addition of radiation therapy to chemotherapy in early stage peripheral T-cell lymphoma, not otherwise specified Zhang XM , Li YX , Wang WH , Jin J, Wang SL , Liu YP , Song YW , et al . International Journal of Radiation Oncology, Biology, Physics.2013;85(4). CrossRef
- Outcomes using doxorubicin-based chemotherapy with or without radiotherapy for early-stage peripheral T-cell lymphomas Lee HK , Wilder RB , Jones D, Ha CS , Pro B, Rodriguez MA , Romaguera JE , et al . Leukemia & Lymphoma.2002;43(9). CrossRef
- Comprehensive analysis of peripheral T-cell and natural killer/T-cell lymphoma in Asian patients: A multinational, multicenter, prospective registry study in Asia Yoon SE , Song Y, Kim SJ , Yoon DH , Chen TY , Koh Y, Kang KW , et al . The Lancet Regional Health. Western Pacific.2021;10. CrossRef
- Profiling of peripheral T-cell lymphomas in Kerala, South India: A 5-year study Nair RA , Vasudevan JA , Jacob PM , Sukumaran R. Indian Journal of Pathology & Microbiology.2017;60(2). CrossRef
- The outcome of peripheral T-cell lymphoma patients failing first-line therapy: a report from the prospective, International T-Cell Project Bellei M, Foss FM , Shustov AR , Horwitz SM , Marcheselli L, Kim WS , Cabrera ME , et al . Haematologica.2018;103(7). CrossRef
- Peripheral T cell lymphoma, not otherwise specified (PTCL-NOS). A new prognostic model developed by the International T cell Project Network Federico M, Bellei M, Marcheselli L, Schwartz M, Manni M, Tarantino V, Pileri S, et al . British Journal of Haematology.2018;181(6). CrossRef
- Long-term follow-up of patients with peripheral T-cell lymphomas treated up-front with high-dose chemotherapy followed by autologous stem cell transplantation Corradini P, Tarella C, Zallio F, Dodero A, Zanni M, Valagussa P, Gianni AM , et al . Leukemia.2006;20(9). CrossRef
- Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy Coiffier B, Pro B, Prince HM , Foss F, Sokol L, Greenwood M, Caballero D, et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2012;30(6). CrossRef
- Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study O'Connor OA , Pro B, Pinter-Brown L, Bartlett N, Popplewell L, Coiffier B, Lechowicz MJ , et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2011;29(9). CrossRef
- A phase II study of cyclophosphamide, etoposide, vincristine and prednisone (CEOP) Alternating with Pralatrexate (P) as front line therapy for patients with peripheral T-cell lymphoma (PTCL): final results from the T- cell consortium trial Advani RH , Ansell SM , Lechowicz MJ , Beaven AW , Loberiza F, Carson KR , Evens AM , et al . British Journal of Haematology.2016;172(4). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2023
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times