Port-site Metastasis from Gall Bladder Adenocarcinoma with Squamous Differentiation Following Laparoscopic Cholecystectomy: A Rare Case Report
Download
Abstract
Port site cutaneous metastasis of gallbladder cancer is an exceedingly infrequent phenomenon with very few cases reported in the past. Herein, we report a case of port site cutaneous metastases of gallbladder adenocarcinoma in a 57 years old female. Physical examination revealed a non healing ulcer in the epigastric region. FNA was suggestive of poorly differentiated carcinoma. Biopsy demonstrated atypical cells. Immunohistochemical analysis showed diffuse cytoplasmic staining with CK 7 and p63 showed focal nuclear positivity in tumour cells. This confirmed the diagnosis of metastastatic gall bladder adenocarcinoma with squamous differentiation. Diagnosis in such a case can be very challenging as the differential of primary cutaneous malignancies should also be kept in mind.
Introduction
Cutaneous metastasis from internal malignancies is rare,with incidence ranging from 1.0-4.6% [1]. The different patterns of metastasis to skin are classified as Sister (Mary) Joseph nodules (SJNs) i.e. umbilical nodules and non- SJNs. Non SJNs are further divided into non SJNs metastasis after Surgery, non SJNs after injury and non SJNs after lymphadenopathy [2]. The incidence of non SJNs after laparoscopic surgery for malignancy is 1-2% [3].
Surgery is the treatment of choice for resectable disease. Oncology concerns regarding laparoscopic surgery for gall bladder carcinoma evaluated by an expert committee found that the risk of port site recurrence was due to bile leak associated with gall bladder injury which is a risk factor for tumour recurrence and decreased survival [4].
The most common tumours exhibiting cutaneous metastasis are primaries from ovary and breast in females and lung and colon in males [3]. Skin metastasis from carcinoma gall bladder is very uncommon and the incidence ranges from 0.7-9% [4]. We present a case report of a patient with surgical site cutaneous metastasis following laparoscopic cholecystectomy for adenocarcinoma gall bladder.
Case Summary
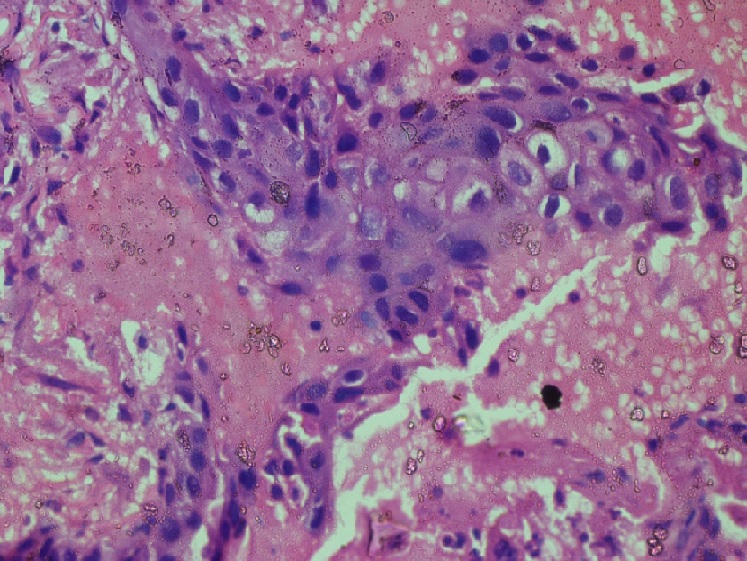
A 57 years old female who was previously diagnosed with Adenocarcinoma Gall Bladder and had undergone surgery for the same 1 year back presented in Surgery Clinics with an ulcer over the midline laparoscopic cholecystectomy scar. The ulcer was present in the epigastric region. It was 8.5 x 3 cm in size, tender, with irregular margins and purulent discharge. CECT revealed an evolving abscess. However, FNA of the lesion was suggestive of poorly differentiated carcinoma with unknown primary. A PET Scan was advised which revealed a supraumbilical epigastric midline scar with ill defined hypermetabolic irregular thickening. Gall Bladder was not visualised. Patient was given 8 cycles of weekly gemcitabine therapy and a repeat PET scan was done for treatment response 10 months later. The repeat scan revealed an unremarkable post operative Gall Bladder fossa with interval progression of pre existing hypermetabolic supraumbilical laparoscopic cholecystectomy port site irregular thickening. Biopsy from surgical site ulcer showed mildly dysplastic stratified squamous epithelium with pseudoepitheliomatous hyperplasia. Few small nests and singly scattered atypical cells exhibiting pleomorphism, hyperchromasia, prominent nucleoli and abundant cytoplasm were seen in the superficial dermis (Figure 1 and 2).
Figure 1. Nests and Singly Scattered Atypical Cells were Seen in the Superficial Dermis. Hematoxylin and Eosin x10X.
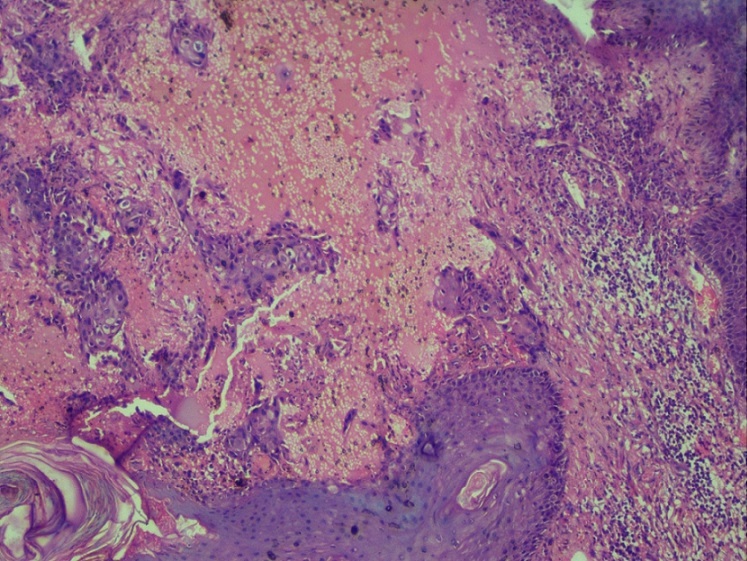
Figure 2. The Atypical Cells Show Pleomorphism, Hyperchromasia, Prominent Nucleoli and Abundant Cytoplasm Infiltrating the Superficial Dermis. Hematoxylin and Eosin x40X.
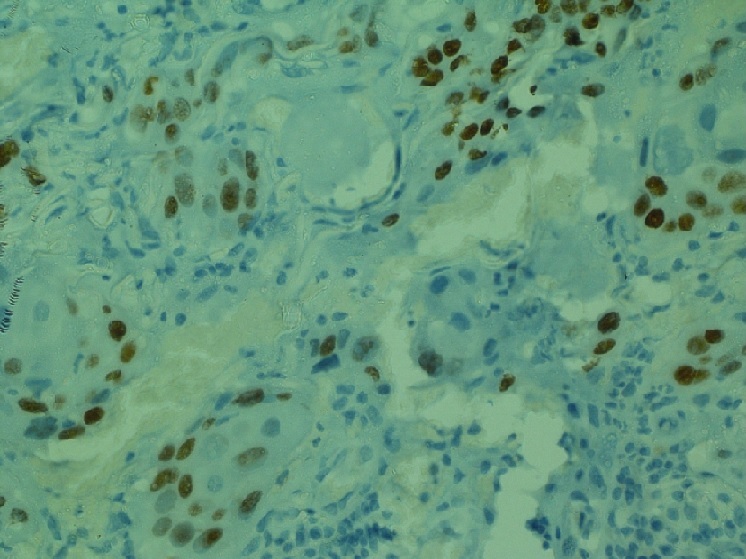
Cytokeratin 7 showed diffuse cytoplasmic positivity (Figure 3) while Cytokeratin 20 and p63 was focally positive in few tumour cells (Figure 4).
Figure 3. Cytokeratin 7 Showed Diffuse Cytoplasmic Positivity in the Tumor Cells. IHC CK 7 x 40X.
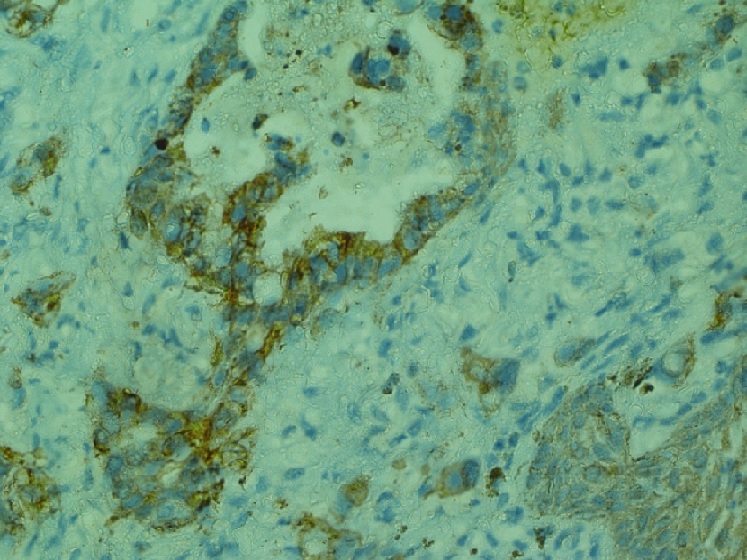
Figure 4. p63 was Focally Positive in Few Tumour Cells. IHC p63 x 40X.
This confirmed the diagnosis of metastastatic gall bladder adenocarcinoma with squamous differentiation. The patient was given additional locoregional radiotherapy of 5000 cGy. The patient is currently under regular follow up and doing well.
Discussion
Cutaneous metastasis from visceral malignancies is relatively rareand may present as either patients with a known malignancy who develop cutaneous metastases later in the course of disease or patients presenting simultaneously with cutaneous metastases as well as primary malignancy or in rare cases, only cutaneous metastases is present without any apparent malignancy [5]. As per epidemiological data, gall bladder malignancies are the sixth most common malignancies involving the gastrointestinal tract with the most common histological type being adenocarcinoma [6]. Typically gall bladder carcinoma shows loco regional spread to liver, lymph nodes, adjacent organs and peritoneum with cutaneous metastasis being extremely rare [6]. As per patient data, the most common histological type of gall bladder cancer with skin metastasis is also adenocarcinoma [7].
Multiple instances of port site metastasis in gall bladder adenocarcinoma have been reported in the past [7-8]. However, in most of the cases, gall bladder carcinoma was unsuspected preoperatively. The time gap between laparoscopic cholecystectomy and port site metastasis can vary upto 4 years [9].
The metastatic cutaneous lesions are usually non- tender, erythematous or nodular resembling inflammatory skin lesions and commonly involve thoracic and abdominal skin, the extremities, neck, head, and scalp [6,9]. Padilla et al has reported a single case with an ulcerated epidermal invasion similar to our case [10].
Tumour cells may spread via different routes like direct implantation, hematogenous or lymphatic dissemination and extra nodal extension from lymph node capsule to surrounding subcutaneous tissue. The most likely mechanism for cutaneous metastasis at surgical incisions and laparoscopic port site is direct implantation of exfoliated tumour cells. The inflammatory responses that normally occur during the wound healing process involving macrophages and angiogenic factors, are exploited by tumor cells thus facilitating growth of tumour tissue. Mechanisms that play a role in port site metastases include contamination of the wound directly, pneumoperitoneum, aerosilization of tumour cells, host factors like increasing age, immunosuppression, obesity and advanced stage of disease [2].
The diagnosis in our case was confirmed after histopathology and clinic-radiological correlation. Certain metastatic tumours simulate primary skin malignancies, hence application of immunohistochemistry is necessary to rule out these differentials. We applied p63 to rule out squamous differentiation.
It has been observed that preoperative irrigation of port site with a cytotoxic solution, meticulous handling of resected specimen, judicious use of retractor bags and peritoneal closure at port site can prevent the metastasis. [10] Cutaneous metastases from gall bladder malignancies represent advanced disease and are associated with a very poor prognosis.The intent of treatment in such a patient is palliative. Hence early diagnosis is of utmost importance. In Conclusion, confirmation of port site cutaneous metastasis of gall bladder adenocarcinoma is very rare and can be challenging. Complete excision with histopathological examination and immunostaining is the method of choice to achieve an early diagnosis and a favourable prognosis.
References
- Cutaneous Metastasis after Surgery, Injury, Lymphadenopathy, and Peritonitis: Possible Mechanisms Otsuka I. International Journal of Molecular Sciences.2019;20(13). CrossRef
- Laparoscopic Surgery for Gallbladder Cancer: An Expert Consensus Statement Han HS , Yoon YS , Agarwal AK , Belli G, Itano O, Gumbs AA , Yoon DS , et al . Digestive Surgery.2019;36(1). CrossRef
- Adenocarcinoma of the gall bladder presenting with a cutaneous metastasis Kaur J, Puri T, Julka PK , Gunabushanam G, Iyer VK , Singh MK , Ramam M. Indian Journal of Dermatology, Venereology and Leprology.2006;72(1). CrossRef
- Cancer of the liver, bile duct, and gall bladder, Leibel and Phillips Textbook of Radiation Oncology, 3rd ed, Philadelphia Goodman K, Wagman R, Ho . Elselvier Saunders.2010;:829-831.
- An unusual case of cutaneous metastases from gall bladder cancer Sun KK , Shen XJ , Zhao H. Transl Cancer Res.2017;6(5):1005-1008.
- Mohaimeed K, Mohamed A. Port-Site metastasis from gallbladder carcinoma after laparoscopic cholecystectomy, report of three cases Emran F, Shami S, Abukhater M. Inter J Surg.2009;25(2):12-15.
- Treatable solitary giant port site metastasis following cholecystectomy for carcinoma gall bladder Joshi N, Banerjee A, Mody B, Shah SR . Int J Ortho Rheum.2020;1(1):1-3.
- A Rare Case of Carcinoma Gallbladder with Port Site Recurrence And Isolated Sternal Metastasis Mishra H, Hadi R, Masood S, Khurana R, Mishra R. IOSR- JDMS.2017;16(8):25-27.
- Axillary metastases after port site recurrences of gallbladder carcinoma: a case report Nijhuis JJHT , Bosscher MRF , Liem MSL . World Journal of Surgical Oncology.2020;18. CrossRef
- Cutaneous metastatic adenocarcinoma of gallbladder origin Padilla RS , Jarmillo M, Dao A, Chapman W. Archives of Dermatology.1982;118(7).
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2023
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times