Factors Affecting Survival of Patients with Synchronous Metastatic Colorectal Cancer in a Tertiary Hospital in Indonesia: A Retrospective Study
Download
Abstract
Background and Objective: Despite colorectal cancer (CRC) being one of the most frequent cancers in Indonesia, limited data exists regarding the survival and prognostic factors of Indonesian metastatic CRC patients. This study aimed to investigate the survival outcome and factors influencing local CRC patients presenting with a metastatic stage at diagnosis.
Materials and Methods: A retrospective cohort study was conducted using data from 441 cases of synchronous metastatic CRC treated between January 2016 and December 2019 at Dr. Sardjito Hospital, Yogyakarta, Indonesia. Secondary data were collected from the CRC clinical registry database. Demographic, clinicopathological, and treatment data were collected. Survival status was obtained from the registry database and communication with patients or their families. Kaplan-Meier curves were used to estimate overall survival (OS). The Cox proportional hazards regression model was applied to analyze potential factors affecting survival.
Results: The median follow-up in the study was 17 months. The median overall survival was 13 months. Two-year overall survival was 37%, and the estimated 5-year overall survival was 16.1%. Multivariate Cox analysis identified poor performance status (HR 2.639, 95% CI 1.438-4.842, p = 0.002), elevated carcinoembryonic antigen (CEA) (HR 2.795, 95% CI 1.509-5.176, p = 0.001), and higher histological grade (HR 2.019, 95% CI 1.112-3.667, p = 0.021) as factors associated with poorer overall survival.
Conclusion: Based on the findings, poor performance status, high CEA levels, and higher histological grade were associated with unfavorable overall survival among patients with synchronous metastatic colorectal cancer in Yogyakarta, Indonesia.
Introduction
Colorectal cancer (CRC) is a malignancy with the third most common incidence globally based on the GLOBOCAN database 2020 [1]. In Indonesia, CRC is ranked 4th for cancer incidence and 5th for the cause of death due to cancer [2]. Approximately 22% of CRC patients are diagnosed at stage IV [3]. Stage 4 colorectal cancer patients have poor overall survival, around 10% for five years [3]. A retrospective cohort study using the Surveillance Epidemiology and End Results (SEER) database reported that age at diagnosis, marital status, race, primary tumor site, tumor grade, CEA level, T status, N status, surgery for the primary lesion, chemotherapy, and location of metastases (bone, brain, liver and lung) independently influenced prognosis [4].
There is little information on CRC survival in Indonesia and even less on mCRC. A study in eastern Indonesia reported an association between age, type of histopathology, stage and mode of surgery on overall survival of CRC [5]. Still, it did not focus on patients with metastatic disease. In Yogyakarta province, CRC is the third most common cancer according to the cancer registry data, with 39% of patients have been diagnosed at stage 4 [6]. The purpose of this study is to determine the survival of this group of patients and identify factors affecting it.
Materials and Methods
Data collection
This study used secondary data from the hospital based cancer registry (HBCR) of colorectal in Dr. Sardjito General Hospital Yogyakarta. HBCR abstracted 32 variables using canreg5 software. Based on initial data from canreg5, more detailed data were collected through medical records as the CRC clinical registry, including patients diagnosed in 2016-2019. The present study recruited all patients with synchronous metastatic disease data from the clinical registry database, including demographic, clinical, pathology and first line treatment information. Data collection was undertaken between August 2020 and February 2022. We included 441 mCRC patients’ data from the registry database. The joint ethics committee from the Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada/Dr. Sardjito Hospital, Yogyakarta, has approved this study (reference number KE/FK/0549/E.C./2020). Based on ethical clearance policy, informed consent is not required for retrospective studies
Key Variables
We determined age as “young” if <50 years and “old” if ≥50 years. gender (male versus female), education (
Tumor location was categorized into right sided colon (caecum, ascending colon, hepatic flexure, and transverse colon) and left sided colon (splenic flexure, descending colon, sigmoid colon and rectosigmoid colon). Other tumor parameters included histological grade (1, 2, 3, or 4), morphological subtypes (adenocarcinoma, mucinous carcinoma, or signet ring cell carcinoma), T status (1, 2, 3, 4, and X if it was not determined or unknown), N status (0, 1, 2, and X if it was not determined or unknown), M status (0, 1, and X if it was not determined or unknown), and metastatic stage (A or B-C). Disease stage was determined according to TNM classification of the 8th edition American Joint Committee of Cancer (AJCC) [9]. Primary tumor resection was categorized into two, namely yes and no.
Chemotherapy treatments were categorized into non-oxaliplatin based and oxaliplatin based regimens. Included in the non-oxaliplatin based regimens are single capecitabine (common dose: 2000-2500 mg/m2/day day 1-14 every 21 days for 8 cycles), De gramont or 2FU/LV (common dose: fluorouracil 400 mg/m2 IV bolus days 1-2, fluorouracil 600mg/m2 continuous IV days 1-2, folinicacid 200 mg/m2 every 14 days for 12 cycles), and FOLFIRI (common dose: irinotecan 180 mg/m2 day 1, folinic acid 200 mg/m2 day 1, fluorouracil 400-800 mg IV bolus day 1, fluorouracil 2400-3000 mg/m2 IV continuously for 36 hours, every 14 days for 6-8 cycles). In comparison, the oxaliplatin based regimens are FOLFOX (common dose: oxaliplatin 85 mg/m2 day 1, folinic acid 200 mg/m2 day 1-2, fluorouracil 400 mg/m2 IV bolus day 1-2, fluorouracil 600 mg/m2 continuous IV for 22 hours days 1-2, repeated every 14 days for 12 cycles) and CAPOX (common dose: oxaliplatin 135 mg/m2 day 1, capecitabine 2000-2500 mg/m2 day 1-14, repeated every 21 days for 8 cycles). The use of targeted therapy was categorized into no targeted therapy and with targeted therapy. Targeted therapy referred to bevacizumab (5 mg/kg day 1, usually administered with FOLFOX 12 cycles maximum or cetuximab (400 mg/kg on first administration, then 200 mg/kg, repeated every week, 12 cycles maximum (based on Indonesia national insurance regulation) according to the KRAS mutation status. The addition of bevacizumab or cetuximab was based on the consideration of the oncologist.
Overall survival (O.S.) was determined as months difference between date of diagnosis and date of death from any causes or the date from current information from medical record. If the patients did not visit the outpatient clinic for more than six months, we contacted them or their families by telephone or mail. We used estimation date in 7 patients who died and the family could only remember the year of death. We determined Jun 30 of the death year as the date of death. While we could not contact the patients or their families, their survival status was determined by the date of their last visit to the hospital.
Statistical analysis
Variables were compared by Cox proportional hazards regression model. Variables with a p-value <0.05 were included in the multivariate analysis. The Kaplan-Meier method was used to calculate the O.S. Comparisons between groups of interest were analyzed using a log-rank test. A p-value of <0.05 was considered statistically significant. All statistical analyses were performed using SPSS statistical software (IBM SPSS Statistics for Windows, version 28 (IBM Corp., Armonk, N.Y., USA).
Results
We analyzed 441 eligible patients. The majority of subjects were ≥50 years old (318, 72.1%), males (244, 55.3%), ≥ junior high school educational attainment (245, 55.6%) and being married (391, 88.7%). Sixty-seven percent had private or premium payer or government insurance for a civil servant. Most subjects had ECOG 0-1 performance status (228, 51.7%). At total of 183 cases (41.5%) had 18.5-23 kg/m2 BMI and 310 cases (70.3%) had a pretreatment hemoglobin level ≥10 g/dL. CEA level >5 ng/mL were in 227 subjects (51.5%). Most subjects had left-sided tumor (333, 75.5%) and histological grading 1 (152, 34.5%). Adenocarcinoma was the prominent morphological subtypes (n =390, 88.4%). T3 was the majority of tumor status (213, 48.3%) and N1 was the majority of N status (142, 32.2%). A total of 257 (58.3%) patients did not undergo primary tumor resection, 291 patients received targeted therapy (66.0%). and 335 patients (76.0%) had a IV-A metastatic disease. The baseline characteristics of study subjects are listed in Table 1.
| Variables | Frequency (N) | Percentage (%) |
| Age (years) | ||
| <50 | 123 | 27.9 |
| ≥50 | 318 | 72.1 |
| Gender | ||
| Male | 244 | 55.3 |
| Female | 197 | 44.7 |
| Education | ||
| < junior high school | 134 | 30.4 |
| ≥ junior high school | 245 | 55.6 |
| Unknown | 62 | 14.1 |
| Marital status | ||
| Single | 12 | 2.7 |
| Married | 391 | 88.7 |
| Widower/widow | 31 | 7 |
| Unknown | 7 | 1.6 |
| Insurance | ||
| Government insurance for the poor | 114 | 25.9 |
| Private insurance, premium payer, or government insurance for civil servant | 297 | 67.3 |
| Self-pocket | 23 | 5.2 |
| Unknown | 7 | 1.6 |
| ECOG | ||
| 0-1 | 228 | 51.7 |
| 2 | 94 | 21.3 |
| 04-Mar | 46 | 10.4 |
| Unknown | 73 | 16.6 |
| BMI (kg/m2) | ||
| <18.5 | 146 | 33.1 |
| 18.5-23 | 183 | 41.5 |
| ≥23 | 81 | 18.4 |
| Unknown | 31 | 7 |
| Hemoglobin level (g/dL) | ||
| <10 | 98 | 22.2 |
| ≥10 | 310 | 70.3 |
| Unknown | 33 | 7.5 |
| CEA (ng/mL) | ||
| ≤5 | 56 | 12.7 |
| >5 | 227 | 51.5 |
| Unknown | 158 | 35.8 |
| Tumor location | ||
| Right | 82 | 18.6 |
| Left | 333 | 75.5 |
| Unknown | 26 | 5.9 |
| Histological grading | ||
| 1 | 152 | 34.5 |
| 2 | 131 | 29.7 |
| 04-Mar | 46 | 10.4 |
| Unknown | 112 | 25.4 |
| Morphological subtypes | ||
| Adenocarcinoma | 390 | 88.4 |
| Mucinous carcinoma | 19 | 4.3 |
| Signet ring cell carcinoma | 8 | 1.8 |
| T status | ||
| 1 | 4 | 0.9 |
| 2 | 23 | 5.2 |
| 3 | 213 | 48.3 |
| 4 | 120 | 27.2 |
| X | 81 | 18.4 |
| N status | ||
| 0 | 138 | 31.3 |
| 1 | 142 | 32.2 |
| 2 | 50 | 11.3 |
| X | 111 | 25.2 |
| Targeted therapy | ||
| No | 291 | 66 |
| Yes | 150 | 34 |
| Resection of primary tumor | ||
| No | 257 | 58.3 |
| Yes | 75 | 17 |
| Unknown | 109 | 24.7 |
| Metastatic stage | ||
| IVA | 335 | 76 |
| IVB | 91 | 20.6 |
| IVC | 15 | 3.4 |
Note, ECOG, Eastern Cooperative Oncology Group; BMI, body mass index; CEA, carcinoembryonic antigen, T, tumor; N, nodal.
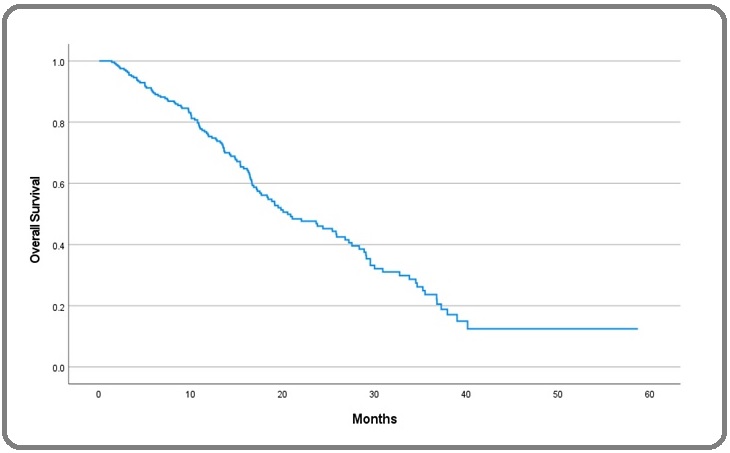
The median duration of follow-up for the whole cohort was 17 months (ranged 0.0-72 months). The median overall survival was 13 months. The 2-year survival was 37%, and the estimated 5-year survival was 16.1% (Figure 1).
Figure 1. Kaplan-Meier curve of OS in mCRC.
Univariate cox regression showed that ECOG, BMI, CEA, hemoglobin, tumor location, histological grading, N status, resection of the primary tumor, targeted therapy and metastatic stage were potential survival predictors.
Multivariate cox regression demonstrated that ECOG 3-4 was associated with poorer prognosis (HR =2.639, 95% confidence interval/CI 1.438-4.842, p 0.002). A higher CEA level was associated with increased mortality (HR = 2.795, 95% CI 1.509-5.176, p = 0.001).
A higher histological grade was also associated with poorer survival (histological grade 3-4, HR 0.021, 95%CI 1.112-3.667, p = 0.021). Due to missing data, only in 177 cases (40.1%) can multivariate analysis be performed. The univariate and multivariate analyses for overall survival are listed in Table 2.
| Variables | Univariate analysis | Multivariate analysis | ||||
| HR | 95% CI | p* | HR | 95% CI | p* | |
| Age (years) | ||||||
| <50 | ||||||
| ≥50 | 1.179 | 0.877-1.584 | 0.275 | |||
| Gender | ||||||
| Male | ||||||
| Female | 1.037 | 0.802-1.341 | 0.781 | |||
| Education | ||||||
|
|
|
|
|
|
|
|
| ≥junior high school | 1.043 | 0.777 -1.402 | 0.778 | |||
| Marital status | ||||||
| Single | ||||||
| Married | 0.952 | 0.563-1.609 | 0.855 | |||
| Widower/Widow | 1.085 | 0.481-2.447 | 0.844 | |||
| Insurance type | ||||||
| Government insurance for the poor | ||||||
| Private insurance, premium payer, or government insurance for civil servant | 0.998 | 0.737-1.351 | 0.99 | |||
| Self-pocket | 0.727 | 0.359-1.471 | 0.375 | |||
| ECOG | ||||||
| 0-1 | ||||||
| 2 | 1.336 | 0.955-1.87 | 0.091 | 1.245 | 0.734-2.111 | 0.417 |
| 04-Mar | 2.471 | 1.655-3.688 | 0 | 2.651 | 1.444-4.865 | 0.002 |
| BMI (kg/m2) | ||||||
| <18.5 | ||||||
| 18.5-22.9 | 0.878 | 0.651-1.183 | 0.392 | 0.782 | 0.502-1.217 | 0.276 |
| ≥23 | 0.774 | 0.53-1.13 | 0.185 | 0.904 | 0.508-1.609 | 0.731 |
| CEA (ng/mL) | ||||||
| <=5 | ||||||
| >5 | 1.854 | 1.214-2.83 | 0.004 | 2.762 | 1.49-5.121 | 0.001 |
| Hemoglobin (mg/dL) | ||||||
| <10 | ||||||
| ≥10 | 0.524 | 0.384-0.716 | <0.001 | 0.767 | 0.446-1.319 | 0.338 |
| Tumor location | ||||||
| Right | ||||||
| Left | 0.819 | 0.595-1.126 | 0.219 | 0.728 | 0.417-1.271 | 0.264 |
| Histological grading | ||||||
| 1 | ||||||
| 2 | 0.81 | 0.587-1.116 | 0.198 | 0.755 | 0.482-1.184 | 0.221 |
| 04-Mar | 1.428 | 0.945-2.157 | 0.091 | 2.01 | 1.107-3.649 | 0.022 |
| Pathological morphology | ||||||
| Adenocarcinoma | ||||||
| Mucinous carcinoma | 0.962 | 0.51-1.813 | 0.904 | |||
| Signet ring cell carcinoma | 1.12 | 0.278-4.515 | 0.874 | |||
| T status | ||||||
| 1 | ||||||
| 2 | 0.661 | 0.148-2.957 | 0.588 | |||
| 3 | 0.637 | 0.157-2.587 | 0.528 | |||
| 4 | 0.818 | 0.2-3.342 | 0.78 | |||
| x | 0.804 | 0.193-3.346 | 0.764 | |||
| N status | ||||||
| 0 | ||||||
| 1 | 1.057 | 0.767-1.456 | 0.734 | 0.807 | 0.5-1.304 | 0.382 |
| 2 | 1.195 | 0.766-1.863 | 0.432 | 0.93 | 0.482-1.795 | 0.829 |
| x | 1.528 | 1.078-2.166 | 0.017 | 1.053 | 0.57-1.944 | 0.869 |
| Resection of primary tumor | ||||||
| No | ||||||
| Yes | 1.374 | 0.976-1.934 | 0.068 | 1.197 | 0.676-2.12 | 0.536 |
| Unknown | 1.023 | 0.661-1.583 | 0.919 | 1.41 | 0.641-3.103 | 0.393 |
| Targeted therapy | ||||||
| No | ||||||
| Yes | 0.787 | 0.605-1.023 | 0.073 | 1.066 | 0.704-1.615 | 0.762 |
| Metastatic stage | ||||||
| A | ||||||
| B-C | 1.544 | 1.544-2.098 | 0.006 | 1.345 | 0.817-2.215 | 0.243 |
Statistical analysis performed by Cox proportional hazard model with p*< 0.05; ECOG, Eastern Cooperative Oncology Group; BMI, body mass index; CEA, carcinoembryonic antigen, T, tumor; N, nodal.
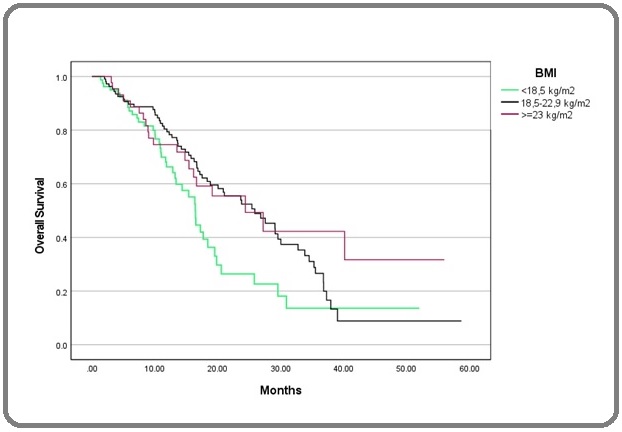
Kaplan-Meier curves also showed statistically significant differences based on ECOG, CEA, and histological grade (Figure 2-4).
Figure 2. Kaplan-Meier Curve of OS in ECOG 0-1, 2, and 3-4. OS, Overall Survival, ECOG, Eastern Cooperative Oncology Group.
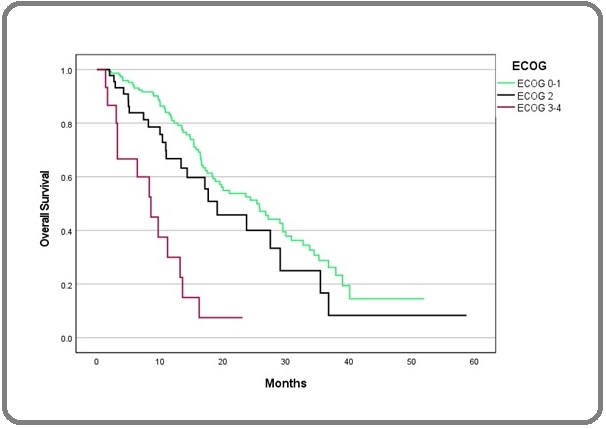
Figure 3. Kaplan-Meier Curve of OS Based on CEA Level. OS, Overall Survival; CEA, Carcinoembryonic Antigen.

Figure 4. Kaplan-Meier Curve of OS Based on BMI. OS, overall survival; BMI, body mass index.
Discussion
Metastatic CRC is still a challenge in the field of oncology. In Yogyakarta, Indonesia, nearly 40% of CRC patients present at stage 4 (6). To the best our knowledge, this is the first study conducted to determine the factors that affect the overall survival of mCRC patients in Yogyakarta. We analyzed 411 mCRC patients treated at Dr. Sardjito Hospital, a tertiary hospital in Yogyakarta.
Five years O.S. in this study was 16.1% with a median survival of 13 months. There 41% of participants did not receive targeted therapy. The addition of targeted therapy was at the discretion of the oncologist. The reasons why these patients did not receive targeted therapy were not investigated further in this study. Although the resources for cancer services in Indonesia were limited, as was often found in developing countries, the 5-year overall survival in this study was comparable to the Surveillance, Epidemiology, and End Results (SEER) report, which showed 5-year O.S. for metastatic colorectal cancer in the USA was 14.7% [10,11]. Meanwhile, in Europe, the 5-year overall survival for mCRC was 22% [12].
Based on multivariate cox regression, the variables that independently affect overall survival were ECOG score, CEA and histological grade. The ECOG score, an assessment of performance status, has been known to be one of the most influencing factors for survival in cases of metastatic colorectal cancer [13]. In this study, patients with ECOG 3-4 were independently associated with a worse prognosis with HR 8,472 (p < 0.001, 95% CI: 3.748-19151). Since cases with metastases usually present with poor performance status associated with visceral crisis, clinicians tend to give less aggressive therapy or reduced chemotherapy doses [13]. However, we did not record whether the patients received a reduced dose or not in this study.
CEA can be measured in serum quantitatively and can be used as a diagnostic and prognostic marker [14]. Increased preoperative CEA has been known to be associated with poorer overall survival [15], as confirmed by our study. The relationship between histological grade based on tumor differentiation and disease prognosis has also been well-recognized, supporting our findings [16]. Those with colorectal adenocarcinomas that are grade 3 often have a worse prognosis than patients whose tumors are grade 1 or 2. Patients with grade 3 colorectal adenocarcinomas had a 45.5% 5-year survival rate, while the 5-year survival rate of grades 1 and 2 were 71.4% and 59.5%, respectively [17].
The strength of this study is that it is the first study on factors affecting survival in mCRC in Indonesia. Missing data is a weakness of retrospective studies; this study was no exception. There were 16.6% missing data on ECOG, 35.8% on CEA and 25.4% on histological grading. Thus only 40.1% of cases could be done multivariate analysis (n=177). Another limitation was that it was unknown why patients did not receive targeted therapy and whether patients with poor performance status received reduced chemotherapy doses. Despite these limitations, we found that ECOG score, CEA and histological grading showed their prognostic ability. In conclusion, this study showed ECOG score, CEA levels and histological grade were independent factors affecting survival in mCRC.
Funding
The authors received funding from the Institutional Links grant, ID 527558574, under the Newton Institutional Link-Indonesia KLN Fund partnership, funded by the UK Department for Business, Energy and Industrial Strategy and Indonesia Ministry of Research Technology & Higher Education and delivered by the British Council.
Institutional Review Board Statement
This study was conducted under Ethical clerence (KE/ FK/0549/E.C./2020; Medical and Health Research Ethics Committee (MHREC), FKKMK, UGM-Dr. Sardjito General Hospital).
Acknowledgments
The authors thank Yana Suryani, Rosita Yunanda Purwanto, Riani Witaningrum and Nugira Dinantia for technical assistance and coordination
Conflicts of Interest
All authors declare that they have no conflicts of interest.
References
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries Sung H, Ferlay J, Siegel RL , Laversanne M, Soerjomataram I, Jemal A, Bray F. CA: a cancer journal for clinicians.2021;71(3). CrossRef
- World Health Organization. Cancer Incident in Indonesia Int Agency Res Cancer.2020;858:1-2.
- Metastatic patterns and survival outcomes in patients with stage IV colon cancer: A population-based analysis Wang J, Li S, Liu Y, Zhang C, Li H, Lai B. Cancer Medicine.2020;9(1). CrossRef
- Nomogram for predicting overall survival in colorectal cancer with distant metastasis Liu Z, Xu Y, Xu G, Baklaushev VP , Chekhonin VP , Peltzer K, Ma W, Wang X, Wang G, Zhang C. BMC gastroenterology.2021;21(1). CrossRef
- Colorectal cancer survival rates in Makassar, Eastern Indonesia: A retrospective Cohort Study Labeda I, Lusikooy RE , Mappincara , Dani MI , Sampetoding Sa, Kusuma MI , Uwuratuw JA , et al . Annals of Medicine and Surgery.2021;74. CrossRef
- RKBR Maret 2021. 2021;(8 July 2021) Available from: https://canreg.fk.ugm.ac.id/laporan-data/registrasi-kanker-berbasis-rumah-sakit-dr-sardjito-fkkmk-ugm/rkbr-maret-2021/..
- ECOG Performance Status Scale The ECOG Performance Status Scale circulates in the public domain and is therefore available for public use . Comparing the ECOG Performance Status and the Karnofsky Performance Status Scales. ECOG-ACRIN [Internet]. 2022;0–1 Available from: https://ecog-acrin.org/resources/ecog-performance-status/..
- WHO/IASO/IOTF . The Asia-Pacific perspective: redefining obesity and its treatment. Health Communications Australia; Melbourne: 2000. 2000; .
- AJCC Cancer Staging Manual (8th edition). Springer International Publishing: American Joint Commission on Cancer; 2017. 2AD. 2017 p Amin MB , Edge S, Greene F, Byrd DR , Brookland RK , Gershenwald JE , et al . .
- Global Health Equity : Cancer Care Outcome Disparities in Countries L, Souza JA De , Hunt B, Asirwa FC , Adebamowo C, Lopes G. 2016;34(1):4-6.
- Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER, Cancer Stat Facts: Colorectal Cancer 2011-2017. Accessed 4. 2022;(April):2022. .
- Overall and stage-specific survival of patients with screen-detected colorectal cancer in European countries: A population-based study in 9 countries Cardoso R, Guo F, Heisser T, De Schutter H, Van Damme N, Nilbert MC , Christensen J, et al . The Lancet Regional Health. Europe.2022;21. CrossRef
- Systematic review of prognostic factors related to overall survival in patients with stage IV colorectal cancer and unresectable metastases Stillwell AP , Ho YH , Veitch C. World Journal of Surgery.2011;35(3). CrossRef
- The Roles of Carcinoembryonic Antigen in Liver Metastasis and Therapeutic Approaches Lee JH , Lee SW . Gastroenterology Research and Practice.2017;2017. CrossRef
- The role of preoperative CEA in the management of colorectal cancer: A cohort study from two cancer centres Baqar AR , Wilkins S, Staples M, Angus Lee CH , Oliva K, McMurrick P. International Journal of Surgery (London, England).2019;64. CrossRef
- Histological grading of colorectal cancer: a simple and objective method Ueno H, Mochizuki H, Hashiguchi Y, Ishiguro M, Kajiwara Y, Sato T, Shimazaki H, Hase K, Talbot IC . Annals of Surgery.2008;247(5). CrossRef
- Clinicopathological characteristics of poorly differentiated adenocarcinoma of the colon and rectum Takeuchi K, Kuwano H, Tsuzuki Y, Ando T, Sekihara M, Hara T, Asao T. Hepato-Gastroenterology.2004;51(60).
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2023
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times