Treatment Outcomes of Stereotactic Body Radiotherapy for Early-stage Non-small Cell Lung Cancer and Lung Metastasis
Download
Abstract
Background and rationale: Stereotactic body radiotherapy (SBRT) is a highly precise localized high-dose per fraction radiation treatment used mainly in lung cancer. Despite SBRT’s increasing use, no clear predictive factors of outcome exist.
Objectives: To report local control rates, patterns of failure, and incidence of treatment- related toxicity, and to determine factors predicting SBRT outcomes for primary and secondary lung tumors at Ramathibodi Hospital using competing risk analysis.
Materials and methods: This retrospective study included all patients diagnosed with primary early non-small cell lung cancer (NSCLC) and lung metastasis in our radiosurgery and radiotherapy database registry between January 2009 and September 2018.
Results: Fifty-nine patients (98 lung tumors) were studied; primary NSCLC and lung metastasis were 15.3% and 84.7%, respectively. Median follow-up was 16.8 months. The overall 1-year local control rate was 90.8%. The most common pattern of failure was distant failure (46.9%). The incidence of radiation pneumonitis (RP) grade ≥2 was 9.2%, and one of four patients with an ultra-central tumor developed grade 5 pulmonary toxicity. The predictive factor for local failure was the mean biological equivalent dose (BED) of the planning target volume (PTV), and for RP grade ≥2, the tumor’s maximal diameter. BED PTV mean <100 Gy had higher local failure than BED PTV mean ≥100 Gy (adjusted subdistribution hazard ratio 8.26; 95% confidence interval (CI) 1.76–38.68, p=0.007). Patients with tumors with maximal diameters ≥5 cm compared with those with maximal diameters <5 cm had more RP grade ≥2 (adjusted subdistribution hazard ratio 5.34; 95% CI 1.52–18.69, p=0.009). The overall 1-year survival rate was 73.5%.
Conclusions: Local control of lung tumors using SBRT was high with acceptable toxicity. BED PTV mean was a local control predictor. Large tumors correlated with symptomatic RP grade ≥2. SBRT should be used judiciously for ultra-central lung tumors.
Introduction
Stereotactic body radiotherapy (SBRT) or stereotactic ablative radiotherapy, used mainly in lung cancer, is a highly precise local radiation treatment that delivers a high dose per fraction and has a short overall treatment time. The local control rate in early-stage lung cancer and lung metastases treated with SBRT is very high, 85%–95% [1]. Several studies have established that a dose greater than 100 Gy of the prescribed biological equivalent dose (BED) with α/β = 10 (BED10) was a predictor of local control [2, 3] along with other clinical factors related to local control such as pretreatment performance status, tumor volume, total dose, and dose prescription [4-7].
Since the early 1990s, when SBRT was developed, many SBRT adverse effects have been demonstrated, ranging from mild fatigue and transient esophagitis to fatal events such as pneumonitis and pulmonary hemorrhage. Although there have been few severe adverse events reported, dosimetric factors have been the significant predictors of SBRT-related complications [8-10]. Radiation pneumonitis (RP) is very common in patients with lung tumors, but few have severe symptoms. Yamashita et al. reported that the important SBRT dose-volume factors that could predict RP were the percentage of the lung volume covered by the minimum dose of 20 Gy (V20) and the mean lung dose. Rib fracture is rare, but there is an increased interest in being able to predict this. A previous study reported that the maximum rib dose was the best predictor of rib fracture [9]. Cardiac complications are also rare but occur more frequently in patients with a history of heart problems. Stam et al. discovered that the maximum dose delivered to the left atrium and the dose delivered to 90% of the superior vena cava were the significant factors associated with non- cancer related death with lung SBRT [10].
Two preliminary studies from Ramathibodi Hospital regarding lung SBRT have been published [11, 12]. Both had the same endpoint, the tumor response, evaluated using World Health Organization criteria. These studies concluded that lung SBRT could be used acceptably.
With increasing use of SBRT, it is still unclear as to what factors can be used to predict outcomes. In this study, we reviewed the efficacy, toxicity, and predictive factors in patients with lung tumors treated with SBRT at Ramathibodi Hospital.
Materials and Methods
This retrospective cohort study was performed at Ramathibodi Hospital, a 900-bed tertiary care university hospital, and was approved by the Local Ethics Committee on Human Rights related to research involving human subjects at our institution. All patients provided written informed consent prior to SBRT.
Patients
All patients diagnosed with early-stage non-small cell lung cancer (NSCLC) or lung metastasis treated with SBRT between January 2009 and September 2018 at Ramathibodi Hospital were selected for this study. Inclusion criteria were histologically confirmed early-stage NSCLC (T1-2N0M0), lung metastasis with known primary malignancy, and Eastern Cooperative Oncology Group performance status of ≤2. Exclusion criteria were missing data and re-irradiation to the in-field region. Of the 96 patients, 37 were excluded because of missing records resulting from CyberKnife system failure (n =34) and in-field re-irradiation (n =3). The final study included 59 patients with 98 lung lesions (Figure 1), including primary NSCLC (15.3% of patients) and lung metastasis (84.7% of patients).
Figure 1. Flow Diagram. SBRT, stereotactic body radiotherapy.
Methods
All patients underwent SBRT using one of the three linear accelerators as follows:
1. CyberKnife G4 system, a lightweight linear accelerator mounted on a robotic arm (Accuray Inc., Sunnyvale, CA) with radiosurgery systems and a MultiPlan 2.0 planning system, as well as ray tracing as a treatment planning algorithm, which began being used in treating lung SBRT in January 2009.
1. Varian EDGE, a gantry-based linear accelerator with a stereotactic radiosurgery (SRS)/SBRT-based treatment system (Varian Medical Systems, Palo Alto, CA) and the Eclipse treatment planning system with the Analytical Anisotropic Algorithm (AAA), which began being used in treating lung SBRT in June 2016.
2. Varian RapidArc, a gantry-based linear accelerator, and the Eclipse treatment planning system (Varian Medical Systems) with the AAA, which began being used in treating lung SBRT in 2016.
We delineated additional structures such as a 3-cm shell outside the planning target volume (PTV); lungs minus GTV, left atrium, superior vena cava, and ribs. BED at different points of target volumes were calculated using α/β =10 with dose per fraction below 15 Gy and α/β =20 with dose per fraction higher than 15 Gy [13]. The dose was prescribed at the isodose line covering PTV and evaluated at PTV D95%.
The primary endpoint, local control rate at 1 year, and the secondary endpoints, including patterns of failure, incidence of SBRT-related toxicities, overall survival rate, factors predicting local failure, and SBRT-related complications were retrospectively reviewed from the medical records, imaging studies, and SBRT plans.
A thoracic radiation oncologist and a thoracic radiologist evaluated tumor recurrence and SBRT-related toxicities on selective imaging studies or SBRT plans. The criteria and grading for radiation pneumonitis (RP) using high-risk CT features were based on a prior publication [14].
Statistical analysis
We performed analysis of the primary endpoint, the local control rate at 1 year, using Kaplan–Meier survival and competing risk analyses with cumulative incidence curves.
For the secondary endpoints, including patterns of failure, incidence of SBRT-related toxicities, overall survival rate, factors predicting local failure, and SBRT-related complications, continuous variables are shown as medians and ranges. Categorical variables are shown as frequencies and percentages. Univariate and multivariate analyses were performed using competing risk regression.
Competing risk analysis refers to a type of survival analysis that aims to precisely estimate the marginal probability of an event in the presence of competing events. Competing risks were defined as any event preventing the occurrence of an event of interest. In our study, death before the time-to-event of interest was considered a competing event. Cumulative incidence curves were estimated. Fine and Gray’s test was used to determine the significance between curves. A P-value of ≤0.05 was considered a statistically significant finding. All statistical analyses were performed using STATA statistical Software version 14.2, StataCorp LP, College Station, TX .
Results
For the 59 patients, the overall median follow-up time was 17.7 months (5.2–67.3 months) for patients with a primary NSCLC and 16.5 months (0.1–71.7 months) for those with lung metastasis. There were variations in demographics between patients with the two types of lung tumors. The primary NSCLC group had older patients, more comorbidities, and poorer performance status compared with those in the lung metastasis group. The majority of tumor origins and tumor histopathologies were primary lung cancer (49%) and adenocarcinoma (82.7%), respectively. The median maximal diameter of the tumor was 2.3 cm (0.1–8 cm). The tumor locations of the primary NSCLCs were more central to ultra-central when compared with the lung metastasis group. There were no differences in SBRT treatment plans between patients with primary and secondary lung tumors. No thoracic surgery was performed in patients with primary lung tumors because most were medically inoperable. None of the patients received immunotherapy at that time (Table 1).
| Parameters | Primary NSCLC | Lung metastasis | Total |
| (N=15) | (N=83) | (N=98) | |
| Patient characteristics | |||
| Age, years, median (range) | 80 (54–87) | 61 (27–85) | 61 (27–87) |
| Sex, N (%) | |||
| Male | 9 (60) | 51 (61.4) | 60 (61.2) |
| Female | 6 (40) | 32 (38.6) | 38 (38.8) |
| Performance status, N (%) | |||
| ECOG PS ≤1 | 8 (53.3) | 79 (95.2) | 87 (88.8) |
| ECOG PS >1 | 7 (46.7) | 4 (4.8) | 11 (11.2) |
| Comorbid diseases, N (%) | |||
| None | 1 (6.7) | 20 (24.1) | 21 (21.4) |
| Heart | 3 (20) | 6 (7.2) | 69 (70.4) |
| Interstitial pneumonitis | 1 (6.7) | 0 | 9 (9.2) |
| DM, HT, DLP | 10 (66.7) | 41 (49.4) | 1 (1) |
| COPD | 5 (33.3) | 6 (7.2) | 11 (11.2) |
| Secondary malignancy | 7 (46.7) | 31 (37.3) | 51 (52) |
| Smoking, N (%) | |||
| <30 | 9 (60) | 74 (89.2) | 83 (84.7) |
| ≥30 | 6 (40) | 9 (10.8) | 15 (15.3) |
| Tumor characteristics | |||
| Origin, N (%) | |||
| Lung cancer | 15 (100) | 33 (39.8) | 48 (49) |
| Colorectal cancer | 0 | 27 (32.5) | 27 (27.6) |
| Other | 0 | 23 (27.7) | 23 (23.4) |
| Histology, N (%) | |||
| Adenocarcinoma | 13 (86.7) | 68 (81.9) | 6.2 (0.1–134.6) |
| Squamous cell carcinoma | 2 (13.3) | 7 (8.4) | 9 (9.2) |
| Others | 0 | 6 (7.2) | 6 (6.1) |
| Maximal diameter, cm, median (range) | 3.6 (0.8–7.4) | 2.1 (0.1–8) | 2.3 (0.1–8) |
| Maximal volume, cc, median (range) | 16.8 (2.2–134.6) | 5 (0.1–96.4) | 6.2 (0.1–134.6) |
| Lobe, N (%) | |||
| Right upper lobe | 4 (26.7) | 17 (20.5) | 21 (21.4) |
| Right middle lobe | 2 (13.3) | 17 (20.5) | 19 (19.4) |
| Left upper lobe | 4 (26.6) | 19 (22.9) | 23 (23.5) |
| Right lower lobe | 2 (13.4) | 19 (22.9) | 21 (21.4) |
| Left lower lobe | 3 (20) | 11 (13.2) | 14 (14.3) |
| Distribution, N (%) | |||
| Peripheral | 9 (53.3) | 60 (72.3) | 69 (69.4) |
| Central | 4 (26.7) | 21 (25.3) | 25 (25.5) |
| Ultra-central | 2 (13.3) | 2 (2.4) | 4 (4.1) |
| Treatments | |||
| Pre-SBRT treatments, N (%) | |||
| Thoracic surgery | 0 | 24 (28.9) | 24 (24.5) |
| Targeted therapy | 1 (6.7) | 33 (39.8) | 34 (34.7) |
| Chemotherapy | 4 (26.7) | 53 (63.9) | 57 (58.2) |
| Hormonal therapy | 2 (13.3) | 1 (1.2) | 3 (3.1) |
| Post-SBRT treatments, N (%) | |||
| Thoracic radiotherapy | 0 | 14 (16.9) | 14 (14.3) |
| Targeted therapy | 2 (13.3) | 23 (27.7) | 25 (25.5) |
| Chemotherapy | 2 (13.3) | 42 (50.6) | 44 (44.9) |
| Hormonal therapy | 2 (13.3) | 1 (1.2) | 3 (3.1) |
| Planning characteristics | |||
| BED max, Gy, median (range) | 136.2 (64.4–144.8) | 130.8 (45–191.5) | 131.6 (45–191.5) |
| BED PTV D 95%, Gy, median (range) | 100.8 (50.8–136.8) | 100.8 (38.4–152.6) | 100.8 (48.4–152.6) |
| BED PTV mean, Gy, median (range) | 114.6 (40.7–123.6) | 106.8 (25.7–158) | 109.4 (25.7–158) |
| Total dose, Gy, median (range) | 50 (37–50) | 50 (25–60) | 50 (25–60) |
| Dose per fraction, Gy, median (range) | 10 (3.7–14) | 10 (4–26) | 10 (3.7–26) |
| Isodose line, %, median (range) | 76 (70–80) | 76 (63–90) | 76 (63–90) |
| Mean EQD2 at shell 3 cm [9], Gy | 27.3 (15–32.2) | 24.5 (8–49.5) | 25.5 (8–49.5) |
| V5 lungs, % | 16.3 (6.7–37.7) | 20 (0–71.4) | 19 (0–71.4) |
| V20 lungs, % | 3.4 (1.7–12.3) | 5 (0–13.5) | 4.6 (0–23.1) |
| MLD, Gy | 3.5 (1.8–7.7) | 4.7 (0–13.5) | 4.5 (0–13.5) |
| Maximum dose of the LA, Gy | 8 (0.2–20.8) | 9.4 (0–46.4) | 9.3 (0–46.4) |
| SVC D90%, Gy | 0.7 (0.1–4.1) | 0.7 (0.1–10.4) | 0.7 (0.1–10.4) |
| Maximum dose delivered to the rib, Gy | 44.6 (31.3–61.8) | 47.2 (2.5–61.8) | 45.7 (2.5–61.8) |
NSCLC, non-small cell lung cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; DM, diabetes mellitus; HT, hypertension; DLP, dyslipidemia; COPD, chronic obstructive pulmonary disease; SBRT, stereotactic body radiotherapy; BED, biological equivalent dose; D, dose; EQD2, equivalent total dose in 2-Gy fraction; LA, left atrium; MLD, mean lung dose; SVC, superior vena cava.
3D and 4D CT simulations were used in 2009–2016 and 2016–2018, respectively. All 4D CT simulations were free breathing with 10 phases of the respiratory motion management. The GTV, PTV, and the organ at risk volumes were delineated. The 3–5 mm margin of the GTV equaled that of the PTV. Dose prescriptions varied (Table in appendices).
Local control rates
Kaplan–Meier survival analysis showed that the overall 1-year local control rate was 89.2%, and for primary and secondary lung tumors they were 91.7% and 88.7%, respectively. In the competing risk analysis, the overall 1-year local control rates were 90.8% with 93.4% for primary lung tumor and 90.1% for patients with lung metastasis, respectively (Figure 2).
Figure 2. Cumulative Incidence Failures were Plotted in Competing Risk Survival Analysis. (A) Overall 1-year local failure was 90.8%. (B) 1-year local failure in patients with primary NSCLC (solid line) and lung metastasis (dashed line) were 93.4% and 90.1%, respectively. NSCLC, non-small cell lung cancer.
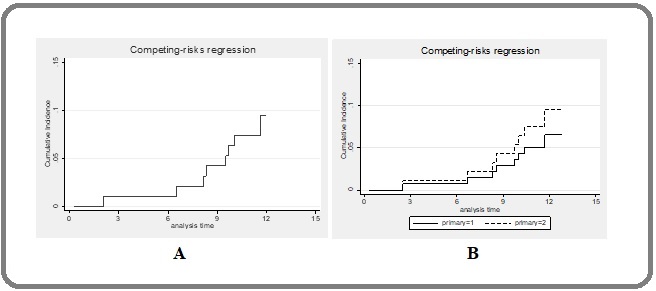
Thus, there was no substantial difference between these survival analyses.
Patterns of failure
The most common pattern of failure was distant failure, 46.9%, of which 55.4% was found in the lung metastasis group. The local and regional failure patterns were 12.2% and 6.1%, respectively. No regional or distant failures in patients with primary NSCLC were detected. The median time for locoregional failure was 16.8 months (0.1–71.7 months), and 0.3 months (0.1–67.4 months) for distant failure.
Treatment-related toxicities
For nine lung tumors (9.2%), the pulmonary toxicity RP grade ≥2 was found in eight lesions (8.2%), and pulmonary hemorrhage grade 5 was found in one lesion in a patient with an ultra-central primary NSCLC. The definition of ultra-central tumors is tumors for which the PTV touches or overlaps the following organs at risk: central bronchial tree, esophagus, pulmonary vein, or pulmonary artery 15 (Figure S1 and S2 in appendices). Radiologic change in RP without symptoms was found in 90.8% of patients. Symptomatic RP grades 1, 2, 3, and 4 were observed in 1%, 6%, 0%, and 2% of patients, respectively. There was no incidence of rib fracture or of cardiovascular toxicity (Table 2).
| Toxicity | Primary NSCLC | Lung metastasis | Total |
| (N=15) | (N=83) | (N=98) | |
| Pulmonary, N (%) | 4 (26.7) | 5 (6) | 9 (9.2) |
| - Radiation pneumonitis grade ≥2 | 3 (20) | 5 (6) | 8 (8.2) |
| - Hemorrhage | 1 (6.7) | 0 | 1 (1) |
| Cardiovascular | 0 | 0 | 0 |
| Rib fracture | 0 | 0 | 0 |
SBRT, stereotactic body radiotherapy; NSCLC, non-small cell lung cancer.
Of the four patients with an ultra-central lung tumor, one (25%) developed a pulmonary toxicity grade 5, and it was determined that a pulmonary hemorrhage was the cause of death. Reviewing the treatment plan for this patient, dose-volume constraints of the lungs, the heart, and the superior vena cava were acceptable for all parameters. However, the hot spot of the maximum dose was at the proximal bronchial tree region abutting the pulmonary vessels.
Factors predicting local failure/control
In univariate analysis, maximum BED, BED PTV mean, prescribed dose per fraction, and pre-SBRT thoracic surgery were significant predictive factors of local control (Table 3).
| Variables | SHR | 95% CI | P-value* |
| Patient characteristics | |||
| Age: ≤60 as a reference | |||
| >60 | 0.72 | 0.25–2.26 | 0.6 |
| Sex: male as a reference | |||
| Female | 0.82 | 0.25–2.69 | 0.74 |
| ECOG PS: ≤1 as a reference | |||
| >1 | 0.64 | 0.08–5.04 | 0.67 |
| Comorbidity: no as a reference | |||
| Heart | 0.65 | 0.33–1.27 | 0.2 |
| Interstitial pneumonitis | N/A | N/A | N/A |
| DM, HT, DLP | 1.24 | 0.4–3.84 | 0.7 |
| COPD | 0.52 | 0.12–2.22 | 0.37 |
| Secondary malignancy | 0.48 | 0.13–1.81 | 0.28 |
| Smoking: <30 as a reference | |||
| ≥30 | 1.05 | 0.24–4.60 | 0.94 |
| Tumor characteristics | |||
| Type: primary lung tumors as a reference | |||
| Secondary lung tumors | 2.23 | 0.28–17.51 | 0.45 |
| Origin: lung as a reference | |||
| Colorectal cancer | 0.43 | 0.09–2.10 | 0.29 |
| Others | 0.75 | 0.32–1.63 | 0.46 |
| Tumor characteristics | |||
| Pathology: adenocarcinoma as reference | |||
| Squamous cell carcinoma | 1.35 | 0.16–11.32 | 0.78 |
| Others | 1.29 | 0.43–3.86 | 0.65 |
| Maximal diameter: <5 cm as reference | |||
| ≥5 cm | 2.87 | 0.77–10.69 | 0.12 |
| Maximal volume: ≤16 cc as reference | |||
| 16 cc | 11.7 | 0.37–3.66 | 0.79 |
| Lobe: upper lobes as reference | |||
| Lower lobes | 0.59 | 0.16–2.23 | 0.44 |
| Distribution: peripheral as reference | |||
| Central | 0.32 | 0.04–2.54 | 0.28 |
| Ultra-central | 1.7 | 0.78–3.71 | 0.19 |
| Pre- and post-SBRT treatments | |||
| Pre-SBRT treatments: no as reference | |||
| Thoracic surgery | 3.55 | 1.12–11.32 | 0.03* |
| Targeted therapy | 1.31 | 0.45–4.43 | 0.56 |
| Chemotherapy | 1.18 | 0.32–.35 | 0.8 |
| Hormonal therapy | N/A | N/A | N/A |
| Post-SBRT treatments: no as reference | |||
| Thoracic radiotherapy | 1.67 | 0.52–5.34 | 0.39 |
| Targeted therapy | 0.99 | 0.30–3.24 | 0.99 |
| Chemotherapy | 0.95 | 0.51–1.78 | 0.8 |
| Hormonal therapy | N/A | N/A | N/A |
| Planning characteristics | |||
| BED max: ≥115 Gy as reference | |||
| <115 Gy | 3.94 | 1.19–13.10 | 0.03* |
| BED PTV D95%: ≥100 Gy as reference | |||
| <100 Gy | 2.06 | 0.65–6.59 | 0.22 |
| Planning characteristics | |||
| BED PTV mean: ≥100 Gy as reference | |||
| <100 Gy | 7.02 | 1.54–31.98 | 0.01* |
| Dose per fraction: ≥10 Gy/fx as reference | |||
| <10 Gy/Fx | 4.33 | 1.41–13.32 | 0.01* |
| Isodose line: ≤69% as reference | |||
| >69% | 0.34 | 0.04–3.02 | 0.33 |
| Shell 3 cm: ≥20.8 Gy as reference | |||
| <20.8 Gy | 1.4 | 0.45–4.36 | 0.57 |
*P<0.05; Considered Statistically Significant. SHR, subhazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; SBRT, stereotactic body radiotherapy; BED, biological equivalent dose; PTV, planning target volume; NSCLC, non-small cell lung cancer; DM, diabetes mellitus; HT, hypertension; DLP, dyslipidemia; COPD, chronic obstructive pulmonary disease; SBRT, stereotactic body radiotherapy; D, dose; EQD2, equivalent total dose in 2-Gy fraction; LA, left atrium; SVC, superior vena cava.
However, multivariate analysis showed that only BED PTV mean was a significant predictive factor of local control. The mean BED of the PTV <100 Gy had more local failure compared with the dose ≥100 Gy (adjusted subdistribution hazard ratio 5.41; 95% confidence interval 1.14–25.69, p=0.034) (Table 4).
| Variables | Adjusted SHR | 95% CI | P-value* |
| Pre-SBRT thoracic surgery: yes as a reference | |||
| No | 6.15 | 1.00–37.58 | 0.05 |
| BED max: ≥115 Gy as a reference | |||
| <115 Gy | 0.86 | 0.15–4.87 | 0.87 |
| BED PTV mean: ≥100 Gy as a reference | |||
| <100 Gy | 8.26 | 1.76–38.68 | 0.007* |
| Dose per fraction: ≥10 Gy/Fx as a reference | |||
| <10 Gy/Fx | 1.05 | 0.89–1.24 | 0.58 |
P<0.05; considered statistically significant. SHR, subhazard ratio; CI, confidence interval; BED, biological equivalent dose; PTV, planning target volume.
The 1-year local failure rates beween mean BED of the PTV ≥100 Gy and <100 Gy were 3% and 15.5%, respectively, p=0.034 (Figure 3A).
Figure 3. Cumulative Incidence Curves of Factors Predicting Local Failure and Radiation Pneumonitis Grade ≥2. (A) Mean BED PTV ≥100 Gy (solid line) and ≥ 100Gy (dash). (B) Maximal diameter of the tumor <5 cm (solid) and ≥5 cm (dashed line).BED, biological equivalent dose; PTV, planning target volume.
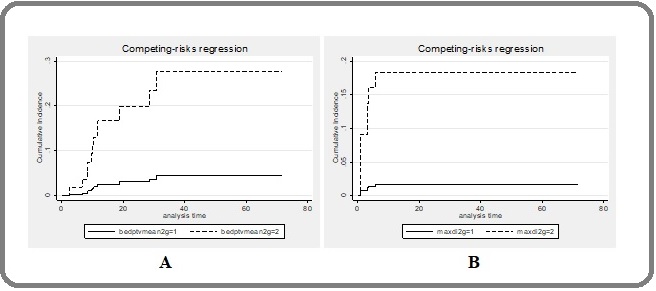
Factors predicting RP
Univariate analysis showed that comorbidities such as diabetes mellitus, hypertension, and dyslipidemia, as well as tumor origin, histopathology, maximal diameter of the tumor, maximal volume of the tumor, V5 lungs, and mean lung dose were factors predicting ≥grade 2 RP (Table 5).
| Variables | SHR | 95% CI | P-value* |
| Patient characteristics | |||
| Age: ≤60 as reference | |||
| >60 | 0.45 | 0.11–1.81 | 0.26 |
| Sex: male as reference | |||
| Female | 2.75 | 0.69–11.07 | 0.15 |
| ECOG PS: ≤1 as a reference | |||
| >1 | 1.11 | 0.15–8.41 | 0.92 |
| Comorbidity: no as a reference | |||
| Heart | N/A | N/A | N/A |
| Interstitial pneumonitis | N/A | N/A | N/A |
| DM, HT, DLP | 0.12 | 0.02–0.97 | 0.047* |
| COPD | 0.83 | 0.66–1.03 | 0.09 |
| Secondary malignancy | 0.77 | 0.69–11.11 | 0.15 |
| Smoking: <30 as a reference | |||
| ≥30 | 1.86 | 0.40–8.69 | 0.43 |
| Tumor characteristics | |||
| Type: primary lung tumors as a reference | |||
| Secondary lung tumors | 0.3 | 0.07–1.16 | 0.08 |
| Origin: lung as a reference | |||
| Colorectal and other cancers | 0.99 | 0.25–3.83 | 0.99 |
| Pathology: adenocarcinoma as a reference | |||
| Squamous cell carcinoma | 8.44 | 2.18–32.74 | 0.002* |
| and others | |||
| Maximal diameter: <5 cm as a reference | |||
| ≥5 cm | 12.33 | 1.54–98.68 | 0.02* |
| Maximal volume: ≤16 cc as a reference | |||
| 16 cc | 4.9 | 1.00–24.29 | 0.05 |
| Lobe: upper lobes as a reference | |||
| Lower lobes | 0.6 | 0.12–2.98 | 0.53 |
| Distribution: peripheral as a reference | |||
| Central and ultra-central | 2.31 | 0.59–9.01 | 0.23 |
| Variables | SHR | 95% CI | P-value* |
| Pre- and post-SBRT treatments | |||
| Pre-SBRT treatments: no as reference | |||
| Thoracic surgery | N/A | N/A | N/A |
| Targeted therapy | 0.25 | 0.03–1.92 | 0.18 |
| Chemotherapy | 0.64 | 0.16–2.57 | 0.53 |
| Hormonal therapy | N/A | N/A | N/A |
| Post-SBRT treatments: no as reference | |||
| Thoracic radiotherapy | 3.79 | 1.01–14.27 | 0.049* |
| Targeted therapy | 0.27 | 0.03–2.11 | 0.21 |
| Chemotherapy | 0.9 | 0.42–1.96 | 0.8 |
| Hormonal therapy | N/A | N/A | N/A |
| Planning characteristics | |||
| BED max: ≥115 Gy as reference | |||
| <115 Gy | N/A | N/A | N/A |
| BED PTV D95%: ≥100 Gy as reference | |||
| <100 Gy | 0.62 | 0.13–3.06 | 0.56 |
| BED PTV mean: ≥100 Gy as reference | |||
| <100 Gy | N/A | N/A | N/A |
| Planning characteristics | |||
| Dose per fraction: ≥10 Gy/Fx as reference | |||
| <10 Gy/Fx | N/A | N/A | N/A |
| Isodose line: ≤69% as reference | |||
| >69% | 0.34 | 0.04–3.02 | 0.33 |
| Shell 3 cm: ≥20.8 Gy as reference | |||
| <20.8 Gy | N/A | N/A | N/A |
| V5 lungs: <30% as reference | |||
| ≥30% | 6.42 | 1.37–30.11 | 0.02* |
| V20 lungs: <10% as reference | |||
| ≥10% | 20.45 | 2.63–159.06 | 0.004* |
| MLD: <6 Gy as reference | |||
| ≥6 Gy | 6.74 | 1.44–31.58 | 0.02* |
*P<0.05; considered statistically significant. SHR, subhazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; DM, diabetes mellitus; HT, hypertension; DLP, dyslipidemia; COPD, chronic obstructive pulmonary disease; SBRT, stereotactic body radiotherapy; BED, biological equivalent dose; PTV, planning target volume; MLD, mean lung dose.
Multivariate analysis found that the only significant predictive factor for pneumonitis was the maximal diameter of the tumor. Patients with tumors with a maximal diameter ≥5 cm had a higher rate of ≥grade 2 RP (adjusted subdistribution hazard ratio 5.34 (95% confidence interval 1.52–18.69), p=0.009 (Table. 6).
| Variables | Adjusted | 95% CI | P-value* |
| SHR | |||
| Pathology: adenocarcinoma as a reference | |||
| Squamous cell carcinoma and others | 2.33 | 0.48–11.34 | 0.29 |
| Maximal diameter: <5 cm as a reference | |||
| ≥5 cm | 5.34 | 1.52–18.69 | 0.009* |
| V5 lungs: <30% as a reference | |||
| ≥30% | 4.69 | 0.8–27.69 | 0.09 |
*P<0.05; considered statistically significant. SBRT, stereotactic body radiotherapy; SHR, subhazard ratio; CI, confidence interval.
Incidence of RP was 18% when the maximal diameter of the tumor was ≥5 cm and 2% when they were <5 cm, p=0.009 (Figure 3B).
Overall survival
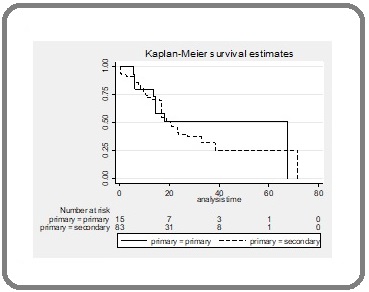
The 1-year overall survival was 73.5%; 80% for patients with a primary NSCLC, and 72.3% for those with a lung metastasis. The median overall survival was 16.8 months (0.1–71.7 months) (Figure 4).
Figure 4.Kaplan–Meier Survival Analysis of Overall Survival in Patients with Primary NSCLC (solid line) and Lung Metastasis (dashed line). NSCLC, non-small cell lung cancer.
Discussion
We analyzed the data using Kaplan–Meier survival estimates and competing risk analysis, and local control rates at 1 year after lung SBRT were 89.2 % and 90.8%, respectively. Our results were comparable with those from previous studies [3, 16]. Patterns of failure were also similar with those reported previously [4, 5, 17]. The most common pattern of failure was still distant failure (46.9%). Siva et al. [7] discussed novel therapies, especially immunotherapy combined with SBRT, that would reduce distant failure with the abscopal effect; however, it remained unclear as to the physio-biological mechanism. Radiation dose was still a factor in predicting local outcomes in his study.
In our study, we found that the BED PTV mean was the only factor predicting local failure. In 2014, Kestin et al. [18] studied the dose-response relationship for local tumor control in early-stage NSCLC by calculating the area under the curve of the prescribed BED, BED PTV mean, and BED GTV mean parameters. The first two highest area under the curves were the prescribed BED and the BED PTV mean. Thus, the study reported that these two parameters had the highest correlation with local recurrence. In 2016, a study identifying the optimal dose parameters predictive of local/lobar control after SBRT in early-stage NSCLC was conducted [19]. Using competing risk analysis, minimum BED PTV D95% was determined to be a statistically significant factor predicting local recurrence, while the BED PTV mean trended towards significance as one factor (p=0.05). Because of intensity- modulated radiation therapy- or volumetric-modulated arc therapy-approached SBRT era, the dose within the PTV was much more homogeneous than 3D conformal radiotherapy (3D-CRT)-approached SBRT. Thus, they further analyzed the correlation between local control and both dose indexes, minimum BED PTV D95%, and BED PTV mean and found that BED PTV mean was highly correlated with BED PTV D95%. The conclusion was that both dose indexes should be considered for SBRT plan optimization.
Most patients tolerated SBRT-related toxicity. The incidence of RP grade ≥2 was 8.2%, which was comparable to the historical data [3]. In reviewing the treatment plan of the patient who developed grade 4 RP, V20 lungs and mean lung dose were higher than the dose constraints in the reviewed literature [3], but they were not found to be predictive factors of symptomatic RP grade ≥2. In our study, we found that the maximal diameter of a tumor was a statistically significant factor predicting RP, which correlated with the historical data in that the larger the tumor, the higher the incidence of RP [20, 21]. A study in 2018 reported that tumor size might not impact RP directly but that it correlated with the ipsilateral lung dose that was most strongly correlated with symptomatic RP [22]. Thus, further study will be needed to understand these confounding correlations.
There have been several reports exploring the cause of death in patients with ultra-central lung tumors [2, 23]. Pulmonary hemorrhage and cardiac condition, such as myocardial infarction and congestive heart failure, were common causes of death. However, one study found that a high-risk indicator for grade 5 treatment-related mortality was administering a maximum BED3 ≥180 Gy3 at the proximal bronchial tree [23]. In our study, of the four patients with ultra-central lung tumors, three received a proximal bronchial BED3 <160 Gy; however, the fourth patient who died received a dose of 243.9 Gy at the proximal bronchial tree area, which may have been the cause of their grade 5 pulmonary toxicity.
Limitations of the study
Our study had some limitations. This was a retrospective study, and data for several patients were missing. In addition, the sample size was small with a limited number of events that made it difficult to predict outcomes accurately.
In conclusions, the local control rate of early NSCLC and lung metastasis using SBRT was high. Our results were comparable to historical data with acceptable toxicities. We found that BED PTV mean was the best predictive factor of local tumor control and that tumors with a maximal diameter ≥5 cm might lead to a RP grade ≥2.
Because there was the possible risk of developing grade 5 treatment-related mortality, SBRT should be used with caution for ultra-central lung tumors.
References
- Systemic review of the patterns of failure following stereotactic body radiation therapy in early-stage non-small-cell lung cancer: clinical implications Chi A, Liao Z, Nguyen NP , Xu J, Stea B, Komaki R. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology.2010;94(1). CrossRef
- Outcomes and toxicity of stereotactic body radiation therapy for advanced stage ultra-central non-small cell lung cancer Cong Y, Sun B, Wang J, Meng X, Xuan L, Zhang J, Liu J, Shen G, Wu S. Thoracic Cancer.2019;10(7). CrossRef
- Local tumor control probability modeling of primary and secondary lung tumors in stereotactic body radiotherapy Guckenberger M, Klement RJ , Allgäuer M, Andratschke N, Blanck O, Boda-Heggemann J, Dieckmann K, et al . Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology.2016;118(3). CrossRef
- Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study Onishi H, Araki T, Shirato H, Nagata Y, Hiraoka M, Gomi K, Yamashita T, et al . Cancer.2004;101(7). CrossRef
- Steep dose-response relationship for stage I non-small-cell lung cancer using hypofractionated high-dose irradiation by real-time tumor-tracking radiotherapy Onimaru R, Fujino M, Yamazaki K, Onodera Y, Taguchi H, Katoh N, Hommura F, Oizumi S, Nishimura M, Shirato H. International Journal of Radiation Oncology, Biology, Physics.2008;70(2). CrossRef
- Planning benchmark study for SBRT of early stage NSCLC : Results of the DEGRO Working Group Stereotactic Radiotherapy Moustakis C, Blanck O, Ebrahimi Tazehmahalleh F, Ka Heng Chan M, Ernst I, Krieger T, Duma M, et al . Strahlentherapie Und Onkologie: Organ Der Deutschen Rontgengesellschaft ... [et Al].2017;193(10). CrossRef
- Stereotactic Ablative Body Radiotherapy for Lung Metastases: Where is the Evidence and What are We Doing With It? Siva S, Slotman BJ . Seminars in Radiation Oncology.2017;27(3). CrossRef
- Radiation pneumonitis after stereotactic radiation therapy for lung cancer Yamashita H, Takahashi W, Haga A, Nakagawa K. World Journal of Radiology.2014;6(9). CrossRef
- Dose-effect analysis of radiation induced rib fractures after thoracic SBRT Stam B, Bijl E, Peulen H, Rossi MMG , Belderbos JSA , Sonke JJ . Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology.2017;123(2). CrossRef
- Dose to heart substructures is associated with non-cancer death after SBRT in stage I-II NSCLC patients Stam B, Peulen H, Guckenberger M, Mantel F, Hope A, Werner-Wasik M, Belderbos J, et al . Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology.2017;123(3). CrossRef
- Preliminary experience of CyberKnife treatment of primary non-small cell lung cancer Swangsilpa T, Yongvithisatid P, Pairat K, Dechsupa P, Dhanachai M, Dangprasert S, Narkwong L, Sitathanee C, et al . Journal of the Medical Association of Thailand = Chotmaihet Thangphaet.2012;95(10).
- Preliminary experience of CyberKnife treatment of lung metastasis: the question about real clinical benefit Swangsilpa T, Yongvithisatid P, Pairat K, Dechsupa P, Dhanachai M, Dangprasert S, Narkwong L, et al . Journal of the Medical Association of Thailand = Chotmaihet Thangphaet.2013;96(5).
- What would be the most appropriate α/β ratio in the setting of stereotactic body radiation therapy for early stage non-small cell lung cancer Chi A, Wen S, Liao Z, Fowler J, Xu J, Nguyen NP , Welsh JS , Komaki R. BioMed Research International.2013;2013. CrossRef
- High-risk CT features for detection of local recurrence after stereotactic ablative radiotherapy for lung cancer Huang K, Senthi S, Palma DA , Spoelstra FOB , Warner A, Slotman BJ , Senan S. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology.2013;109(1). CrossRef
- SUNSET: Stereotactic Radiation for Ultracentral Non-Small-Cell Lung Cancer-A Safety and Efficacy Trial Giuliani M, Mathew AS , Bahig H, Bratman SV , Filion E, Glick D, Louie AV , et al . Clinical Lung Cancer.2018;19(4). CrossRef
- Outcomes after stereotactic body radiotherapy for lung tumors, with emphasis on comparison of primary lung cancer and metastatic lung tumors Yamamoto T, Jingu K, Shirata Y, Koto M, Matsushita H, Sugawara T, Kubozono M, et al . BMC cancer.2014;14. CrossRef
- Long-term Results of Stereotactic Body Radiation Therapy in Medically Inoperable Stage I Non-Small Cell Lung Cancer Timmerman RD , Hu C, Michalski JM , Bradley JC , Galvin J, Johnstone DW , Choy H. JAMA oncology.2018;4(9). CrossRef
- Dose-response relationship with clinical outcome for lung stereotactic body radiotherapy (SBRT) delivered via online image guidance Kestin L, Grills I, Guckenberger M, Belderbos J, Hope AJ , Werner-Wasik M, Sonke J, et al . Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology.2014;110(3). CrossRef
- Planning Target Volume D95 and Mean Dose Should Be Considered for Optimal Local Control for Stereotactic Ablative Radiation Therapy Zhao L, Zhou S, Balter P, Shen C, Gomez DR , Welsh JD , Lin SH , Chang JY . International Journal of Radiation Oncology, Biology, Physics.2016;95(4). CrossRef
- Clinical and dosimetric predictors of radiation pneumonitis in a large series of patients treated with stereotactic body radiation therapy to the lung Baker R, Han G, Sarangkasiri S, DeMarco M, Turke C, Stevens CW , Dilling TJ . International Journal of Radiation Oncology, Biology, Physics.2013;85(1). CrossRef
- Predictive parameters of symptomatic radiation pneumonitis following stereotactic or hypofractionated radiotherapy delivered using volumetric modulated arcs Bongers EM , Botticella A, Palma DA , Haasbeek CJA , Warner A, Verbakel WFAR , Slotman B, Ricardi U, Senan S. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology.2013;109(1). CrossRef
- Impact of Tumor Size on Local Control and Pneumonitis After Stereotactic Body Radiation Therapy for Lung Tumors Parker SM , Siochi RA , Wen S, Mattes MD . Practical Radiation Oncology.2019;9(1). CrossRef
- Safety and Effectiveness of Stereotactic Ablative Radiotherapy for Ultra-Central Lung Lesions: A Systematic Review Chen H, Laba JM , Zayed S, Boldt RG , Palma DA , Louie AV . Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer.2019;14(8). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2024
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times