A Prospective, Multicenter, Observational Registry (ReCoRD) on Demography, Molecular Profile, Clinical Features, and Treatment Outcomes in Individuals with Metastatic Colorectal Cancer
Download
Abstract
Background and objective: Globally, approximately 20% of newly diagnosed colorectal cancer cases present with metastasis, and 25% develop metastasis later. The 5-year survival rate for patients with metastatic colorectal cancer (mCRC) is less than 20%. While many studies using population-based cancer registries from India are available for various cancers, data on mCRC in India is scarce. The ReCoRD registry aims to gather real-world data on demographics, treatment patterns, and outcomes in Indian patients with mCRC.
Materials and Methods: This was a prospective, multicenter, observational study enrolling 1000 participants with mCRC across up to 15 centers for a period of two years. Recruitment ceased two years after the first patient enrollment. A minimum follow-up of 12-18 months was conducted post-enrollment. Data on demographics, clinical features, molecular profile, treatment options, and treatment outcomes were collected from the study sites by investigators. The Kaplan-Meier method was used to analyze survival data, and the median time computed by this method was presented with a 95% confidence interval.
Conclusion: While mCRC remains largely incurable, survival rates have improved with the development of newer cytotoxic chemotherapeutic drugs and targeted agents. The ReCoRD registry’s comprehensive data on demographics, tumor characteristics, molecular aspects, treatment, and treatment outcomes in individuals with mCRC can guide clinicians in decision-making and treatment based on Indian patient data.
Introduction
Globally, colorectal cancer (CRC), the third-most prevalent cancer, has the second-highest mortality rate among all types of cancers [1]. According to Global Cancer Observatory (Globocan) 2020, globally, there were 1,931,590 CRC (10% of all cancers) cases in 2020. A total of 935,173 deaths due to CRC were recorded in 2020, which accounted for 9.4% of the total cancer-related deaths [2]. A higher occurrence of CRC is observed in middle- and high-income countries [1]. India contributes 6.86% to the global burden of cancer (Globocan 2020) [3]. In India, CRC ranks 5th following breast, lip, and oral cavity, cervix uteri, and lung cancer, respectively [4]. Although CRC has a low age-standardized rate in India (5.1/per 100,000 population for women and 7.2/per 100,000 population for men), the absolute number of patients with CRC is still large considering the country’s population, which is more than a billion [5]. Poor prognosis due to delayed presentation can be avoided in the case of CRC, a largely preventable cancer, with better screening methods [6].
Epidemiological data on metastatic CRC (mCRC) is scarce. Out of all newly diagnosed CRC cases, 20% of the patients present with metastasis (synchronous metastasis) at diagnosis, and 25% develop metastasis later (metachronous metastasis). The 5-year survival rate after diagnosis is <20% for mCRC patients [7,8].
Around 70% of mCRC patients present with liver metastasis, and 15%–20% with metastasis in the distant lymph nodes, lungs, and peritoneum. Metastasis to the liver is common in both colon cancer (71%) and rectal cancer (60%). A retrospective cohort study by Holch et al. demonstrated that peritoneal metastasis was six times more common in colon cancer than in rectal cancer, and metastasis in the lungs was twice as common in rectal cancer compared to colon cancer [7]. The prognosis in mCRC patients is determined by the number of metastatic sites and localization of metastasis [7,8]. Genomic profiling identifies somatic variants, which play a role in determining personalized treatment for mCRC patients. In patients with mCRC, who possess Kirsten rat sarcoma viral oncogene (KRAS)/neuroblastoma ras viral oncogene homolog (NRAS)/B-type Raf proto-oncogene (BRAF) wild-type tumors, treatment with epidermal growth factor receptors (EGFR) such as panitumumab and cetuximab, and chemotherapy can extend the survival time by 2–4 months compared to chemotherapy alone. In patients having a BRAF V600E mutation EGFR, and BRAF inhibitors extend the survival time, while immunotherapy improves the treatment outcome in patients demonstrating microsatellite instability. This emphasizes the importance of molecular profiling in directing treatment specific to an individual [8].
Considering the complex and multidisciplinary nature of therapy required to manage mCRC, an opinion from a specialist and tertiary care center is desirable. Deciding on palliative or curative therapy is important for mCRC patients and depends on the tumor burden. In patients presenting with oligometastases, resection may be the most suitable option. The use of conversion therapies to reduce the size of tumors is also gaining significant importance. Fluoropyrimidines, irinotecan, and oxaliplatin are the preferred chemotherapeutic agents either in three-drug or two-drug regimens. Additionally, a biologic is chosen depending on the patient and tumor-specific factors. Based on the patient’s presentation, mCRC typically needs multiple types of therapy [9].
Data obtained from state cancer registries form the backbone of most cancer control programs by allowing for informed decisions regarding the allocation of resources to specific populations. Furthermore, surveillance on the patterns of occurrence of cancer guides activities aimed at cancer prevention at the local, state and national levels. The challenges faced in cancer control are unique to every state, and population-specific measures promoting cancer survival can be made based on data from high-quality cancer registries. Such data identify gaps in cancer diagnosis and management, present a comprehensive picture of the cancer burden, and support public health efforts toward cancer prevention, improving quality of life, and extending survival times [10].
Numerous studies using population-based cancer registries from India are available on various cancers [11].
However, data on mCRC in India is scarce. Therefore, the purpose of the ReCoRD registry is to obtain real world data on demographics, treatment pattern and outcomes in Indian patients with mCRC.
Rationale
The registry will help to generate and evaluate the real-world data about demographics, clinical profile, molecular profile, treatment characteristics and treatment outcomes in mCRC patients undergoing treatment:
• to assess the effectiveness of first-line treatment
• to understand variations in treatment and outcomes;
• to examine factors that influence prognosis; and
• to observe the course of the disease.
Objectives
The objectives of this trial are:
1. To understand the clinical profile (e.g., baseline demographics, clinical presentations, comorbid conditions, and performance status) of mCRC patients undergoing treatment.
2. To understand tumor characteristics (tumor location, site of metastasis, number of metastatic sites, molecular profile (Kirsten ras [KRAS] including KRAS G12C, v-raf murine sarcoma viral oncogene homolog B1 [BRAF], microsatellite instability/microsatellite stable [MSI/MSS], and human epithelial growth factor receptor 2 [HER2]) of mCRC patients undergoing treatment.
3. To understand the current treatment pattern of mCRC patients.
4. To understand treatment outcomes in mCRC patients undergoing treatment.
Trial design
This is a prospective, multicenter, observational (non-interventional) study.
Materials and Methods
Study setting
The study intends to include 1000 participants with mCRC enrolled in the registry in up to 15 centers for 2 years. The recruitment will be stopped 2 years following the first patient enrollment. A minimum follow-up of 12–18 months will be carried out post-enrollment in the registry. Table 1 mentions key aspects of the registry.
| Key Aspect | Details |
| Trial identifier and registry name | DRL-IND-GGI-010-MCRC/2022 |
| CTRI registration number | CTRI/2022/07/043968 |
| Protocol version | 1 |
| Version date | 22-Feb-22 |
| Sponsor and contact for public and scientific queries | Dr. Rahul Rathod |
| Head – Clinical Operations and Ideation, | |
| Dr. Reddy’s Laboratories Limited | |
| Address: #7-1-27, Ameerpet, | |
| Hyderabad – 500016 | |
| Rahul.Rathod@drreddys.com | |
| 040-49048400 |
CTRI, Clinical Study Registry India
The patients are attended to as per the local standards of care, along with the collection of the necessary data. All participating hospitals need to notify new patients about the registry till the registry is terminated. Data collection and storage are conducted via a customized electronic case report form (eCRF) (web-based), which is available to the source data providers, followed by a 1-year telephonic follow-up to collect the survival status of enrolled participants.
Eligibility criteria
Inclusion criteria
1. Metastatic adenocarcinoma of the colon or rectum
2. Inoperable and eligible for first-line systemic therapy
3. Age ≥18 years
4. Signed informed consent
Exclusion criteria
1. Participants who received their treatment at study sites before enrollment except patients with metachronous mCRC
2. Non-metastatic CRC
Data on demography, clinical presentation, family history, tumor characteristics, and treatment plan from the patient’s medical records are collected by the designated staff and this data-collection process is being supervised by investigators/site coordinators. Data on patient demographics and baseline clinical characteristics such as age, sex, height, weight, annual income, treatment expense (out of pocket/medical insurance), presence of risk factors, clinical signs and symptoms at presentation, comorbid conditions, diagnostic test/method, baseline laboratory values, tumor marker, and Eastern Cooperative Oncology Group (ECOG) performance status are being collected [12]. Tumor characteristics such as tumor pathology, tumor location, metastatic lesion details, and tumor staging are to be noted. Molecular marker details such as the presence of rat sarcoma (RAS) mutation, including KRAS, KRAS G12C, NRAS, and BRAF mutation; NTRK; MSI/MSS status; HER2 mutation; or any other mutation as noted in medical records are being collected as well. Treatment details such as first-line/upfront therapy, treatment assessment, and details during follow-up visits will be noted. The response assessment, such as treatment outcomes, will be followed up. The flowchart with the steps to be followed in the trial is depicted in Figure 1.
Figure 1. Flowchart Indicating the Various Steps to be Followed in the Trial.
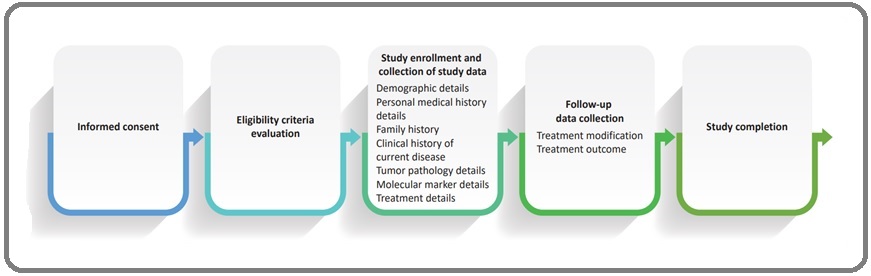
The schedule of data collection is mentioned in Table 2.
| Study procedures | Enrolment and baseline data collection | Follow-up data collection (~3–6 months) | End of registry (12–18 months) |
| Informed consent | √ | ||
| Inclusion/exclusion criteria | √ | ||
| Medical and surgical history (including history related to diagnosis of mCRC) | √ | ||
| Prior treatment details for CRC | √ | ||
| Demographic details | √ | ||
| Comorbid conditions (diabetes, HTN, etc) | √ | ||
| Height, body weight (kg), and BMI | √ | ||
| Tumor pathology | |||
| Molecular marker details | √ | √ | √ |
| Laboratory report details for mCRC | √ | √ | √ |
| Current treatment details for mCRC | √ | ||
| Treatment modification/ treatment changes for mCRC | √ | √ | |
| Treatment outcome | √ | √ |
Sample Size
All subjects enrolling in the study with at least one follow-up data collection completed post-first-line therapy are to be considered for analysis.
Sample size justification: Since this is a real-world disease registry, formal sample size calculation has not been planned. To assess the registry objectives, up to 1000 participants with mCRC will be enrolled in the registry from a maximum of 15 centers for 2 years. The enrollment will be stopped 2 years from the date of the first patient enrollment in the registry or once 1000 participants are recruited, whichever occurs first.
Study withdrawal
Patients may stop participating in the trial without justification and without affecting the right to medical care thereafter. In addition, the study investigator may withdraw such patients’ participation if needed.
Reasons for withdrawal
The patient does not fulfill the necessary criteria for participating in the study.
Patients’ voluntary withdrawal of consent.
Study termination by the sponsor.
The patient does not attend follow-up visits. Other reasons not included above.
Statistical Analysis
Continuous data such as age and family income will be represented by the number of observations (n), mean, standard deviation (SD), median, minimum (min), and maximum (max). In addition, categorical data (e.g., gender and state) will be depicted with count and percentage (%) in each category.
Various parameters of tumor characteristics (tumor location, site of metastasis, number of metastatic sites, and molecular profile [KRAS including KRAS G12C, BRAF, MSI/MSS, HER2]) will be presented descriptively. The presence or absence of RAS mutation, including KRAS G12C, BRAF mutation, MSI/MSS status, HER2 mutation, or any other mutation listed in medical records, will be presented using count (%). The symptoms at the presentation of metastatic disease, side involved (right- or left-sided cancer), and ECOG performance status will be presented using count and percentage. Descriptive statistics will be used to present the number of metastatic sites and treatment duration. Discontinuation of treatment, the reason for such discontinuation, and all spontaneously reported adverse events will be presented descriptively. The laboratory results will also be presented descriptively using n, mean, median, min, and max. Imputation for missing data will not be done and will be presented as missing data.
The Kaplan-Meier Plot will be utilized to examine the survival data, and the median time computed by the Kaplan-Meier technique will be presented with a confidence interval of 95%.
If there is a need for additional statistical analysis, the appropriate analysis will be conducted and changes will be mentioned in the Statistical Analysis Plan amendment.
Approval by the participating sites’ Institutional Review Board and the Ethics Committees (IRB ECs) will be sought before the study begins and all local and national legal requirements concerning clinical studies will be followed.
The current study is registered with the Clinical Study Registry-India (www.ctri.nic.in). After the completion of the study, one or more manuscripts for joint publications will be prepared in collaboration among the investigators.
Discussion
Globally, over 1.9 million cases of CRC were reported in 2020, per Globocan 2020 [2]. Colorectal cancer is a common gastrointestinal malignancy in Indian men and women [3]. Around 15%–30% of patients with CRC present with metastasis commonly involving the liver, lung, peritoneum, and lymph nodes [1].
The overall survival rate of patients with mCRC depends on tumor sidedness, the metatstatic and the mutation status. In cases of oligometastatic mCRC, a short course of radiotherapy followed by surgery is recommended. Liver metastasis requires a multi-modality approach such as chemotherapy and local ablative technique/radiofrequency ablation. For larger metastatic lesions of the liver or lesions close to blood vessels, microwave ablation or stereotactic radiotherapy is preferred. Managing lung metastasis is more challenging and involves resection, radiofrequency ablation, and radiotherapy. Peritoneal metastasis is by far not treatable. However, hyperthermic intraperitoneal chemotherapy and cytoreductive surgery improve the survival time in patients with peritoneal metastasis. A systematic review has demonstrated that a difference exists in the prevalence of mutations in BRAF and KRAS genes according to the location of the tumor. These mutations were observed more often in right-sided colon cancer compared to cancer on the left side. Moreover, epidemiological, molecular, and clinical characteristics differ based on the side affected by the primary tumor. A worse prognosis is observed in right-sided colon tumors than in left-sided tumors. Additionally, National Comprehensive Cancer Network (NCCN) guidelines have specifically recommended EGFR inhibitors in mCRC on the left side and in KRAS/BRAF/NRAS wild-type mCRC. There is no specific recommendation for the treatment of right-sided and KRAS/BRAF/ NRAS-mutant mCRC. This is important considering the management of mCRC is shifting toward targeted therapy and personalized medicine [13]. Characteristics of the tumor, the patient, and the treatment drive the therapeutic choices in the first line setting. Tumor mutations such as BRAF and RAS as well as tumor biology are important factors in deciding the treatment. Doublet chemotherapy with EGFR antibody and, additionally, FOLFOXIRI and bevacizumab in selected patients, is the treatment of choice in RASwt or BRAFwt patients. Left-sided tumors, in RAS wt or BRAFwt patients, benefit the most from EGFR antibody treatment along with chemotherapy or from bevacizumab with chemotherapy. The tumors on the right side in these patients are associated with a worse prognosis irrespective of the treatment they received as the first line. However, the choice of treatment in these patients would be bevacizumab with chemotherapy triplet (FOLFOXIRI) [14, 15].
Chemotherapy remains the backbone in the management of mCRC. Most patients demonstrate tumor shrinkage and 15%–20% of patients demonstrate tumor resolution following chemotherapy. Chemotherapy is usually administered 8–10 weeks post-surgery. Additionally, fluoropyrimidines, irinotecan, oxaliplatin, and biologics are used in the management of mCRC [9]. Although mCRC remains incurable in most cases, survival rates have improved with the advent of newer cytotoxic chemotherapeutic drugs and targeted agents [16].
Differences in the cancer profiles in different countries and regions demonstrate the existence of significant geographic diversity. This is due to distribution patterns influenced by lifestyles, physical activity, environmental determinants, occupation, and other risk factors. The prevalence of risk factors differs across populations and accounts for the varying prevalence of cancer in different geographic regions. In lower- and middle-income countries (LMIC) poverty-related and infection-related cancers are more, whereas in high-income countries cancer is related to lifestyle. The burden of cancer is the highest in LMIC with a trend toward a further increase in cancer cases in the future. An appraisal of this situation helps in designing a framework for action. Registries on cancer collect data over a duration of time. They do so in a systematic manner and provide cancer statistics which by estimating current trends, and future evolution aid in the assessment and control of cancer in the local population. The profile and magnitude of cancer are evaluated by the incidence, prevalence, years of life lost, disability-adjusted life years, and mortality. Such registries also help in monitoring the success of cancer interventions [10, 17].
In India, cancer is a major public health concern and therefore, the National Cancer Registry Program (NCRP) was started by the Indian Council of Medical Research.
The population-based cancer registries collect data on the incidence, trend, and mortality, and help in planning cancer control programs to reduce the cancer burden in the community [18]. The challenge is that cancer registries cover less than 15% of the Indian population. Expanding the network of cancer registries in India and developing a strong infrastructure for surveillance would be a key step to bridging the gap [19].
The ReCoRD registry aims to assess the characteristics of mCRC in India, which may help in informed decision- making based on real-world safety and effectiveness data. In conclusion, metastatic colorectal cancer is associated with poor survival rates. The molecular profile of patients with mCRC helps determine their treatment and treatment outcomes. Several registries on various other types of cancer are available; however, data on the demography, molecular profile, clinical features, and treatment outcomes in mCRC cases in India are scarce. The ReCoRD registry intends to obtain real-world data on demographics, treatment pattern and outcomes in Indian
patients with mCRC.
Acknowledgements
General
We would like to thank BioQuest Solutions for the supporting with manuscript development and editorial assistance.
Funding Statement
The financial support for the study was provided by Dr. Reddy’s Laboratories Limited.
Conflict of Interest
Sunil Kumar Yadav Y, Rohit Desai, Pranav Sopory, Femina Dawer, Rajan Mittal, Kumar Gaurav, Rahul Rathod, Akhila Paspulate are employees of Dr Reddy’s Laboratories, Hyderabad, Telangana, India.
Ethical Declaration
Ethics approval for the study was obtained at each participating centre. The institutional ethics committees at each of the following centres approved the study:
• AIG Hospitals, Hyderabad
• MVRCCRI, Calicut
• Indo American Hospital, Hyderabad
• Sahyadri Hospital, Pune
• Manipal Hospitals, Bangalore
• RGCIRC, New Delhi
• Regency Hospital, Kanpur
• Sushrut Hospital, Mumbai
Authors Contribution
All authors have contributed equally to the conception, design, drafting, review and approval of manuscript.
Data Availability
This is not applicable as this is protocol and not Research study.
Study Registration
This protocol was registered at Clinical Study Registry India - CTRI/2022/07/043968
References
- Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials Arnold D, Lueza B, Douillard JY , Peeters M, Lenz HJ , Venook A, Heinemann V, et al . Annals of Oncology: Official Journal of the European Society for Medical Oncology.2017;28(8). CrossRef
- Incidence of colorectal cancers in India: A review from population-based cancer registries Asthana S, Khenchi R, Labani S. Current Medicine Research and Practice.2021;11(2). CrossRef
- Population Based Cancer Registry of India – the Challenges and Opportunities Behera P, Patro BK . Asian Pacific journal of cancer prevention: APJCP.2018;19(10). CrossRef
- Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review Biller LH , Schrag D. JAMA.2021;325(7). CrossRef
- Primary tumor sidedness has an impact on prognosis and treatment outcome in metastatic colorectal cancer: results from two randomized first-line panitumumab studies Boeckx N, Koukakis R, Op de Beeck K, Rolfo C, Van Camp G, Siena S, Tabernero J, et al . Annals of Oncology: Official Journal of the European Society for Medical Oncology.2017;28(8). CrossRef
- Prevalence of RAS and BRAF mutations in metastatic colorectal cancer patients by tumor sidedness: A systematic review and meta-analysis Bylsma LC , Gillezeau C, Garawin TA , Kelsh MA , Fryzek JP , Sangaré L, Lowe KA . Cancer Medicine.2020;9(3). CrossRef
- Cancer Registration in India - Current Scenario and Future Perspectives Chatterjee S, Chattopadhyay A, Senapati SN , Samanta DR , Elliott L, Loomis D, Mery L, Panigrahi P. Asian Pacific journal of cancer prevention: APJCP.2016;17(8).
- Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, Seligmann J, et al . Annals of Oncology: Official Journal of the European Society for Medical Oncology.2023;34(1). CrossRef
- Colorectal cancer: Globocan 2020 Available at: https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf. Accessed on: 08 February 2023..
- Incidence of colorectal cancer in North-Western India over 5 Years Daga P, Rawal T, Gupta PK , Khatri NK . Journal of Radiation and Cancer Research.2021;12(4). CrossRef
- Colorectal cancer Dekker E, Tanis PJ , Vleugels JLA , Kasi PM , Wallace MB . Lancet (London, England).2019;394(10207). CrossRef
- ECOG-ACRIN Cancer research group Available at: https://ecog-acrin.org/resources/ecog-performance-status/. Accessed on: 09 February 2023..
- Pattern and Dynamics of Distant Metastases in Metastatic Colorectal Cancer Holch JW , Demmer M, Lamersdorf C, Michl M, Schulz C, Einem JC , Modest DP , Heinemann V. Visceral Medicine.2017;33(1). CrossRef
- India: Globocan 2020 Available at: https://gco.iarc.fr/today/data/factsheets/populations/356-india-fact-sheets.pdf. Accessed on 28 March 2023..
- Treatment sequencing in metastatic colorectal cancer Modest DP , Pant S, Sartore-Bianchi A. European Journal of Cancer (Oxford, England: 1990).2019;109. CrossRef
- Colorectal Cancer in India: An Audit from a Tertiary Center in a Low Prevalence Area Patil PS , Saklani A, Gambhire P, Mehta S, Engineer R, De'Souza A, Chopra S, Bal M. Indian Journal of Surgical Oncology.2017;8(4). CrossRef
- Rising colorectal cancer in young adults: A warning for all! Let us adopt a healthy lifestyle and colorectal cancer screening Patel A, Hande V. Indian Journal of Cancer.2022;59(3). CrossRef
- The history and use of cancer registry data by public health cancer control programs in the United States White MC , Babcock F, Hayes NS , Mariotto AB , Wong FL , Kohler BA , Weir HK . Cancer.2017;123 Suppl 24(Suppl 24). CrossRef
- The role of registration in cancer control and prevention Cancer bioinformatics. https://www.intechopen.com/chapters/79380. Accessed on 28 March 2023..
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2023
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times