A Comparative Study of Hypofractionated Radiotherapy Versus Conventional Radiotherapy for Early Glottic Cancer (T1-2N0M0)
Download
Abstract
Background and objective: Cancer is a major health concern in India, with approximately 1.1 million cases diagnosed annually. Laryngeal cancer constitutes about 1% of the total cancer burden and accounts for 0.3% of all cancer deaths. Glottic tumors typically metastasize after directly invading adjacent structures with better drainage. Glottic cancer boasts a high cure rate, regardless of the treatment modality employed. Radiotherapy is generally favored in most centers despite comparable cure rates for selected T1 and T2 glottis tumors. This study aimed to compare the radiation-induced acute and late treatment-related toxicities of hypofractionated radiotherapy and conventional radiotherapy in early glottic cancer (T1-2N0M0).
Material and Methods: This study was conducted at the Acharya Tulsi Regional Cancer Treatment and Research Institute, Sardar Patel Medical College, Bikaner. It included 50 histologically confirmed cases of early glottic cancer in patients under 70 years of age. Patients were treated with radiotherapy and randomized into two arms: Arm A (Study) and Arm B (Control). Arm A received hypofractionated radiotherapy (55 Gy/20 fractions in 2.75 Gy/fraction over 4 weeks) and Arm B received conventional radiotherapy (66 Gy/33 fractions in 2 Gy/fraction over 6.5 weeks). Voice quality and toxicities were assessed at the end of treatment and at 1, 2, 3, and 6 months follow-up. Data were analyzed using percentages, means, chi-square tests, and p-values.
Results: The majority of patients were in their sixth decade of life and all were male. Most patients had an ECOG Performance Score of 1. Hoarseness alone was present in 46 (92%) patients, while 4 (8%) presented with hoarseness and dysphagia, which were comparable between groups. Histologically, all patients had squamous cell carcinoma (SCC). In the study arm, 11 (44%) patients were T1A, 9 (36%) were T1B, 4 (20%) were T2A, and 1 (4%) was T2B, compared to 5 (20%), 5 (20%), 10 (40%), and 5 (20%) respectively in the control arm. In the study arm, 25 (100%) patients received 58.4 Gy (2 GyEq), while in the control group, 15 (60%) received 66 Gy and the remaining 10 (40%) received 64 Gy. All 25 (100%) patients in both arms completed treatment. At the end of treatment, only 3 (12%) patients in the study arm and 5 (20%) in the control arm had normal voice. At the 1-month follow-up, 7 (28%) versus 9 (36%) patients; at the 3-month follow-up, 13 (52%) versus 15 (60%) patients; and at the 6-month follow-up, 21 (84%) versus 22 (88%) patients in the study and control arms respectively had normal voice (X2 = 1.026, p-value = 0.599). Grades of skin reactions, mucositis, and dysphagia decreased from 2 to 1 at the end of treatment, and further to 1 to 0 at 1, 3, and 6 months follow-up in both groups.
Conclusion: Hypofractionated radiotherapy is a safe treatment modality with high local control rates, acceptable long-term toxicities, favorable voice outcomes, and symptomatic relief, offering the added advantage of a shorter treatment duration, which improves patient compliance.
Introduction
Cancer of the Larynx represents about 1% of the total cancer burden and accounts for 0.3% of all cancer deaths. It is the second most common head and neck mucosal cancer [1]. Cancer is a leading health problem in India with approximately 1.1 million cases occurring each year. At our institute (Acharya Tulsi Regional Cancer Treatment and Regional Institute) [2] there were 155 cases of laryngeal cancer out of 11299 cases registered accounting for 1.37% of total malignancies.
Glottis, the hub of voice production accounts for 60-65% of all laryngeal carcinoma [3]. Glottic carcinomas are usually well differentiated. Grow slow, and tend to metastasize late in their course. Glottic tumors typically metastasize after they have directly invaded adjacent structures with better drainage. These tumors do have early extension toward the anterior third of the vocal cord and the anterior commissure with subsequent spread to the opposite cord of anteriorly invade the thyroid cartilage [4].
Glottic cancer has a high rate of cure and regardless of the modality used, T1 and T2 carcinomas have an excellent probability of cure. The aims of treatment for early glottis cancer are cure, laryngeal voice preservation, optimal voice quality with minimal morbidity, expense and inconvenience [5]. Radiotherapy is generally the favoured treatment in most centers despite comparable cure rates for selected T1 and T2 glottis tumors with laser excision, cordectomy and hemi-laryngectomy. The preference of radiotherapy over surgery stems from a less restrictive selection criteria, better quality of voice and comparable local control and survival rates [4].
The selection of one modality over another continues to generate controversy. With both modalities showing similar efficacy with regard to survival and oncologic outcomes, the selection of one modality over other hinges upon vocal function, quality of life, and cost –effectiveness as the main outcome of interest. Radiotherapy and conservation surgery are the two viable treatment modalities employed in the management of early glottic cancer, with concurrent chemoradiotherapy and radical surgery reserved for advanced disease [6]. The present study was conducted to compare local control rates, radiation induced acute and late treatment related toxicities of hypo-fractionated radiotherapy and conventional radiotherapy in early glottic cancer T1-2N0M0”
The aim of this study was to compare local control rates, radiation induced acute and late treatment related toxicities in treatment with total dose of 55Gy and 64 Gy.
Materials and Methods
The present Prospective analytical study was conducted in Acharya Tulsi Regional Cancer Treatment and Research Institute, Sardar Patel Medical College, Bikaner. It was done on 50 histologically proven new cases of early glottic cancer with age <70 years with European Co-operative Oncology Group (ECOG) performance status 0-2. Patients with distant metastases, other concurrent malignancies and pregnant and lactating women were excluded from the study. Complete history with general physical and systemic examination was done. Local examination of larynx was done by direct and indirect laryngoscopy. Complete blood count, Liver and Renal function test, X-ray soft tissue neck and Chest, Ultrasonography abdomen and CT Scan and MRI of head and neck were done wherever required. Tissue diagnosis was done by biopsy.
Study Design
All 50 enrolled patients of T1-2 N0 M0 were treated by radiotherapy and randomized into either of the two arms Arm A(Study) and Arm B(Control). On ARM A (Study Arm) Hypo-fractionated radiotherapy 55Gy/20# in 2.75Gy/# in 4 weeks (5# per week) and on ARM B (Control Arm) Conventional radiotherapy 66Gy/33# in 2Gy/#6.5 weeks (5# per week) was given. Treatment with radiation therapy was given using Cobalt-60 energy source on Theratron-780 E/780C, Bhabhatron II or using 6 MV photon beam on Linear Accelerator. Field size taken: 5x5 cm/6x6.
Biological effective dose (BED) of hypo-fractionated arm was compared with that of conventional arm. For Hypo-fractionated group, BED was for early effects was 70.125 and for late effects was 105.39 and for Conventional group, BED was for early effects was 79.20 and for late effects was 109.56.
Voice quality was done using the Voice Handicap Index (VHI) questionnaire while toxicities were graded using the RTOG guidelines. At the end of treatment, patients were examined by direct and indirect laryngoscopy, assessed for local response using the RECIST criteria, improvement in symptoms using VHI questionnaire, and toxicities using RTOG guidelines. After 1 month, 2 months, 3 months and 6 months, patients were again assessed in detail as in first visit. Data was analyzed using tools like percentage, mean, chi square test and p-value. Chi-square and p-value were calculated by statistical online software (http:/quantpsy. org) p-value of <0.05 was considered significant. Data is presented in the form or tables and bar diagrams.
Results
In the present study, Majority of the patients were in their 6th decade of life with age in range of 25-70 years and median age was 54 years. All patients were male. ECOG Performance status ranged from 0-2. Majority of the population study had ECOG Performance Score of 1. 46 (92%) of patients presented with hoarseness alone while 4 (8%) of them presented with hoarseness and dysphagia, which were comparable in both the groups. Histologically, throughout the study population, patients had Squamous Cell carcinoma (SCC). 35 (70%) patients had MDSCC, 14 (28%) of them had WDSCC and only 1 (2%) had PDSCC. In this population, 6 patients in study group and 15 patients in Control group had anterior commissure involvement. 92% of the patients gave a history of smoking, only 13 (26%) patients gave a history of chewing tobacco and 34 (68%) patients gave a history of alcohol consumption, which was comparable in both the groups (Table 1).
| Demographic Characteristics | Study Group | Control Group | |
| Age Groups | 25-35 Years | 0 | 1 |
| 36-45 Years | 3 | 1 | |
| 46-55 Years | 2 | 6 | |
| 56-65 Years | 14 | 13 | |
| Sex | Male | 25 | 25 |
| Female | 0 | 0 | |
| ECOG | 0 | 11 | 8 |
| 1 | 14 | 15 | |
| 2 | 0 | 2 | |
| Symptoms | Hoarseness of Voice | 23 | 23 |
| Dysphagia & Hoarseness of Voice | 2 | 2 | |
| Histo-pathological Examination | WDSCC* | 10 | 4 |
| MDSCC# | 15 | 20 | |
| PDSCC@ | 0 | 1 | |
| Anterior Commissure Involvement | Yes | 6 | 15 |
| No | 19 | 10 | |
| Smoking | Never | 1 | 7 |
| ≤ 10 Years | 0 | 0 | |
| > 10 Years | 24 | 18 | |
| History of Tobacco Use | No | 17 | 20 |
| Occasional | 8 | 5 | |
| History of Alcohol Consumption | No | 5 | 11 |
| Occasional | 20 | 14 |
* WDSCC - Moderately differentiated squamous cell carcinoma; # MDSCC - Well differentiated squamous cell carcinoma; @ PDSCC - Poorly differentiated squamous cell carcinoma
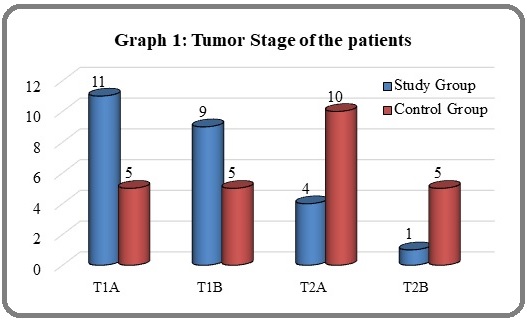
Graph 1 shows tumor stage of the patients. In study vs control arm where 11 (44%) vs 5 (20%) patients were T1A, 9 (36%) vs 5 (20%) in T1B, 4 (20%) vs 10 (40%) in T2A and 1 (4%) vs 5 (20%) in T2B respectively. There was high incidence of T2 stage in control group.
All patients were N0 and M0. Amongst the population, 20 patients were having stage II while the rest 30 were having stage I. In Study group 20 patients were of stage I and 5 patients were having stage II while in control group 10 and 15 patients were having stage I and II respectively (Figure 1).
Figure 1. Tumor Stage of the Patients.
Overall Treatment time
For study arm, most of the population 13 (52%) completed treatment in 28 days, 8 (32%) in 29 days while the rest 2 (8%) in 27 and 30 days each respectively. Overall treatment time ranged from 47-51 days with 7 (28%) patients completing treatment in 49 &50 days each, 6 (24%) in 47 days, 2 (8%) in 48 days, 3 (12%) in 51 days.
Total Treatment dose
In the study arm 25 (100%) patients received 58.4 Gy (2GyEq) whereas in control population 15 (60%) received 66 Gy and the rest 10 (40%) received 64 Gy. All 25 (100%) patients in both arms completed treatment, which is comparable in both groups.
Treatment Response
Treatment response evaluation for disease control using RECIST criteria at subsequent follow up, all 25 patients in study arm and 25 patients in control arm had complete response.
Table 2 shows voice quality assessment of patients done using the voice handicap index (VHI) questionnaire. At the end of treatment, only 3 (12%) patients in study arm and 5 (20%) in control arm had normal voice. At 1st month of follow up, 7 (28%) vs 9 (36%) patients, at 3rd month of follow up, 13 (52%) vs 15 (60%) patients and at 6th month follow up, 21 (84%) vs 22 (88%) patients in study and control arm respectively had normal voice (X2 = 1.026, p value = 0.599).
| Voice Quality | EOT | 1st month F/U | 3rd month F/U | 6th month F/U | ||||
| Study (%) | Control (%) | Study (%) | Control (%) | Study (%) | Control (%) | Study (%) | Control (%) | |
| Normal | 3 (12) | 5 (20) | 7 (28) | 9 (36) | 13 (52) | 15 (60) | 21 (84) | 22 (88) |
| Improved | 10 (40) | 10 (40) | 16 (64) | 12 (48) | 12 (48) | 10 (40) | 4 (16) | 3 (12) |
| Same | 12 (48) | 10 (40) | 2 (8) | 4 (16) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
As compared, at the end of the treatment, at 1st, 3rd and 6th months follow up, grades of skin reactions, mucositis and dysphagia reduced from 2 to 1 and later 1 to 0 (Table 3).
| Toxicities | Grade | EOT | 1st month F/U | 3rd month F/U | 6th month F/U | ||||
| Study | Control | Study | Control | Study | Control | Study | Control | ||
| Skin Reaction | 0 | 0 | 0 | 15 | 13 | 25 | 25 | 25 | 25 |
| 1 | 13 | 11 | 10 | 11 | 0 | 0 | 0 | 0 | |
| 2 | 12 | 14 | 0 | 1 | 0 | 0 | 0 | 0 | |
| Mucositis | 0 | 0 | 0 | 15 | 14 | 25 | 25 | 25 | 25 |
| 1 | 10 | 8 | 8 | 10 | 0 | 0 | 0 | 0 | |
| 2 | 15 | 17 | 2 | 1 | 0 | 0 | 0 | 0 | |
| Dysphagia | 0 | 18 | 13 | 24 | 22 | 25 | 25 | 25 | 25 |
| 1 | 6 | 10 | 1 | 3 | 0 | 0 | 0 | 0 | |
| 2 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
Discussion
Glottic cancer is the most common laryngeal cancer and the intent of treatment is cure. In the present study, all 50 (100%) patients were histologically proven to have squamous cell carcinoma (SCC). Review shows that squamous cell carcinoma is the most common histology [1]. Review of literature on symptomatology highlights hoarseness as the most common presenting symptom while dysphagia, odynophagia, ear ache does not generally occur. The main complain at presentation given by 92% of patients in this study was hoarseness and 8% complained of hoarseness and dysphagia.
In this study, 21 (42%) of the patients had anterior commissure involvement. Studies have shown how anterior commissure involvement is poor predictor of local control and an indicator or recurrence [7,8]. LeQt et al. [9] stated that the five – year local control was 80% for patients with AC involvement and 88% for those without.
About 84% of patients had associated lifestyle habits of tobacco consumption either in the form of smoking or chewing, with 26% of them having mixed habits. Browman et al. [10] in their study concluded that patients with head and neck cancer who continued to smoke during treatment had reduced response to radiation. At the Christie and royal Marsden Hospital [11], in their study of 200 patients with T1 glottic cancer using hypo-fractionated regime, they found that severe late radiation complication was seen in only one patient who continued to smoke heavily after treatment.
In treating early glottic cancer, options include open partial laryngectomy, transoral laser excision, and radiotherapy. Favorable outcomes could be achieved by any of these modalities, and the local control rates at 5 years ranged from 85% to 95% in T1, and from 60% to 80% in T2 stages. The choice of treatment modality usually depends on T-stage, anterior commissure involvement, preference of patient and physician, general status of patient [9]. Among these therapies, radiotherapy may be preferred to surgery if the tumor size becomes bigger, considering the post-therapy voice quality,while open partial laryngectomy is usually considered for treating local recurrence following other modalities [12]. Less restrictive patient selection criteria also favor radiotherapy to surgery for patients who may not be fit for surgery [13].
Patients in the study arm completed their treatment in a range of 27-30 days while those in the control arm completed theirs in a range of 47 – 51 days. All 50 patients in this study completed their treatment. Literature reports that overall treatment time affects local control [9,14].
In this study none of the patients, within the follow up period of 6 months, in either arm had recurrences at local site. Literature states that recurrences occur within 5 years of treatment. Recurrence is more common in patients with anterior commissure involvement [14]. In a study by Ernis et al [15], five year regional control, CSS and OS rates were 95.4, 95.7% and 78.8% respectively. Arif et al [13] in their study reported, local control rate (LCR) at 5 years was 91% and 5yr overall survival (OS) was 86%. Patients with T1a and T1b had 95% and 88% LCR respectively.
Arunsingh et al [16], in 59 patients of histologically proven early glottis squamous cell carcinoma treated at Tata Memorial Hospital Kolkata during September 2011 to July 2014 that were analyzed, they found that hypo-fractionated radiotherapy is safe with acceptable toxicity rates and good overall control. In a study by Laskar et al [17], the local control and overall survival at 10 years were 84 and 86.1%, respectively, for TI glottic carcinoma. They concluded that radical radiotherapy schedules incorporating a higher dose per fraction yield acceptable local control rates and late toxicity. At the Christie and royal Marsden Hospital11, once daily radiotherapy over 3 weeks gives excellent local control in patients with T1 glottic squamous-cell carcinoma and has a low rate of severe complications. Results of our study are in concordance with those of many trials done on hypo-fractionated regime. The study group was small and the follow up period was short as compared to other trials which had larger study groups and longer follow up periods.
In our study, toxicities occurred in to the form of skin reactions, mucositis and dysphagia. At the end of one month, Grade 2 reactions were 48% vs 56% respectively in study and control arm (X2 = 0.762, p value 0.383). Grade 2 mucositis was 60% vs 68% in study vs control arm (X2 = 0.000, p value 0.01). Grade 2 dysphagia was seen in 4% vs 8% patients (X2 = 3.125, p value 0.210). Ernis et al. [13], reported RTOG grade 3 skin toxicity occurred in 9 (6.8%) of patients. RTOG grade 3 mucositis requiring enteral nutrition, occurred in 13 (9.8%) patients. Arunsigh et al. [16], reported in their study 7 (12.3%) patients had grade II skin reactions while 15 (26.8%) had grade II or more dysphagia. Yamakazi et al. [18], observed diffuse coating and/or edema of the vocal cords was recognized in 10 patients (11%) in study arm and 9 (10%) in control arm. Tai Gyu Kim et al.[19], stated that a vast majority of the patient experienced radiation mucositis and dermatitis presenting from 2 weeks of radiotherapy start, and complained of throat pain on swallowing and voice change.
In terms of voice quality, 80% vs 76% patients had normal voice by the 6th month of follow up and patients continued to report improvement in voice quality in subsequent follow ups. Verdonck-de Leeuw at al. [20] reported that voice characteristics improved after radiotherapy.
In conclusion, Hypo-fractionated radiotherapy is a safe modality of treatment with high local control rates, acceptable long term toxicities, favorable voice outcomes and symptomatic relief with added advantage of shorter treatment time which offers better patient compliance.
Good local control and acceptable toxicities have favored hypo-fractionated regime as the established treatment for early glottis cancer. Such favorable outcomes and advantage of shorter treatment time warrant for trials and studies of hypo-fractionated regimes in advanced stage glottic cancer and other malignancies too. In busy set ups where there is heavy patient load in the outpatient department and machines, hypo-fractionated radiotherapy is recommended in other malignancies.
References
- Perez & Brady's principles and practice of radiation oncology Halperin EC , Brady LW , Wazer DE , Perez CA . Lippincott Williams & Wilkins.2013.
- Annual report of Acharya Tulsi Regional Cancer Regional Cancer Treatment & Research Institute, Bikaner 2012 .
- The epidemiology of cancers of the upper alimentary and upper respiratory tracts Wynder EL . The Laryngoscope.1978;88(1 Pt 2 Suppl 8).
- Lingual thyroglossal duct cyst presenting in infancy Samuel M, Freeman NV , Sajwany MJ . Journal of Pediatric Surgery.1993;28(7). CrossRef
- Definitive Radiotherapy for Squamous Cell Carcinoma of the Glottic Larynx Mendenhall WM , Dagan R, Bryant CM , Amdur RJ , Mancuso AA . Cancer Control: Journal of the Moffitt Cancer Center.2016;23(3). CrossRef
- Spread and barriers to spread of cancer with in larynx. Silver CE, et al (Eds): Laryngeal cancer Kirchner JA. Thieme Medical Publishers, New York.1991;2:6-13.
- Definitive radiotherapy for early stage glottic cancer by 6 MV photons Tong CC , Au KH , Ngan RKC , Cheung FY , Chow SM , Fu YT , Au JSK , Law SCK . Head & Neck Oncology.2012;4. CrossRef
- Radiotherapy for T1 glottic carcinoma: impact of anterior commissure involvement Maheshwar AA , Gaffney CC . The Journal of Laryngology and Otology.2001;115(4). CrossRef
- Influence of fraction size, total dose, and overall time on local control of T1-T2 glottic carcinoma Le QT , Fu KK , Kroll S, Ryu JK , Quivey JM , Meyler TS , Krieg RM , Phillips TL . International Journal of Radiation Oncology, Biology, Physics.1997;39(1). CrossRef
- Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer Browman GP , Wong G, Hodson I, Sathya J, Russell R, McAlpine L, Skingley P, Levine MN . The New England Journal of Medicine.1993;328(3). CrossRef
- Three weeks radiotherapy for T1 glottic cancer: the Christie and Royal Marsden Hospital Experience Gowda RV , Henk JM , Mais KL , Sykes AJ , Swindell R, Slevin NJ . Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology.2003;68(2). CrossRef
- Management of T1-T2 glottic carcinomas Mendenhall WM , Werning JW , Hinerman RW , Amdur RJ , Villaret DB . Cancer.2004;100(9). CrossRef
- Synovial Cell Sarcoma of the Hypopharynx Jamshed A, Loya A, Tirmazi AH , Hussain R. Head Neck Surg.2013;4(2):86-88.
- T1-T2N0 squamous cell carcinoma of the glottic larynx treated with radiation therapy Mendenhall WM , Amdur RJ , Morris CG , Hinerman RW . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2001;19(20). CrossRef
- Definitive hypofractionated radiotherapy for early glottic carcinoma: experience of 55Gy in 20 fractions Ermiş E, Teo M, Dyker KE , Fosker C, Sen M, Prestwich RJ . Radiation Oncology (London, England).2015;10. CrossRef
- Arunsingh M, Roy B, Achari R, Tamilselvan S, Shrimali R, Mallick I, Chatterjee S. PO-1115 Hypofractionated radiotherapy in early glottic cancers ñ analysis of image guidance and clinical factors on outcome. Radiotherapy and Oncology. 2015(115):S604. .
- Radiation therapy in T1-T2 glottic carcinoma: influence of various treatment parameters on local control/complications Dinshaw KA , Sharma V, Agarwal JP , Ghosh S, Havaldar R. International Journal of Radiation Oncology, Biology, Physics.2000;48(3). CrossRef
- Radiotherapy for early glottic carcinoma (T1N0M0): results of prospective randomized study of radiation fraction size and overall treatment time Yamazaki H, Nishiyama K, Tanaka E, Koizumi M, Chatani M. International Journal of Radiation Oncology, Biology, Physics.2006;64(1). CrossRef
- Definitive Radiation Therapy foe Early Glottic Cancer : Experience of Two Fractionation Schedules Kim TG , Ahn YC , Nam HR , Chung MK , Jeong HS , Son YI , Baek CH . Clinical and Experimental Otorhinolaryngology.2012;5(2):97-100.
- Consequences of voice impairment in daily life for patients following radiotherapy for early glottic cancer: voice quality, vocal function, and vocal performance Verdonck-de Leeuw IM , Keus RB , Hilgers FJ , Koopmans-van Beinum FJ , Greven AJ , Jong JM , Vreeburg G, Bartelink H. International Journal of Radiation Oncology, Biology, Physics.1999;44(5). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2023
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times