Myelomatous Pleural Effusion: A Case Series
Download
Abstract
Background: Extramedullary disease in multiple myeloma typically occurs at advanced stages and is associated with poor survival. Common extramedullary sites include the nasal cavity, lymph nodes, lungs, central nervous system, liver, spleen, skin, and orbit. Pleural effusions in multiple myeloma are unusual and rarely due to the myeloma itself, occurring in less than 1% of cases.
Case Presentation: This case series described eight patients diagnosed with myelomatous pleural effusion at the Department of Medical Oncology in a tertiary cancer care center in India, between 2012 and 2018. Clinical presentation, diagnosis, treatment, and survival data were extracted from medical records.
Results: The eight patients with myelomatous pleural effusion represented 0.45% of all multiple myeloma cases treated during the study period. Five of the eight patients were female, with a median age of 58 years. Three patients presented with myelomatous pleural effusion at initial diagnosis, while five developed it during disease progression. IgG was the most common immunoglobulin subtype. The International Staging System (ISS) stage was I in one patient and III in seven. After the diagnosis of multiple myeloma, all patients developed myelomatous pleural effusion at a median of 7 months, and all died within 2 weeks.
Conclusion: Pleural effusions in multiple myeloma should be investigated to rule out myelomatous pleural effusion. Myelomatous pleural effusion is a rare manifestation of myeloma, characterized by an aggressive course and poor prognosis.
Introduction
Multiple myeloma (MM) is a malignant disease characterized by the proliferation of neoplastic plasma cells, which replace the normal bone marrow and produce excessive amounts of immunoglobulin [1]. Patients with MM commonly present with bone pain, pathologic fractures, weakness of limbs, anemia, infections, hypercalcemia, spinal cord compression, and renal failure. The development of pleural effusion (PE) in MM is unusual and the incidence is less than 6% [2]. Pleural effusions in MM are seldom a direct consequence of the myeloma itself and are often a result of co-existing systemic illness. Pleural effusion directly due to myeloma involvement or myelomatous PE is rarely observed, occurring in less than 1% of MM cases [3]. Myelomatous PE is associated with a poor prognosis. In this retrospective study, we review the clinical characteristics and treatment outcomes of eight patients with myelomatous PE treated at a tertiary cancer center.
Materials and Methods
Eight patients were diagnosed with myelomatous PE in the Department of Medical Oncology at a tertiary cancer care center in India, during the 7-year period (2012 to 2018). The details of clinical presentation, diagnosis, treatment and survival were abstracted from medical records. Patients were evaluated with baseline blood tests, serum protein electrophoresis and immunofixation electrophoresis (IFE), serum immunoglobulin assay, serum free light chain assay, urine protein electrophoresis and immunofixation electrophoresis, bone marrow biopsy and aspiration and skeletal survey. They were treated with multiagent chemotherapy regimens. Elderly patients received two drug combinations for induction chemotherapy. Remission status was assessed at regular intervals during induction, post induction, post-autologous bone marrow transplantation and during maintenance therapy. Radiotherapy was administered for symptom relief as and when indicated.
Results
A total of 1748 patients were diagnosed with MM during the study period. Eight patients were diagnosed with myelomatous PE, constituting 0.45% of all MM patients. The median age was 58 years (range 43–76 years), and there were 3 males and 5 females. Presenting symptoms and baseline characteristics are summarised in Table 1.
| Case no | Age/ Sex | Clinical feature at presentation | Time of onset | Laterality | IFE | BM PC% | ISS stage | CRAB |
| 1 | 63/ F | Difficulty walking, soft tissue swelling back, fatigue, decreased responsiveness | At presentation | bilateral | IgG-λ | 83 | III | C+R+AB+ |
| 2 | 60/ M | Low backache, Neck node enlargement, pleural effusion and altered sensorium | At presentation | unilateral | IgA-κ | 25 | III | C+R+A+B |
| 3 | 76/ M | Fever, cough, dyspnoea | At presentation | unilateral | IgG-λ | 13 | III | CR+A+B+ |
| 4 | 70/ F | Anterior chest wall swelling | At progression | bilateral | IgG-λ | 40 | III | CRA+B+ |
| 5 | 56/ F | Left lateral rectus palsy, Fatigue, fever, epistaxis | At progression | bilateral | λ light chain | 30 | III | CR+A+B+ |
| 6 | 43/ F | Pain in lower limbs and Low backache | At progression, | unilateral | IgG-λ | 4 | I | CRA+B+ |
| 7 | 44/ F | Swelling in front of the chest | At progression | unilateral | IgG-κ | 28 | III | C+R+AB+ |
| 8 | 53/ M | Low backache, difficulty in passing urine, bilateral lower limb weakness | At progression | unilateral | IgG-λ | 20 | III | CR+AB+ |
M: Male, F: Female, IFE: Immunofixation Electrophoresis, BM: Bone Marrow, PC: Plasma Cells, ISS: International Staging System, CRAB: Hypercalcemia, Renal impairment, Anaemia and Bone lesions
The presenting symptoms were low back ache in three patients, swelling of the chest wall in two, fatigue in two and one each with neck swelling, difficulty in walking, cranial nerve palsy and respiratory tract infection.
Out of eight patients, seven had lytic bone lesions, five had anemia (Haemoglobin < 10gm/dL), six had renal impairment (Serum Creatinine > 2gm/dL), and three patients had hypercalcemia > 11 gm/dL. Serum lactate dehydrogenase was elevated in five patients. The International staging system (ISS) stage was stage I in one patient and stage III in seven patients. IFE revealed that the monoclonal immunoglobulin was IgG-λ in five patients and IgG-κ, IgA-κ and λ light chain disease in one each. Cytogenetic data was not available.
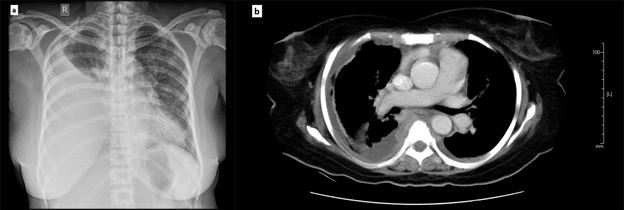
Three patients had myelomatous PE at the time of initial diagnosis and five developed PE at disease progression. Five patients had unilateral PE and three had bilateral PE as diagnosed by chest roentgenogram or computerized tomogram (Figure 1).
Figure 1. (a). Chest X-ray anterior-posterior view showing right sided pleural effusion in a patient with multiple myeloma (b). CT scan showing pleural effusion involving the right side in the same patient with collapse of the underlying lung.
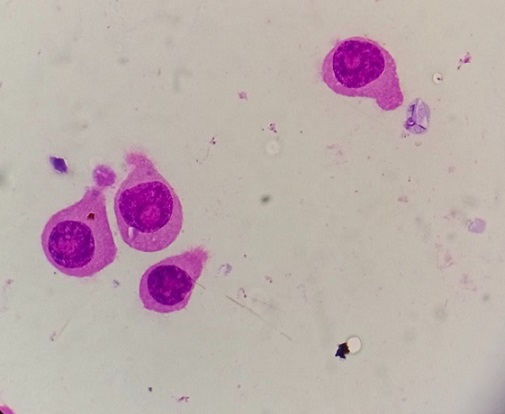
In all eight patients, pleural fluid cytology showed atypical and immature plasma cells, confirming the myelomatous nature of the effusions (Figure 2).
Figure 2. Pleural fluid cytology (1000x) showing plasma cells with round-to-ovoid shape, abundant deep blue cytoplasm with a pale perinuclear area corresponding to the Golgi apparatus. These cells have a round, eccentrically placed nucleus with coarse chromatin arranged in a clock face (cartwheel) pattern.
Details of treatment and outcome are summarised in Table 2.
| Case no. | Chemo prior to myelomatous PE | Chemo after myelomatous PE | Radiotherapy | Outcome | Overall Survival (months) | OS since diagnosis of pleural effusion |
| 1 | - | Vd x 4 weeks | Skull and thorax, 30Gy/10# | Had disease progression | 1.7 | 4 weeks |
| 2 | - | - | - | Died before starting treatment | 0.2 | 1 week |
| 3 | - | BMP x 1 cycle | - | Had disease progression and deteriorated | 2.5 | 14 weeks |
| 4 | BMP→Progression →BLD | - | 20Gy/ 5# to the paraspinal mass | General condition deteriorated rapidly and died | 24 | 1 week |
| 5 | CyBord | Received steroids alone | WBRT | Poor general condition | 6 | 1 week |
| 6 | BLD followed by → ASCT → Len maintenance → Progressive disease → TD | - | Lumbar spine, 8Gy/1# Pelvis, 30Gy/10# | Died during subsequent re-evaluation | 25.4 | 2 weeks |
| 7 | RVd → Progressive disease | DVD x 1cycle | Neck, 8Gy/1# | Died shortly after starting DVD chemotherapy | 2.4 | 1 week |
| 8 | CyBord → progressive disease | VAD x 1 cycle | Thoracic spine, 20Gy/5# | Performance status deteriorated and received supportive care | 8.6 | 1 week |
PE: Pleural Effusion, Chemo: Chemotherapy, RT: Radiotherapy, OS: Overall Survival, Cybord: Bortezomib, Cyclophosphamide, Dexamethasone, Vd: Bortezomib, Dexamethasone, RVd: Bortezomib, Lenalidomide, Dexamethasone, BMP: Bortezomib, Melphalan, Prednisolone, DVD: Liposo- mal doxorubicin, Vincristine, Dexamethasone, VAD: Vincristine, Adriamycin, Dexamethasone, Len: Lenalidomide.
Of the three patients who were diagnosed with myelomatous PE at the time of presentation, one patient received Bd (Bortezomib + Dexamethasone), another received BMP regimen (Bortezomib + Melphalan + Prednisolone), while the third was given only steroids and best supportive care. Patient #1 received palliative RT to the skull and chest for a dose of 30 Gray (Gy) in 10 fractions. All three patients died within a period of 3 months.
Of the five patients who developed myelomatous PE at relapse, two had received CyBord (Cyclophosphamide + Bortezomib + Dexamethasone), two received RVd (lenalidomide + bortezomib + dexamethasone) and one patient was treated with BMP (Bortezomib + Melphalan + Prednisolone) as first-line treatment. Patient #6 who attained a very good partial response (VGPR) after induction chemotherapy underwent autologous stem cell transplantation followed by maintenance chemotherapy with lenalidomide. All five of these patients received palliative radiotherapy. After the diagnosis of MM, these patients developed PE at a median of 7 months (range 1 - 24 months). All patients died within 2 weeks of diagnosis of myelomatous PE.
Discussion
Extramedullary disease usually occurs in the advanced stages of myeloma and predicts poor survival [4]. Commonly involved extramedullary sites in MM include the nasal cavity, lymph nodes, lung, central nervous system, liver, spleen, skin and orbit [5]. Involvement of the serous cavities is uncommon in MM. Pleural effusions are reported to occur in about 6% of patients and often are the result of a coexisting illness like cardiac failure, amyloidosis, pulmonary embolism, pneumonia or a second malignancy [6]. The diagnosis of myelomatous PE is based on the demonstration of malignant plasma cells in the pleural fluid and it is even rarer presenting in less than one percent of MM cases [3].
In this series, five out of the eight were females. The median age was 58 years which is about a decade younger than the median age of presentation in published Western data [7]. IgD MM is rare, but myelomatous PE is said to be more common in IgD MM [8,9]. In our series IgG was the most common monoclonal subtype, which is similar to the data by Kim YJ et al [10]. However, none of our patients had the IgD type of MM. Seven patients in our series (87%) had ISS stage III disease, similar to the study by Yanamandra U et al [9].
Pathogenesis of myelomatous PE is not well defined, it may be due to invasion from adjacent skeletal lesions, extension from chest wall plasmacytomas, tumor infiltration of the pleura, or mediastinal lymph node involvement causing lymphatic obstruction [2]. Identifying Myelomatous PE is essential as it has both prognostic and therapeutic considerations. Management of myelomatous PE includes systemic chemotherapy for MM along with local management of the pleural effusion such as therapeutic thoracentesis or pleurodesis. Initial therapy should focus on performing a diagnostic and therapeutic thoracocentesis causing an overall respiratory symptomatic improvement. Patients with myelomatous PE are usually treated with bortezomib-based combination chemotherapy combination regimens like any other MM. Extramedullary disease in myeloma has very poor outcomes, particularly in cases with myelomatous PE. This remains true irrespective of whether the myelomatous effusion developed at diagnosis or during the course of the disease [8,10]. In our series all patients succumbed within 3 months of developing myelomatous PE. A study by Yanamandra et al. showed similar outcomes with 90.9% mortality and median survival of 2.47 months [9]. Table 3 shows the outcomes of patients with myelomatous PE published in recent years.
| Reference | Year of Study | Total Myeloma cases | No. of MPE Cases | % of MPE | Median age (years) | M:F | Survival after diagnosing MPE (months) |
| Cho YU et al [8] | 1989-2008 | 734 | 19 | 2.6 | 58 | 2.1:1 | 3.9 |
| Wang et al [3] | 2004-2014 | 319 | 2 | 0.62 | 63 | 1:01 | - |
| Uday Yanamandra et al [9] | 2010-2015 | 415 | 11 | 2.65 | 50 | 1.2:1 | 2 |
| Byun et al [11] | 2011-2015 | 575 | 7 | 1.2 | 64 | 1.3:1 | 5 |
| Present study | 2012-2018 | 1758 | 8 | 0.45 | 58 | 0.6 | 1 |
MPE: myelomatous pleural effusion, M:F: Male: Female
Once patients develop myelomatous PE, the disease progresses rapidly with deterioration of performance status, irrespective of the choice of chemotherapy [12,13]. It is not known if patients who develop myelomatous PE have unique genetic mutations that promote pleural involvement and result in poor outcomes.
In conclusion, pleural effusions in MM patients should be investigated to rule out myelomatous PE. Myelomatous PE is rare, having a poor prognostic outlook and an aggressive natural course.
References
- Multiple myeloma Kyle RA , Rajkumar SV . Blood.2008;111(6). CrossRef
- Massive pleural effusion due to IgG multiple myeloma Lang KJ , Lidder S, Aitchison R. Hematology Reviews.2009;1(2). CrossRef
- Pleural Effusion in Multiple Myeloma Wang Z, Xia G, Lan L, Liu F, Wang Y, Liu B, Ding Y, Dai L, Zhang Y. Internal Medicine (Tokyo, Japan).2016;55(4). CrossRef
- Myelomatous Pleural Effusion: A Rare Involvement in Myeloma Alkan O, Tiryaki TO , Kalayoglu-Besisik S. Journal of Medical Cases.2020;11(3). CrossRef
- Extramedullary disease in multiple myeloma Bansal R, Rakshit S, Kumar S. Blood Cancer Journal.2021;11(9). CrossRef
- Myelomatous pleural effusion-A case report Miller J, Alton P. Respiratory Medicine Case Reports.2011;5. CrossRef
- Consensus document for management of multiple myeloma. New Delhi. Division of Publication and Information, ICMR; 2017. Chapter 2 Kaur T , Malhotra H , Sirohi B . Epidemiology.;:p.3-4.
- Myelomatous pleural effusion: a case series in a single institution and literature review Cho Y, Chi H, Park C, Jang S, Seo E, Suh C. The Korean Journal of Laboratory Medicine.2011;31(4). CrossRef
- Clinicopathological Profile of Myelomatous Pleural Effusion: Single-center Real-world Experience and Review of Literature Yanamandra U, Deo P, Sahu KK , Nampoothiri RV , Gupta N, Prabhakaran A, Dhibhar DP , et al . Clinical Lymphoma, Myeloma & Leukemia.2019;19(3). CrossRef
- Multiple myeloma with myelomatous pleural effusion: a case report and review of the literature Kim YJ , Kim SJ , Min K, Kim HY , Kim HJ , Lee YK , Zang DY . Acta Haematologica.2008;120(2). CrossRef
- Pleural Effusion in Multiple Myeloma: Characteristics and Practice Patterns Byun JM , Kim KH , Choi IS , Park JH , Kim J, Shin D, Koh Y, Kim I, Yoon S, Lim H. Acta Haematologica.2017;138(2). CrossRef
- Myelomatous pleural effusion involvement in 23 patients with multiple myeloma: A single-center clinical analysis Zhong Y, Zhang Ji, Wang H. Thoracic Cancer.2015;6(3). CrossRef
- Myelomatous effusion with poor response to chemotherapy Kim YM , Lee KK , Oh HS , Park SK , Won JH , Hong DS , Park HS , Park JS , Lee DW . Journal of Korean Medical Science.2000;15(2). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2023
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times