The Outcome of Anaplastic Glioma Treated at a Tertiary Cancer Care Institute in South Asia
Download
Abstract
Background and objective: Anaplastic glioma is an aggressive brain tumor frequently diagnosed in young adults. Data on anaplastic gliomas from India is limited. This study aimed to evaluate prognostic factors of anaplastic glioma and correlate them with patient outcomes.
Materials and methods: This retrospective observational study included 57 patients with anaplastic glioma treated at a cancer institute in Chennai, India, between 1996 and 2015. Data was extracted from the institute's hospital-based electronic health record tumor registry. Factors analyzed included age, sex, histology, tumor location, type of surgery, adjuvant treatment, and radiation treatment technique. Progression-free survival (PFS) and overall survival (OS) were calculated using the Statistical Package for Social Sciences (SPSS) software.
Result: The median follow-up time was 34 months. The median PFS and OS were 28 and 41 months, respectively. Univariate analysis showed that improved survival was associated with anaplastic oligodendroglioma histology (HR 0.43, CI 0.22-0.80, p = 0.006), frontal lobe location (HR 1.96, CI 1.07-3.58, p = 0.025), gross total resection (HR 1.94, CI 1.03-3.64, p = 0.034), and the addition of temozolomide (HR 2.09, CI 1.14-3.84, p = 0.014). Multivariate analysis confirmed that anaplastic oligodendroglioma histology (HR 0.49, CI 0.25-0.95, p = 0.037), frontal lobe location (HR 2.14, CI 1.12-4.07, p = 0.021), gross total resection (HR 2.89, CI 1.47-5.69, p = 0.002), and the addition of temozolomide (HR 2.02, CI 1.07-3.81, p = 0.029) remained significant factors for improved OS.
Conclusion: This study reveales that anaplastic oligodendroglioma histology, frontal lobe location, gross total resection, and the addition of temozolomide chemotherapy to radiation are associated with improved outcomes for patients with anaplastic glioma. These factors should be considered when planning treatment strategies for this challenging disease.
Introduction
Primary brain tumors are rare, comprising 1-2% of all cancers. Of these, 80% of primary malignant tumors are gliomas [1]. Anaplastic gliomas (AG) which are classified as World Health Organization (WHO) grade 3 tumors, constitute 6.1% of all primary central nervous system gliomas. Anaplastic glioma includes anaplastic astrocytoma (AA), anaplastic oligodendroglioma(AO), and anaplastic oligoastrocytoma (AOA) [2]. These tumors often occur in young adults and typically recur or progress to grade 4 glioblastoma [3]. Some evidence shows anaplastic glioma is the molecular precursor of glioblastoma [3]. However, these tumors are often uncommon; anaplastic astrocytoma accounts for only 3.2%, and anaplastic oligodendroglioma is 1.2% of primary brain tumors compared with a 20.3% incidence with glioblastoma [4]. Anaplastic oligodendroglioma is a type of glioma that typically occurs in adults and is rarely seen in children. It often arises from oligodendrocytes, myelinating cells in the central nervous system. Oligodendroglioma has unique histological features. It is classified as low-grade oligodendroglioma (Grade II) and high-grade oligodendroglioma (Grade III). Low-grade cells have pathological features of monotonous, regular, rounded, well-demarcated cells with abundant clear cytoplasm and a perinuclear halo, lending them an appearance that classically resembles “fried eggs.”These cells are often seen in conjunction with a background of a fine mesh of vasculature that is thought to resemble “Chicken wire.”High-grade glioma often arises from low-grade glioma and histologically resembles low-grade glioma except having features of mitotic figures, high cellular density, irregular cells, and endothelial hyperplasia and proliferation [5]. Anaplastic astrocytoma is a diffusely infiltrating tumor that arises from certain star-shaped brain cells called astrocytes. It is a rare and aggressive tumor. These astrocytes surround and protect other nerve cells found in the brain and spinal cord. Histologically characterized by nuclear atypia, increased cellularity, significant proliferative activity as manifested by mitoses, and a lack of endothelial proliferation or necrosis, the two pathological hallmarks of glioblastoma [6]. Exposure to ionizing radiation and genetic syndromes like neurofibromatosis type 1 and 2, tuberous sclerosis, and Li-Fraumeni syndrome are only established risk factors. The mixed oligodendroglial tumor has histological features of both oligodendroglia and astrocytic tumors [5].
Management is similar to that of glioblastoma multiforme (GBM). Anaplastic oligodendroglioma is often a chemotherapy-sensitive tumor and is different from anaplastic astrocytoma. This chemosensitivity appears to be linked to the loss of chromosomes 1p and 19q heterozygosity. Treatment of anaplastic astrocytoma has been less variable and often resistant to therapy. The median survival of this anaplastic astrocytoma is shorter, only two years, and as compared to anaplastic oligodendroglioma, it is usually five years [7]. Only limited evidence is available in the management of Anaplastic glioma. Treatment of Anaplastic glioma usually maximal safe resection and involved field radiation concurrent with Temozolamide identical to the management of glioblastoma. Owing to the rarity of the disease, it is difficult to study the factors that affect the long-term outcome of Anaplastic glioma. There is limited data from India on Anaplastic glioma. The primary objective of this study is to measure overall survival and progression-free survival. Secondary objectives to assess the prognostic factor include age, sex, histology, site of disease, adequacy of surgery, the addition of temozolomide, and radiation treatment technique were correlated with outcome.
Materials and Methods
General study details
It was a retrospective study of an individual electronic health record of patients with anaplastic glioma treated from 1996 to 2015. The Institute Ethical Committee (IEC) of the Cancer Institute approved the study on January 20, 2023. IEC project number is IEC/2023/ January 07. As it was a retrospective study, we exempted the requirement for informed consent. We ensured that we conducted the study per the ethical guidelines set by the Declaration of Helsinki and other guidelines such as Good Clinical Practice guidelines and those set by the Indian Council of Medical Research.
Inclusion/exclusion criteria
Inclusion criteria include patients over 18 years of age, newly diagnosed anaplastic glioma, anaplastic astrocytoma, and oligodendroglioma treated with surgery and adjuvant chemoradiation. Exclusion criteria include metastatic disease, previous radiation, and non-anaplastic glioma histology.
Study methodology
The evaluation included history, clinical examination, blood count, biochemistry, chest X-ray, and contrast- enhanced MRI (magnetic resonance imaging) of the brain. Patients underwent either biopsy or maximal safe reaction. The adjuvant therapy was chemoradiation or only radiation, depending on the treating physician’s discretion. Adjuvant radiation included a daily dose of 200 cGy per day and a total dose of 60 Gy over 6-7 weeks. The Chemotherpy regime consisted of either oral Temozolomide (TMZ) 75mg/m2/day with cotrimoxazole prophylaxis half before radiation and 150mg /m2/day for 1- 5 days a month for six months after radiation or adjuvant PCV (procarbazine, carmustine, vincristine) regime after radiation. We assessed the response using contrast-enhanced magnetic resonance imaging.
We followed up with patients for three months in the 1st and 2nd year, six monthly in the 3rd to 5th year, and annually after that with clinical examination and imaging when indicated.
Statistical Analysis
We calculated the progression-free survival (PFS) from the date of diagnosis until the date of progression, date of death, or date of last follow-up. Overall survival (OS) was calculated from the date of diagnosis until the date of death or the date of last follow-up and estimated by the Kaplan-Meier method. The Cox regression model was used to assess prognostic factors. We performed statistical analysis using SPSS version 20, and a p-value <0.05 was considered significant.
Results
General study-related details
We analyzed data from 57 patients with anaplastic glioma treated at Cancer Institute Chennai from 1996 to 2015. The baseline patient’s clinical characteristics are as mentioned in Table 1.
| Variable | n | (%) |
| Age median (range) | 36 (11-74) | |
| Sex | ||
| Male | 40 | 70 |
| Female | 17 | 30 |
| Symptom | 47 | |
| Headache | 27 | 30 |
| Seizure | 17 | 23 |
| Motor deficit | 13 | |
| Site | 25 | |
| Parital | 14 | 54 |
| Frontal | 31 | 21 |
| Temporal | 12 | |
| Histology | ||
| Anaplastic Astrocytoma (AA) | 33 | 56 |
| Anaplastic Oligodentroglioma (AO) | 24 | 44 |
| Surgery | ||
| GTR | 23 | 40 |
| STR | 31 | 55 |
| Biopsy | 3 | 5 |
| Treatment | ||
| CRT -TMZ/Adjuvant TMZ | 27 | 47 |
| RT -PCV | 13 | 23 |
| RT | 17 | 30 |
The median age was 36 (range 11-74).
Outcome
The median PFS and OS were 28 and 41 months, respectively, and the five-year PFS and OS were 32.4% and 34.2%, respectively. The median OS of patients with AO and AA was 59 months and 17 months, respectively. The five-year OS of the patients with AO and AA were 50% and 18.8%, respectively.
Factors affecting the outcome
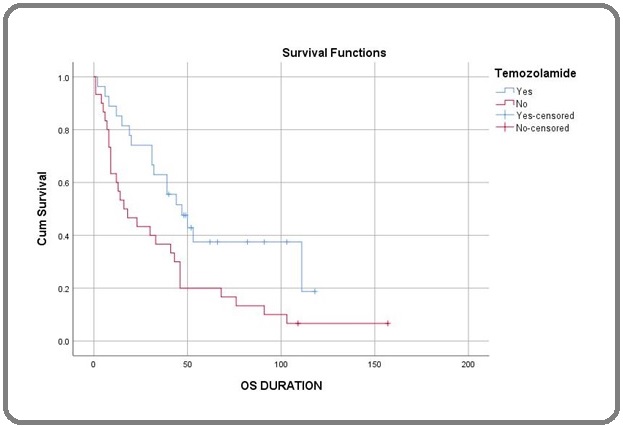
The factors included in the univariate analysis (Table 2) were age, sex, histology, site, type of surgery, treatment modality, and radiotherapy technique. Univariate analysis showed that AO histology (HR-0.43, CI-0.22-0.80, P=0.006), Frontal lobe location (HR-1.96, CI-1.07-3.58, P=0.025), Gross total resection (HR-1.94, CI-1.03-3.64, P=0.034), and addition of Temozolamide with radiation (HR-2.09, CI-1.14-3.84, P=0.014) had a significantly improved outcome.
| Variable | Number | Median survival (months) | 5-years OS (%) | HR | 95% CI | P value |
| Age | ||||||
| ≤36 | 29 | 43 | 40.80 | 1 | ||
| >36 | 28 | 30 | 25.50 | 1.35 | 0.73-2.50 | 0.326 |
| Sex | ||||||
| Male | 40 | 31 | 23.30 | 1 | ||
| Female | 17 | 46 | 40.30 | 0.67 | 0.34-1.30 | 0.23 |
| Histology | ||||||
| AA | 33 | 16 | 21.20 | 1 | ||
| AO | 24 | 47 | 37.20 | 0.43 | 0.22-0.80 | 0.006 |
| Frontal | ||||||
| Yes | 31 | 46 | 36.90 | 1 | ||
| No | 26 | 18 | 19.20 | 1.96 | 1.07-3.58 | 0.025 |
| Gross Total Resection | ||||||
| Yes | 23 | 53 | 47.00 | 1 | 1.03-3.64 | 0.034 |
| No | 34 | 30 | 15.30 | 1.94 | ||
| Temozolamide | ||||||
| Yes | 27 | 47 | 37.50 | 1 | ||
| No | 30 | 16 | 20.00 | 2.09 | 1.14-3.84 | 0.014 |
| Conformal | ||||||
| Yes | 27 | 44 | 33.40 | 1 | ||
| No | 30 | 18 | 24.10 | 1.77 | 0.95-3.27 | 0.063 |
HR-Hazard ratio; CI-Confidence interval; OS-Overall survival
On performing the Multivariate analysis (Table 3), we found patients with Anaplastic Oligodendroglioma histology (HR-0.49, CI-0.25-0.95, P=0.037), Frontal lobe (HR-2.14, CI-1.12-4.07, P=0.021), Gross total resection (HR-2.89, CI-1.47-5.69, P=0.002) and addition of Temozolamide (HR-2.02, CI-1.07-3.81, P=0.029) had a significantly improved outcome.
| Variable | HR | CI | P |
| Histology | |||
| AA | 1 | ||
| AO | 0.49 | 0.25-0.95 | 0.037 |
| Frontal | |||
| Yes | 1 | ||
| No | 2.14 | 1.12-4.07 | 0.021 |
| Gross Total Resection | |||
| Yes | 1 | ||
| No | 2.89 | 1.47-5.69 | 0.002 |
| Temozolomide | |||
| Yes | 1 | ||
| No | 2.02 | 1.07-3.81 | 0.029 |
HR-Hazard ratio; CI-Confidence interval
Figures 1, 2, and 3 show the Kaplan-Meier curve for histology, surgery, and addition of Temozolamide.
Figure 1. Kaplan-Meier Curve for Histology.
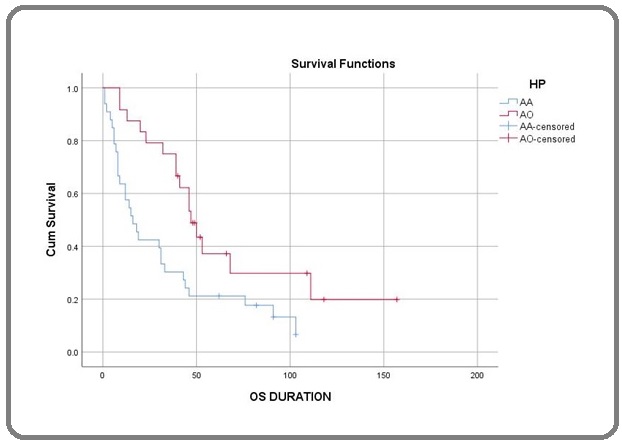
Figure 2. Kaplan-Meier Curve for Surgery.
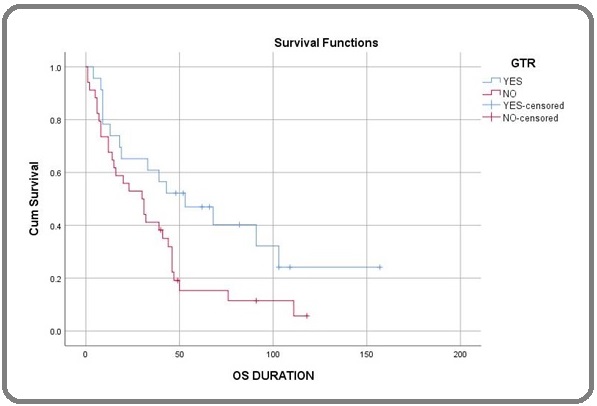
Figure 3. Kaplan-Meier Curve for Temozolamide.
Discussion
Most published literature has combined the patients with AG and GBM, given rarity [3]. However, the biology of both tumors is different, and AG is a molecular precursor of GBM [3]. Anaplastic astrocytoma accounts for 3.2 %, and anaplastic oligodendroglioma is 1.2% of primary brain tumors compared with glioblastoma 20%. The prognosis depends on age, neurological deficit, and ECOG (Eastern Cooperative Oncology Group) performance status [4-7]. Our study showed that younger age (≤36 years) and female sex had non-significant improvements in outcome. One study showed improved outcomes in young (age < 40 years) [8], while the other did not [9]. The female sex has a better outcome, possibly because of a tumor suppressor gene in the additional X chromosome [10].
Anaplastic oligodendroglial tumors had significantly improved survival as compared to anaplastic astrocytoma. The reason is that oligodendroglioma is chemosensitive [11], particularly those with 1p/19q co-deletion, with a median survival of more than ten years [12, 13]. Anaplastic astrocytoma has inferior survival as they are diffuse/ infiltrative, which makes complete resection technically tricky.
Our study showed patients with frontal lobe tumors had a better 5-year OS. Some studies have shown that patients with frontal lobe tumors had better survival than patients with tumors located elsewhere [14-16], while others [9] did not. One possible reason is patients with tumors in the frontal lobe present earlier due to headache, seizure, or cognitive dysfunction. There is an association between 1p/19q co-deletion and tumor location [17].
In our study, the patients who underwent GTR had better survival than those who did not. For example, a study from Cleveland Clinic [18] showed that patients who underwent GTR/STR compared to those who underwent only biopsy had better survival.
In our study, patients treated with adjuvant chemoradiation followed by adjuvant temozolomide showed better outcomes. Deepthi Valiyaveettil [19] et al.’s study also showed that concurrent and adjuvant temozolomide patients had better survival rates. Emory McTyre [20] et al. showed that patients receiving radiation with temozolomide followed by adjuvant temozolomide had improved PFS and OS compared to those who received radiation followed by adjuvant temozolomide.
CATNON trail [21] that had patients without 1p/19q co-deletion showed that patients who received adjuvant temozolomide had improved survival. CODELtrial [22] that had1p/19q co-deleted patients and is for RT followed by PCV versus TMZ concurrent with RT followed by adjuvant TMZ versus TMZ alone.
Our study is one of the first studies from South India to report the outcomes and prognostic factors for anaplastic glioma and confirm the findings reported in the literature. Limitations of our study include retrospective nature, single-center experience, small sample size, and lack of molecular information like IDH mutation, MGMT methylation, 1p19q co-deletion, ATRX, and CGIMP. They may be due to a preliminary study from a low-income country with limited resource access.
In conclusion, among the various clinical and treatment-related prognostic factors, our study revealed that anaplastic oligodendroglioma histology, frontal lobe location, gross total resection, and addition of temozolomide chemotherapy to radiation showed improved outcome.
Institutional Ethical Committee
Approved
Conflicts of Interest
There are no conflicts of interest.
Funding
None.
References
- Primary brain tumours in adults Ricard D, Idbaih A, Ducray F, Lahutte M, Hoang-Xuan K, Delattre J. Lancet (London, England).2012;379(9830). CrossRef
- Anaplastic glioma: how to prognosticate outcome and choose a treatment strategy. [corrected] DeAngelis LM . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2009;27(35). CrossRef
- Indian data on central nervous tumors: A summary of published work Dasgupta A, Gupta T, Jalali R. South Asian Journal of Cancer.2016;5(3). CrossRef
- Survival following adjuvant PCV or temozolomide for anaplastic astrocytoma Brandes AA , Nicolardi L, Tosoni A, Gardiman M, Iuzzolino P, Ghimenton C, Reni M, et al . Neuro-Oncology.2006;8(3). CrossRef
- Anaplastic astrocytoma in adults Stupp R, Reni M, Gatta G, Mazza E, Vecht C. Critical Reviews in Oncology/Hematology.2007;63(1). CrossRef
- Anaplastic astrocytoma: diagnosis, prognosis, and management See SJ , Gilbert MR . Seminars in Oncology.2004;31(5). CrossRef
- Highly anaplastic astrocytoma: a review of 357 patients treated between 1977 and 1989 Prados MD , Gutin PH , Phillips TL , Wara WM , Larson DA , Sneed PK , Davis RL , et al . International Journal of Radiation Oncology, Biology, Physics.1992;23(1). CrossRef
- Oligodendrogliomas: clinicopathological correlations Kros JM . Journal of Neuro-Oncology.1995;24(1). CrossRef
- Oligodendroglioma: incidence and biological behavior in a defined population Mørk SJ , Lindegaard KF , Halvorsen TB , Lehmann EH , Solgaard T, Hatlevoll R, Harvei S, Ganz J. Journal of Neurosurgery.1985;63(6). CrossRef
- The influence of sex and the presence of giant cells on postoperative long-term survival in adult patients with supratentorial glioblastoma multiforme Shinojima N, Kochi M, Hamada J, Nakamura H, Yano S, Makino K, Tsuiki H, et al . Journal of Neurosurgery.2004;101(2). CrossRef
- A phase II trial of primary temozolomide in patients with grade III oligodendroglial brain tumors Gan HK , Rosenthal MA , Dowling A, Kalnins R, Algar E, Wong N, Benson A, et al . Neuro-Oncology.2010;12(5). CrossRef
- Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402 Cairncross G, Wang M, Shaw E, Jenkins R, Brachman D, Buckner J, Fink K, et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2013;31(3). CrossRef
- The impact of molecular and clinical factors on patient outcome in oligodendroglioma from 20 years' experience at a single centre Parkinson JF , Afaghi V, Payne CA , Buckland ME , Brewer JM , Biggs MT , Little NS , et al . Journal of Clinical Neuroscience: Official Journal of the Neurosurgical Society of Australasia.2011;18(3). CrossRef
- Oligodendroglioma: the Rotterdam-Dijkzigt experience Kros JM , Pieterman H, Eden CG , Avezaat CJ . Neurosurgery.1994;34(6). CrossRef
- Oligodendroglioma and anaplastic oligodendroglioma: clinical features, treatment, and prognosis Engelhard HH , Stelea A, Mundt A. Surgical Neurology.2003;60(5). CrossRef
- Oligodendrogliomas: the Mayo Clinic experience Shaw EG , Scheithauer BW , O'Fallon JR , Tazelaar HD , Davis DH . Journal of Neurosurgery.1992;76(3). CrossRef
- Tumor location and growth pattern correlate with genetic signature in oligodendroglial neoplasms Zlatescu MC , TehraniYazdi A, Sasaki H, Megyesi JF , Betensky RA , Louis DN , Cairncross JG . Cancer Research.2001;61(18).
- Prognostic factors in patients with WHO grade 3 gliomas: The Cleveland Clinic experience. Hashemi-Sadraei N, Bawa HS , Satra A, Rahmathulla G, Patel M, Stevens G, Tekautz TM , et al . Journal of Clinical Oncology.2011;29(15_suppl). CrossRef
- Prognostic factors and outcomes in anaplastic gliomas: An institutional experience Valiyaveettil D, Malik M, Joseph D, Ahmed SF , Kothwal SA . South Asian Journal of Cancer.2018;7(1). CrossRef
- Outcomes for Anaplastic Glioma Treated With Radiation Therapy With or Without Concurrent Temozolomide McTyre E, Lucas JT , Helis C, Farris M, Soike M, Mott R, Laxton AW , et al . American Journal of Clinical Oncology.2018;41(8). CrossRef
- EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma Weller M, Bent M, Hopkins K, Tonn JC , Stupp R, Falini A, Cohen-Jonathan-Moyal E, et al . The Lancet. Oncology.2014;15(9). CrossRef
- Results of the interim analysis of the EORTC randomized phase III CATNON trial on concurrent and adjuvant temozolomide in anaplastic glioma without 1p/19q co-deletion: An Intergroup trial. Van Den Bent MJ , Erridge S, Vogelbaum MA , Nowak AK , Sanson M, Brandes AA , Wick W, et al . Journal of Clinical Oncology.2016;34(18_suppl). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2024
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times