Clinico-Epidemiological Profile and Treatment Outcomes of Testicular Germ Cell Tumors: A Retrospective Study from a Tertiary Cancer Center in Northeast India
Download
Abstract
Background and objective: Testicular Germ Cell Tumors (GCTs) are a common malignancy among young adults and are highly curable. However, in India, patients often present at advanced stages, leading to poorer outcomes compared to Western nations where earlier detection is more prevalent. Limited data exists on testicular GCTs from the Indian subcontinent. This retrospective study from the Tata Memorial Centre, Northeast India, explored the epidemiological, clinical, and treatment characteristics of GCTs in this region.
Materials and Methods: This retrospective analysis was conducted on 72 patients diagnosed with testicular GCTs at Tata Memorial Centre - BBCI, Guwahati, from January 2018 to December 2022. Data collected included information related to the patients’ demographics, clinical presentation, staging, treatment, and outcomes.
Results: Among 72 cases, 28 were seminomas and 44 were non-seminomas. Stage I was the most common presentation for seminomas (53.6%), while stage III was most prevalent in non-seminomas (77.2%). According to the International Germ Cell Cancer Collaborative Group (IGCCCG) classification, non-seminomas were categorized as good-risk (25%), intermediate-risk (35%), and poor-risk (40%). Seminomas exhibited a good-risk classification in 54% of cases and intermediate-risk in 46%. Conventional chemotherapy achieved radiologic complete response (CR) in 72% and partial response (PR) in 21% of seminoma patients. Among non-seminoma patients, CR and PR rates were 20% and 61%, respectively. The median recurrence-free survival (RFS) was 43 months. RFS was significantly better in seminomas compared to non-seminomas, stage I compared to stage III, and in the good-risk group compared to the high-risk group.
Conclusion: This study highlights the significant challenge of advanced-stage presentation and high nodal burden in GCT patients from Northeast India. Non-seminomas demonstrated a predominantly partial response to conventional chemotherapy. Future research exploring alternative chemotherapy regimens to improve outcomes in this patient population is warranted.
Introduction
Only 1% of all male malignancies are testicular germ cell tumors (GCTs), making it a rare neoplasm. But among young men, it is the most prevalent solid malignancy. Studies show that the prevalence of testicular GCTs has nearly doubled globally over the past 40 years, although Western countries have seen a decline in mortality rates over that time. The incidence of testicular GCT is the lowest overall in India, at 1.7% [1].
Testicular GCTs are divided into seminoma and non-seminoma as they present distinct epidemiology and natural history, which ultimately guides management strategies. Non-seminoma is further subdivided into 4 types (embryonal carcinoma, yolk cell tumor, teratoma, and choriocarcinoma). Patients with seminoma histology but with elevated alpha-fetoprotein (AFP) or histological diagnosis of mixed GCT are treated as non-seminoma. The Western nations reported an earlier stage at diagnosis while most of the Indian studies have reported an advanced stage at presentation [2,6]. There is a scarcity of literature on GCTs of testis from the Indian subcontinent with reports showing advanced stage of disease at presentation, scrotal violation, and poor compliance to treatment [3-6]. In this study we will report our experience of testicular germ cell tumors presenting at Tata Memorial Centre
- BBCI (Dr. Bhubaneswar Borooah Cancer Institute), Guwahati focusing on epidemiology, histopathology, management, outcomes, and prognosis.
Aims and Objectives
Aims
To evaluate the epidemiology, treatment, outcome, survival, and prognosis of testicular germ cell tumors.
Objectives- Primary objective
To analyze the clinical-epidemiological profile of testicular GCTs in the North-Eastern region of India.
Secondary objectives
To determine the treatment outcomes of surgical procedures, systemic chemotherapy, and their associated toxicity. To determine the recurrence-free survival (RFS).
Materials and Methods
This retrospective observational single institutional study was conducted at BBCI, Guwahati, for the period of 5 years from January 2018 to December 2022. All histologically/serologically confirmed patients with testicular GCTs were included in this study. Patients whose 1) Pre-chemo tumor markers were not available, 2) Retroperitoneal or mediastinal GCT, 3) Histology other than GCT, and 4) Patients with more than one primary cancer were excluded from the study.
The medical records were reviewed for epidemiological data (age, demographic profile), histopathology, stage of the disease, pre-chemo tumor markers (b-HCG, AFP, LDH), S-group, the risk group as per International Germ Cell Cancer Collaborative Group (IGCCCG) classification, detailed treatment protocol including chemotherapy, surgery, and radiotherapy, treatment outcomes and survival.
Patients with seminoma histology, but with elevated AFP or histological diagnosis of mixed GCT were treated as non-seminomas. The study focused on the demographic profile and a clinical presentation concerning age, presenting complaints, histological types and tumor markers, surgical procedures, systemic chemotherapy, associated toxicities, and recurrence-free survival.
Statistical analysis
Statistical evaluation was done using SPSS version 25. Descriptive statistics were used for demographics and clinical characteristics. The chi-square test was used to detect an association between categorical variables. Kaplan-Meier survival curves were plotted for recurrence-free survival. Recurrence-free survival (RFS) was defined as the time from completion of curative surgery/ chemotherapy/ radiotherapy to the time of relapse/ recurrence.
Results
Seventy-two cases of testicular GCTs were studied, of which 28 cases were seminoma and 44 were non-seminoma, respectively. The most affected age group in seminoma was 31-40 years while for non-seminoma, it was 21-30 years. The median age was 40 years (24–77) for seminoma and 27 years (2–73) for non-seminoma. Seven percent of seminoma and 4% of non-seminoma had cryptorchidism. Testicular swelling was the most common symptom and was seen in 89.3% of seminoma and 59.1% of non-seminoma patients. Other presenting symptoms were abdominal pain, abdominal mass, breathing difficulty, left supraclavicular swelling, headache, and vomiting. Seminoma patients mostly had locoregional spread to retroperitoneal nodes. Only 1 patient (3.6%) of the seminoma group had distant metastasis to the lungs. Metastasis was observed in 52% of non-seminoma patients at the initial workup. The lungs were the most common site of metastasis. Eleven percent, 25%, 4% of seminoma, 32%, 34%, and 52% of non-seminoma had N2, N3, and M1 diseases, respectively. In this study, post-op serum tumor marker groups of S0, S1, S2, and S3 were found in 75%, 7.1%, 14.3% and 3.6% of seminoma and 6.8%, 20.4%, 41%, and 31.8% of non-seminoma patients respectively. Post-operative staging revealed seminoma mostly presented in stage I (53.6%) and non-seminoma in stage III (77.2%). As per IGCCCG in patients with advanced disease (i.e., Stage IS, II, III), 25% of non-seminoma were good-risk, 35% were intermediate risk, 40% were poor risk whereas in the seminoma group, 54% were good and 46% were intermediate risk. The poor risk was not applicable to seminoma (Table 1).
| Variables | Seminoma | Non-seminoma | Chi-square test; | |||
| No. | % | No. | % | p-value | ||
| Age | 1 – 10 years | 0 | 0 | 5 | 11.4 | <0.001 |
| 11 – 20 years | 0 | 0 | 2 | 4.5 | ||
| 21 – 30 years | 6 | 21.4 | 22 | 50 | ||
| 31 – 40 years | 8 | 28.6 | 12 | 27.3 | ||
| 41 – 50 years | 7 | 25 | 0 | 0 | ||
| >50 years | 7 | 25 | 3 | 6.8 | ||
| ECOG PS | 0 | 16 | 57.1 | 17 | 38.6 | 0.107 |
| 1 | 11 | 39.3 | 16 | 36.4 | ||
| 2 | 1 | 3.6 | 9 | 20.5 | ||
| 3 | 0 | 0 | 2 | 4.5 | ||
| Presenting complaints | Testicular swelling | 25 | 89.3 | 26 | 59.1 | 0.029 |
| Abdominal pain | 2 | 7.1 | 1 | 2.3 | ||
| Abdominal pain and lump | 1 | 3.6 | 1 | 2.3 | ||
| Breathing difficulty | 0 | 0 | 13 | 29.4 | ||
| Left supraclavicular node | 0 | 0 | 1 | 2.3 | ||
| Headache, Vomiting | 0 | 0 | 2 | 4.6 | ||
| Distant metastasis | Yes | 1 | 3.6 | 23 | 52.3 | <0.001 |
| No | 27 | 96.4 | 21 | 47.7 | ||
| Site of metastases | Lung | 1 | 100 | 17 | 74 | 0.002 |
| Liver | 0 | 0 | 1 | 4.3 | ||
| Non-regional lymph node | 0 | 0 | 2 | 8.7 | ||
| Bone | 0 | 0 | 1 | 4.3 | ||
| Brain | 0 | 0 | 2 | 8.7 | ||
| T staging | T1 | 12 | 42.9 | 14 | 31.8 | 0.805 |
| T2 | 8 | 28.6 | 16 | 36.4 | ||
| T3 | 6 | 21.4 | 11 | 25 | ||
| T4 | 2 | 7.1 | 3 | 6.8 | ||
| N staging | N0 | 15 | 53.6 | 7 | 16 | 0.006 |
| N1 | 3 | 10.7 | 8 | 18.2 | ||
| N2 | 3 | 10.7 | 14 | 31.8 | ||
| N3 | 7 | 25 | 15 | 34 | ||
| Tumor markers | S0 | 21 | 75 | 3 | 6.8 | <0.00001 |
| S1 | 2 | 7.1 | 9 | 20.4 | ||
| S2 | 4 | 14.3 | 18 | 41 | ||
| S3 | 1 | 3.6 | 14 | 31.8 | ||
| Stage of disease | IA | 8 | 28.6 | 0 | 0 | <0.00001 |
| IB | 7 | 25 | 1 | 2.3 | ||
| IS | 0 | 0 | 6 | 13.6 | ||
| IIA | 1 | 3.6 | 1 | 2.3 | ||
| IIB | 2 | 7.1 | 2 | 4.6 | ||
| IIC | 5 | 17.8 | 0 | 0 | ||
| IIIA | 0 | 0 | 5 | 11.3 | ||
| IIIB | 4 | 14.3 | 13 | 29.5 | ||
| IIIC | 1 | 3.6 | 16 | 36.4 | ||
| Risk stratification for advanced disease (Stage IS, II, III) | Good | 7 | 53.8 | 11 | 25.6 | <0.001 |
| Intermediate | 6 | 46.2 | 15 | 34.9 | ||
| Poor | NA | - | 17 | 39.5 | ||
| Not applicable (Stage IA, IB) | 15 | 1 |
Four percent of seminoma and 5% of non-seminoma had scrotal violation due to biopsy done via scrotal route at an outside hospital. Upfront surgery (high inguinal orchidectomy +/- scrotal scar excision) was performed in all seminoma cases and 79.5% of non-seminoma cases. Upfront surgery was not done in one-fifth (20.5%) of non-seminoma cases due to extensive disease at presentation and were operated after neoadjuvant chemotherapy (Table 2).
| Seminoma | Non-seminoma | ||||
| No. | % | No. | % | ||
| Surgery | HIO | 27 | 96.4 | 42 | 95.5 |
| HIO + Scrotal scar excision | 1 | 3.6 | 2 | 4.5 | |
| Upfront surgery | Yes | 28 | 100 | 35 | 79.5 |
| No | 0 | 0 | 9 | 20.5 | |
| Active surveillance after surgery | 1 | 3.6 | 0 | 0 | |
| First line chemotherapy | Carboplatin AUC 7 | 14 | 50 | 0 | 0 |
| BEP | 10 | 35.7 | 21 | 47.7 | |
| EP | 2 | 7.1 | 9 | 20.5 | |
| BEP -> EP | 1 | 3.6 | 7 | 15.9 | |
| VIP | 0 | 0 | 7 | 15.9 | |
| Chemotherapy toxicity | Anemia | 0 | 0 | 2 | 4.5 |
| Neutropenia | 3 | 10.7 | 14 | 31.8 | |
| Thrombocytopenia | 1 | 3.6 | 3 | 6.8 | |
| Febrile neutropenia | 2 | 7.1 | 6 | 13.6 | |
| Mucositis | 1 | 3.6 | 5 | 11.4 | |
| Vomiting | 1 | 3.6 | 5 | 11.4 | |
| Diarrhea | 1 | 3.6 | 3 | 6.8 | |
| Tumor lysis syndrome | 0 | 0 | 1 | 2.3 | |
| Bleomycin toxicity | 1 | 3.6 | 2 | 4.5 | |
| Death | 1 | 3.6 | 1 | 2.3 | |
| Chemo dose reduction due to toxicity | 2 | 7.1 | 4 | 9.1 | |
| Use of G-CSF | Yes | 8 | 28.6 | 21 | 47.7 |
| No | 20 | 71.4 | 23 | 52.3 | |
| Serological response | Yes | 25 | 89.3 | 30 | 68.2 |
| No | 3 | 10.7 | 14 | 31.8 | |
| Radiological response | CR | 20 | 71.4 | 9 | 20.4 |
| PR | 6 | 21.4 | 27 | 61.4 | |
| SD | 1 | 3.6 | 4 | 9.1 | |
| PD | 1 | 3.6 | 4 | 9.1 | |
| Recurrence | Yes | 2 | 7.1 | 10 | 22.7 |
| No | 26 | 92.9 | 34 | 77.3 | |
| Death | Yes | 6 | 21.4 | 16 | 36.4 |
| No | 22 | 78.6 | 28 | 63.6 |
In seminoma, first-line chemotherapy was carboplatin AUC7 in 50%, BEP-based regimen (bleomycin, etoposide and cisplatin) in 35.7%, EP-based regimen (etoposide plus cisplatin) in 7.1%, BEP followed by EP in 3.6% of cases, while in non-seminoma, first-line chemotherapy was BEP in 47.7%, EP in 20.5%, BEP followed by EP in 15.9%, and VIP-regimen (etoposide, ifosfamide, cisplatin) in 15.9% of cases respectively.
Nearly 7%, 15%, and 14% of seminoma and 14%, 43%, and 36% of non-seminoma developed febrile neutropenia (FN), other hematological and non-hematological toxicities respectively, after standard first-line chemotherapy. Toxicities of chemotherapy led to delayed treatment delivery in 10.7% of seminoma cases and 45.5% of non-seminoma cases.
Granulocyte colony-stimulating factor (G-CSF) use was required in 28.6% of seminomas and 47.7% of non-seminomas to maintain the adequate dose intensity.
The primary G-CSF prophylaxis was used only in cases requiring VIP chemotherapy. Bleomycin-induced lung injury was reported in 3.6% of seminomas and 4.5% of non-seminomas.
Rates of radiological complete response (CR) and partial response (PR) in seminoma after first-line chemotherapy were 71% and 21%, respectively. Among non-seminomas, 20% and 61% had radiological CR and PR, respectively. Response evaluation was done using RESIST 1.1 criteria. Two patients with N3 disease (29% of N3) achieved CR in seminoma. In patients with non-seminoma, none with N3 disease achieved CR (Table 2).
12 recurrences were noted, of which 2 were in seminoma and 10 were in the non-seminoma group. The median follow-up period was 36.5 months. In this study on Kaplan Meier survival analysis, we found that the median RFS was 43 months (95% CI; 40.8 – 45.7 months) (Figure 1).
Figure 1. Recurrence-free Survival (RFS) in Months.
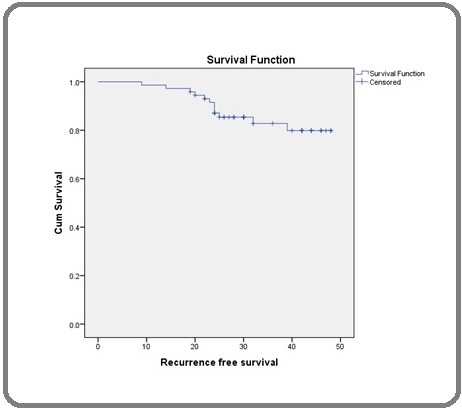
In seminoma, the median RFS was 72 months (95% CI; 71.5 – 73.5 months) while in non-seminoma, the median RFS was 47 months (95% CI; 37 – 56.8 months) which was statistically significant (Log Rank; p=0.03). Similarly, there was a statistically significant difference in median RFS between stage I versus stage III (Log Rank; p=0.03), and good-risk versus high-risk group (Log Rank; p=0.05) as it is presented in Table 2.
Discussion
Except for a few small series, there is a scarcity of recent data on the epidemiology of GCTs from India. The current study showed a significant difference in the participants’ age distribution between seminoma and non-seminoma. Seminoma was more frequently observed in participants between the ages of 31 and 50, with a median age of 40, compared to non-seminoma which was more frequently seen in participants between the ages of 21 and 30, with a median age of 27. Ghazarian AA et al reported similar findings on the median age at diagnosis, noting that the median ages were 36 years and 28 years respectively, for seminoma and non-seminoma [6].
Alpha-fetoprotein (AFP), beta-human chorionic gonadotropin (beta-hCG), and lactate dehydrogenase (LDH) are three relatively sensitive and specific serum biomarkers that are employed in the diagnosis, prognosis, and surveillance of testicular GCTs.7 These tumor markers assist in classifying patients into groups of good-risk, intermediate-risk, and poor-risk together with other prognostic variables [8].
A significant difference in stage-wise distribution between seminoma and non-seminoma groups was observed. Seminoma mostly presented in stage I (53.6% of seminoma cases) while non-seminoma presented in stage III (77.2% of non-seminoma cases) in most of the cases. We observed a statistically significant difference in the risk groups among the seminoma and non-seminoma. Seminoma was more prevalent in the good-risk group and non-seminoma was more prevalent in the poor-risk group.
In our study, 74.4% of non-seminomas belonged to the poor-risk or intermediate-risk group, with 34% of non-seminomas having N3 disease and 52% having metastatic disease at presentation. This figure is substantially higher than that reported from developed countries where the combined poor-risk and intermediate-risk non-seminomas account for 20–30% of cases and that of N3 disease account for only 10–15% [9]. Similarly, 25% of seminomas in our study had N3 nodal disease, a number substantially higher than that reported from the West where it is fewer than 5% [10]. Although these high percentages can be attributed to a referral bias and delay in presentation to a tertiary care center, this is likely to be the case across the country as most of these malignancies are being managed at tertiary care centers only due to a lack of enough infrastructure and trained personnel at the primary and secondary care centers.
After first-line chemotherapy, only 20% of non-seminomas had radiologic CR as per RECIST 1.1. These results were disappointingly low. In previous studies, even in poor-risk subsets, four cycles of bleomycin, etoposide, and cisplatin (BEP) resulted in the achievement of CR in 55–88% of cases [11]. The major challenge in treating non-seminoma at our center has been the presence of bulky nodal disease reflected in the fact that none of the cases with N3 nodal non-seminoma achieved a CR.
In seminomas, which had a predominantly good-risk disease, CR rates were around 71%. This is in line with previous reports of good-risk disease of seminomas where the CR rates with first-line chemotherapy were in the range of 88–97% [12].
Among patients who received first-line chemotherapy with BEP or EP or VIP regimen at our institute, febrile neutropenia occurred in 20% of cases and hematologic toxicity was present in 52% of cases, which is close to previously reported literature [11].
There is an unmet need for a better chemotherapy regimen than the standard BEP for GCTs in first-line settings for patients with high nodal disease burden. The factors that we believe were the major hurdles in achieving optimal outcomes were a high disease burden at presentation, especially in non-seminoma, and a high rate of treatment default. Thirteen percent of cases defaulted to further therapy after first-line chemotherapy. The most common reasons for default were financial constraints and unwillingness for surgery due to the expected complications. This study also highlights that early presentation and referral to a cancer center may go a long way in improving the outcomes of GCT in our country. The use of an alternative chemotherapy regimen to improve outcomes for patients with high nodal disease burden can also be further explored. The major caveat of our study was its retrospective nature.
In conclusion, testicular GCT is a disease of young adults. Patients with localized/locoregional disease and seminoma histology had a better prognosis than those with metastatic disease and non-seminoma histology, which was reflected in recurrence-free survival. Advanced-stage and poor-risk disease at presentation, bulky retroperitoneal nodal disease, and delay in treatment delivery are associated with lower recurrence-free survival. The use of an alternative chemotherapy regimen to improve outcomes for such patients can be further explored.
Acknowledgments
We acknowledge the help extended by the Department of Surgical Oncology, Tata Memorial Centre – BBCI (Dr. Bhubaneswar Borooah Cancer Institute), Guwahati.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Patients’ consent form
Applied for waiver of consent form due to the study’s retrospective nature.
Scientific Committee approval reference number
BBCI-TMC/SC/Appr/250/2023
Institutional Ethics Committee (IEC) approval reference number
BBCI-TMC/Misc-01/MEC/240/2023
References
- Global incidence and outcome of testicular cancer Shanmugalingam T, Soultati A, Chowdhury S, Rudman S, Van Hemelrijck M. Clinical Epidemiology.2013;5. CrossRef
- Etiologic factors in testicular germ cell tumors McGlynn KA , Cook MB . Future oncology (London, England).2009;5(9). CrossRef
- Testicular seminoma: Review of 67 cases from India James FV , Mathew A, Anand RK . Journal of Clinical Oncology.2005;23(16_suppl). CrossRef
- Epidemiology and treatment outcomes of testicular germ cell tumor at tertiary care center in Patna, India: A retrospective analysis Singh D, Singh P, Mandal A. Asian Pacific Journal of Cancer Care.2020;5(1). CrossRef
- Epidemiology of male seminomatous and nonseminomatous germ cell tumors and response to first-line chemotherapy from a tertiary cancer center in India Joshi A, Zanwar S, Shetty N, Patil V, Noronha V, Bakshi G, Prakash G, Menon S, Prabhash K. Indian Journal of Cancer.2016;53(2). CrossRef
- Recent trends in the incidence of testicular germ cell tumors in the United States Ghazarian AA , Trabert B, Devesa SS , McGlynn KA . Andrology.2015;3(1). CrossRef
- Prognostic features and markers for testicular cancer management Leman ES , Gonzalgo ML . Indian journal of urology: IJU: journal of the Urological Society of India.2010;26(1). CrossRef
- Laboratory markers and germ cell tumors Eyben FE . Critical Reviews in Clinical Laboratory Sciences.2003;40(4). CrossRef
- Survival of non-seminomatous germ cell cancer patients according to the IGCC classification: An update based on meta-analysis Dijk MR , Steyerberg EW , Habbema JDF . European Journal of Cancer (Oxford, England: 1990).2006;42(7). CrossRef
- International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.1997;15(2). CrossRef
- Four cycles of BEP versus an alternating regime of PVB and BEP in patients with poor-prognosis metastatic testicular non-seminoma; a randomised study of the EORTC Genitourinary Tract Cancer Cooperative Group Wit R, Stoter G, Sleijfer DT , Kaye SB , Mulder PH , Bokkel Huinink WW , Spaander PJ , Pauw M, Sylvester R. British Journal of Cancer.1995;71(6). CrossRef
- Equivalence of three or four cycles of bleomycin, etoposide, and cisplatin chemotherapy and of a 3- or 5-day schedule in good-prognosis germ cell cancer: a randomized study of the European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group and the Medical Research Council Wit R, Roberts JT , Wilkinson PM , Mulder PH , Mead GM , Fosså SD , Cook P, et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2001;19(6). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2023
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times