Clinicopathological Evaluation of Extragonadal Germ Cell Tumors - A Retrospective Study from a Regional Cancer Center in India
Download
Abstract
Background and objective: Extragonadal germ cell tumors (EGGCTs) are tumors that arise outside the gonads. Their biological behavior and prognosis differ from their gonadal counterparts. This study aimed to analyze the histological subtypes, outcomes, and prognoses of EGGCTs in patients of various age groups.
Material and Methods: This retrospective analysis was conducted on all patients diagnosed with EGGCTs between January 2017 and December 2019 at a regional cancer center in India.
Results: The study included 61 cases of EGGCTs, divided into three age groups: neonatal (birth to six months), prepubertal (more than six months to 12 years), and postpubertal (over 12 years). The prepubertal group was the most frequently affected (44.2%). The age range was from birth to 45 years, with a male-to-female ratio of 1:1.03. The most common primary site was the sacrococcyx (36%), followed by the mediastinum (31.1%). The most prevalent histological subtype was mature teratoma (39.3%), followed by yolk sac tumor (YST) (18%), mixed germ cell tumor (18%), immature teratoma (14.7%), and germinoma (9.8%). Four cases of teratoma exhibited somatic malignancies. The tumor stage distribution was 48 stage I, 8 stage II, and 2 stage III. Statistical analysis revealed a significant association between age group and primary site (p=0.004), histological type (p=0.002), and clinical stage (p=0.034). With a median follow-up of 30 months, an overall survival (OS) rate of 93.2% and an event-free survival (EFS) rate of 90.4% were observed across all age groups. OS rates were 100% (neonatal), 96% (prepubertal), and 88.0% (postpubertal), showing a declining trend with increasing age (p=0.324). Significant prognostic factors for OS included histological subtype (mixed germ cell tumors and teratomas with somatic malignancies had reduced OS) and stage (I vs II and III). Stage was the only significant prognostic factor for EFS (P<0.0001).
Conclusion: This study highlights the clinicopathological characteristics, prognostic parameters, and outcomes of EGGCTs. It emphasizes the importance of detailed pathological reporting for these lesions and the need for comprehensive management strategies tailored to specific age groups and histological subtypes.
Introduction
Extragonadal germ cell tumors (EGGCTs) are relatively uncommon, representing 1% to 5% of all germ cell tumors (GCTs) [1]. Predicting the behavior of these tumors differs significantly from their gonadal counterparts and depends on a combination of factors, including age, anatomical site, histologic subtype, and clinical stage. In this study, we aim to investigate these biologically diverse and rare neoplasms while evaluating their important prognostic parameters.
Materials and Methods
We carried out a retrospective analysis of all cases of EGGCTs diagnosed by both biopsy and resection at our institute from January 2017 to December 2019. Patients with primary gonadal tumors presenting with metastasis to extragonadal location or with streak gonads with or without a history of gonadal germ cell tumors were excluded. Clinical presentation, serum tumor markers (alpha-fetoprotein (AFP), β human Chorionic Gonadotrophin (β hCG) and Lactate Dehydrogenase (LDH)), primary site, clinical staging, treatment, and follow-up information were collected from the medical records. Children’s Oncology Group (COG) staging [2] for pediatric GCTs, Moran and Suster system staging [3] for adult mediastinal GCTs, and soft tissue sarcoma staging by American Joint Committee on Cancer (AJCC-8th edition) for adult retroperitoneal and sacrococcygeal GCTs were followed. All the histopathology slides were retrieved from filing and reviewed by two Onco-pathologists for histological subtype, margin status, coccyx involvement (in cases of sacrococcygeal teratoma), and treatment response (in those who received neoadjuvant chemotherapy (NACT)). The response was scored histologically according to International Germ Cell Consensus Classification Group (IGCCCG) criteria [4] with <10% viable non-teratomatous tumor as a good response. The following immunohistochemical markers were used according to the histomorphology: SALL4, OCT 3/4, CD 117, Glypican 3, CD 30, AFP, and synaptophysin. Recurrence was defined by the re-emergence of serum Alpha-Fetoprotein (AFP) levels in a previously documented normal level or radiological evidence of tumor. Progressive disease refractory to treatment was defined as persistent increase in serum AFP/ increase in tumor size and/or distant metastasis.
The patients were categorized into three subcategories based on the College of American Pathologists (CAP) criteria: congenital/ neonatal (birth to 6 months), prepubertal (7 months to 11 years), and post-pubertal (≥12 years). The data thus obtained were analyzed.
Statistical Analysis
Statistical analysis was done using the software “R”. Baseline parameters were analyzed using de-scriptive statistics. The association of the three subcategories to various parameters like the primary site, histological subtype, and clinical stage were analyzed using the Chi-square test and Fisher Exact test. P value <0.05 was considered to be statistically significant. Overall Survival (OS) and Event Free Survival (EFS) were estimated using Kaplan-Meyer analysis. Overall survival was defined as the time from study entry until death or last follow-up. Event-free survival is defined as the time from study entry till the first occurrence of disease progression, relapse, or until the last contact.
Results
Observation
Our study encompassed 61 cases of EGGCTs. The age range of affected individuals spanned from birth to 45 years with a median age of six years. Among the three defined age groups, the prepuber-tal group comprised the majority (44.2%), followed by post-pubertal (40.9 %) and neonatal (14.7%). The post-pubertal group displayed a male preponderance (2.7:1) whereas the neonatal group exhibited a female preponderance (1:4). Sacrococcyx (22, 36%) was the most common site affected followed by the mediastinum (19, 21.3%), and retroperitoneum (13,31.1%). Additionally, three cases of intracranial germ cell tumors were observed, with one each located in the thalamus, the third ventricle, and posterior falcine. Uncommon sites of involvement included the vagina (2), rectum (1), and stomach (1). The clinical presentation of cases differed according to the site of presentation. Sacrococcygeal lesions typically presented with an external sacral mass (63.6%) or as a presacral mass without externalization (36.3%). Mediastinal tumors manifested with symptoms such as cough (26%), breathlessness (47%), and chest pain (38%). Retroperitoneal tumors presented with abdominal distension (36%) and abdominal pain (58%). Sacrococcyx was the common site involved in the congenital and prepubertal group while mediastinum was the most common site in the post-pubertal group (p=0.004).
| Congenital/ Neonatal | Prepubertal | Postpubertal | Total (%) | P value | |
| Total No. of Cases | 9 (14.7%) | 27 (44.2%) | 25 (40.9%) | 61 | |
| M: F | 1:04 | 1.5:1.7 | 2.7:1 | 01:01.0 | |
| Sites: | |||||
| Sacrococcygeal | 6 | 13 | 3 | 22 (36) | |
| Retroperitoneum | 2 | 6 | 5 | 13 (21.3) | |
| Mediastinum | 1 | 4 | 14 | 19 (31.1) | 0.004 |
| Intracranial | - | - | 3 | 3 (4.9) | |
| Other Sites | - | 4 | - | 4(6.7) | |
| Histological Subtype: | |||||
| Mature Teratoma | 6 | 12 | 6 | 24 (39.3) | |
| Immature Teratoma | 3 | 3 | 3 | 9 (14.7) | |
| Yolk Sac Tumor (YST) | - | 7 | 4 | 11 (18) | |
| Germinoma | - | - | 6 | 6 (9.8) | 0.002 |
| Mixed Germ Cell Tumor | - | ||||
| (Teratoma+Yst) | 3 | 6 | 11 (18) | ||
| (Teratoma+Yst+Ec) | 2 | ||||
| Stage: | |||||
| I | 9 | 25 | 14 | 48 (82.8) | |
| II | 0 | 1 | 7 | 8 (13.80) | 0.034 |
| III | 0 | 1 | 1 | 2 (3.4) |
Histopathological diagnosis
a) Teratomas: Teratomas were the most common histological type across all age groups (54%). This category included 24 cases of mature and nine cases of immature terato-mas. The sacrococcyx was the most common site (39.3%) followed by the retroperito-neum and mediastinum comprising 27.2% each. Two cases of teratoma were noted in the gastrointestinal tract, one in the stomach (Figure 1) and one in the rectum.
Figure 1. Gastric teratoma a) Gross image b) Gastroesophageal junction c) adnexal glands, d) retinal pig-ment epithelium,e) glial tissue f) immature neuroepithelium (H and E, 40x) .

Immature teratomas were graded according to the criteria followed for ovarian teratomas as grade I (4), grade II (2), and grade III tumors (3). In the neonatal group, all cases were diagnosed as teratomas (100%). Four cases of mature teratomas were associated with somatic malignancies, including three with colonic- type adenocarcinoma and one with both carcinoma and sarcoma (squamous cell carcinoma, chondrosarcoma, and neuroendocrine tumor) (Figure 2).
Figure 2. Retroperitoneal Teratoma in a 3-year-old boy (a) Gross Image Showing Tumor with Solid Cystic Areas and Component of Bone b) Microscopy: skin adnexal structures c) Carcinoid tumor showing cells in nests and cords (inset- IHC- synaptophysin positive in neoplastic cells) d) Chondrosarcoma (inset – S100 positive), e) Pancreatic tissue, f) Squamous cell carcinoma. (H and E, 40x) .
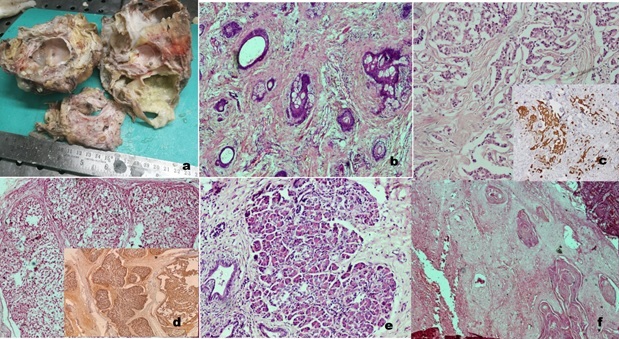
In one case of a mediastinal lesion, a definitive diagnosis of teratoma could not be made despite repeated biopsies due to scanty or not representative tissue. The final diagnosis was established upon resected specimen examination as mature teratoma with adenocarcinoma. Another case of mediastinal teratoma with adenocarcinoma was diagnosed as primary adenocarcinoma, possibly of thymic origin, on biopsy.
b) Germinomas: This histological subtype of GCT was seen exclusively in the post-pubertal group (100%). The most common site was the mediastinum (50%) followed by intracranium (33.3%) and retroperitoneum (16.6%). Elevated serum β-hCG was ob-served in 50% of cases. All the cases exhibited classic histomorphology of germinoma on biopsy and the same was confirmed by immunomarkers.
c) Yolk sac tumor (YST): Out of 11 cases of YST, 7 cases (63%) were noted in the prepu-bertal age group. The sacroccyx was the most common site involved (55.5%) followed by the retroperitoneum (18.1%), mediastinum (9.09%), vagina (18.1%), and intracrani- um (9.09%). All the cases displayed elevated serum AFP levels ranging from 250 U/L to >50,000 U/L and were diagnosed via biopsy, confirmed by SALL4 and Glypican 3 im-munohistochemistry.
d) Mixed germ cell tumor (MGCT): Our study included 11 cases of mixed germ cell tu-mors. The most common site was the mediastinum (54.5%) followed by the sacrococcyx (36.3%) and the retroperitoneum (9.09%). MGCT was observed only in the post-pubertal (54.5%) and prepubertal (45.4%) groups. The mixed germ cell tumor compo-nents comprised teratoma with YST (9 cases), teratoma with YST, and embryonal carci-noma (EC) (2 cases).
No other associated malignancies or syndromes were observed in our study.
Staging, treatment, follow-up, and outcome:
Our study included 48 stage I, eight stage II, and two stage III EGGCT tumors. Intracranial tumors were not staged. All cases of teratomas (both mature and immature) underwent upfront resection while all cases of malignant germ cell tumors received NACT with six cycles of JEB (Carboplatin, Etoposide, and Bleomycin) in patients < 2 years of age and four cycles of BEP (Bleomycin, Etopo- side, and Cisplatin) in patients >2 years. Resection of the residual mass was done following NACT. Recurrent and refractory cases to first-line treatment received salvage chemotherapy with two cy-cles of TIC (Etoposide, Ifosfamide, and Carboplatin). Our follow-up period ranged from 4 months to 68 months with a median follow-up duration of 30 months.
Neonatal group: This group included nine cases (14.7%). All the cases were exclusively teratomas and were categorized as COG stage I. These cases underwent upfront surgery with complete tumor excision.
Coccygectomy was performed in all sacrococcygeal teratomas and coccyx involvement was noted in two out of six cases. None of the neonatal cases displayed recurrence or distant me-tastasis during the follow-up period.
| Case | Age in Years | Location | HPE Diagnosis | Clinical Stage | Treatment | Outcome | Probable Reason |
| No. | / Sex | ||||||
| 1 | 1/M | Sacrococcygeal | Immature teratoma | II | Upfront surgery | *RC -10m (IT+YST) | Positive margin, Inadequate sampling |
| 2 | 8/M | Sacrococcygeal | Mixed GCT (Mature teratoma + YST) | I | NACT+surgery | *RC-1 y MGCT (MT+ YST) | --- |
| 3 | 3.5/F | Vagina | YST | III (inguinal LN +) | NACT+ surgery+ | *RC -6 m (YST) | Incomplete response |
| 4 | 2/F | Vagina | YST | I | NACT+ Surgery+ | *RC -8 m (YST) | Incomplete response |
| 5 | 4/M | Retroperitoneum | Mixed GCT (Immature teratoma+YST) | I | Salvage CT followed by surgery | Lung metastasis and D- 1y | Refractory to treatment, disease progression |
| 6 | 20/F | Retroperitoneum | Immature teratoma | II | Upfront surgery | *RC- 2y (YST) | Positive margin, Inadequate sampling |
| 7 | 28/M | Mediastinum | MT with SM | III (lung) | Upfront surgery | **D-4m | Higher CS |
| 8 | 36/M | Mediastinum | MT with SM | II (Pleura +) | Upfront surgery | **D-7m | Higher CS |
| 9 | 35/M | Mediastinum | MT | II (Pleura +) | Upfront surgery | **D-6m | Higher CS |
*RC- Recurrence, **D- Death IT- Immature teratoma, MT- Mature Teratoma, LN- Lymph nodes, SM- somatic malignancy, CS- clinical stage, CT- Chemotherapy, m- months, y- years (Cases-1 to5- COG staging, Cases 7-9- Moran and suster staging, Case 6-AJCC soft tissue sarcoma staging)
Prepubertal group: The majority (44.2%) of our cases belonged to this group. The incidence of YST was higher in this age group when compared to other groups (63.6%). The stage distribution was as follows: 92.6% of cases were in stage I and 7.4% of cases were in II - III. A single case of retroperitoneal mixed GCT was refractory to treatment, developed lung metastasis, and succumbed despite salvage chemotherapy (Case 5). Two cases of YST, one case of mixed germ cell tumor (immature teratoma +YST), and one immature teratoma developed recurrence 6, 8, 12, and 10 months later, respectively (Case 3, 4, 5, and 1). All four cases received salvage chemotherapy and achieved complete remission.
Post-pubertal group
In our study, 40.9% of cases were post-pubertal adults. Teratomas were the common tumors, followed by germinomas and MGCT, comprising 24% each. The mediastinal tu-mors were staged according to Moran and Suster staging as stage I (78.5%), stage II (14.2%), and stage III (7.1%). Retroperitoneal and sacrococcygeal tumors were staged according to the AJCC soft tissue sarcoma staging system as stage I (37.5%) and stage II (62.5%). All cases of teratomas underwent upfront complete resection and had an uneventful follow-up, except a single case of ret-roperitoneal immature teratoma that recurred as a yolk sac tumor two years later (case 6). All cases of MGCT exhibited a good histological response to NACT and achieved complete remission. 14.2% of cases in this group were associated with somatic malignancies. The mortality rate was higher in this group (12%) and all cases occurred in mediastinal location and presented at stages II and III.
The present study revealed a statistically significant correlation between histological type and stage concerning age. In the neonatal group, all cases were exclusively teratomas. In the prepubertal group, only non-seminomatous tumors were observed, whereas in the post-pubertal group, both seminomatous and non-seminomatous tumors were noted (p=0.002). In the neonatal group, all tumors presented in stage I in contrast to the prepubertal (92.6%) and post-pubertal group (78.5%) (p=0.034). The estimated O.S was 93.4% and EFS was 90.2% across all age groups. Overall survival of 100% was observed in the neonatal, 96.3% in the prepubertal and 88.0% in the post-pubertal group showing a declining trend with increasing age (p=0.324), although not statistically significant (Figure 3).
Figure 3. Overall Survival and Disease-free Survival Analysis in Three Subgroups of Cases. a. Event-free survival (EFS), (p-value= 0.129) b. Overall survival (OS) in months (p value=0.324). Group: 1- neonatal, Group 2- prepubertal, Group 3- post-pubertal.
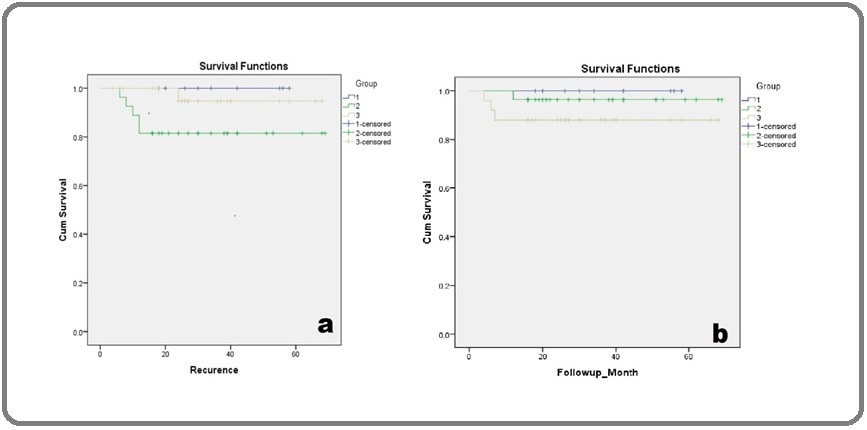
An excellent prognosis was noted in the neonatal group regardless of the tumor location. The prepubertal group showed a higher percentage of recurrence with the least EFS (81.8%) among the three subgroups. We observed poorer OS in mediastinal lesions (84.2%) (p=0.239) and the least EFS in retroperitoneal lesions (84.6%) (p=0.446). Kaplan-Meier analysis identified histological subtype as a significant prognostic parameter with p<0.001 in OS analysis. Mixed germ cell tumors and teratomas with somatic malignancies displayed decreased OS (90.9% and 20% respectively) compared to other tumors (100%). A higher recurrence rate was observed in pure yolk sac tumors, with the least EFS of 81.8% (p=0.762). Stage was a significant parameter in OS and EFS analysis, with stage III tumors exhibiting the lowest survival (50% OS and EFS) (p<0.001).
| Parameters | Estimated OS (%) | P value | Estimated EFS (%) | P value |
| Overall | 93.40 | 90.20 | ||
| Categories | ||||
| ·Neonatal | 100.00 | 100.00 | ||
| ·Prepubertal | 96.30 | 0.324 | 81.50 | 0.129 |
| ·Post-pubertal | 88.00 | 96.00 | ||
| Tumor site | ||||
| ·Sacrococcyx | 100.00 | 90.90 | ||
| ·Retroperotoneum | 92.30 | 0.239 | 84.60 | 0.446 |
| ·Mediastinum | 84.20 | 100.00 | ||
| ·Intracranium | 100.00 | 100.00 | ||
| Clinical stage | ||||
| ·I | 97.90 | 93.80 | ||
| ·II | 75 | <0.001 | 75 | <0.001 |
| ·III | 50 | 50 |
Discussion
EGGCTs arise from the aberrant lodging of primordial germ cells along the midline during their migration [5]. Histologically, EGGCTs exhibit similarities to gonadal germ cell tumors but they differ in their clinical and biological behavior. Shared features with gonadal germ cell tumors in-clude their common occurrence in young individuals, elevated serum markers, responsiveness to cisplatin-based chemotherapy, and the presence of i (12p) abnormality. However, several distinctive features set EGGCTs apart, including their frequent presentations with larger tumor masses, varying prognoses associated with age, tumor location, a predominance of non-seminomatous components, and unique associations with Klinefelter syndrome and hematological neoplasms that often prove refractory to treatment [6].
Congenital/Neonatal Group
In this group, all cases in our study were teratomas. The most prevalent site was the sacrococcyx where we observed a female predominance, aligning with existing literature[4, 5]. Other uncommon sites include the mediastinum, head and neck, and the retroperitoneum [6-11]. Although Issac et al. [12] have reported a 5% frequency of yolk sac tumors in congenital teratomas, we did not encounter any malignant GCTs in this age group. Furthermore, no recurrences or metastases were observed during the follow-up period, resulting in an OS and EFS of 100%. Our results were consistent with Mc. Kenney et al. [9], who observed no recurrences following the complete surgical removal of teratomas during a median follow-up of 36 months.
Childhood (Prepubertal)
In this group, we exclusively observed non- seminomatous germ cell tumors, with YSTs being the most prevalent subtype (69.2%), consistent with the findings of Schneider et al. [13]. Among the 27 prepubertal EGGCTs, four exhibited recurrences, including two yolk sac tumors, one immature teratoma, and one mixed germ cell tumor. Notable, one case of sacrococcygeal immature teratoma with a positive margin developed mixed GCT (YST+teratoma) 10 months post-surgery (case 1). Marina et al. [14] have identified foci of yolk sac tumors in 23 out of 73 cases of immature teratoma upon review, underscoring the need for extensive sampling and margin assessment in all cases of teratomas. This is crucial as some studies have reported the absence of a yolk sac in the primary tumor with the recurrence showing a yolk sac component. [15,16] Two of our cases of vaginal yolk sac tumor showed recurrences 6 months (case 3) and 8 months (case 4) later with an increase in serum AFP levels. Both the cases showed incomplete response to NACT with >50% viable tumor. Consequently, the assessment of viable non-teratomatous components becomes pivotal as it significantly influences prognosis. [17]
Mediastinal tumors in this group predominantly presented as teratomas or YSTs. Among our four mediastinal tumors, three were teratomas and one was a mixed germ cell tumor (teratoma and YST). The prognosis of mediastinal GCTs is closely linked to the extent of surgical excision [18]. In our study, all mediastinal GCTs were at stage I with negative margins, and their follow- up was uneventful. However, Kim et al. [19] showed poorer EFS in patients with mediastinal disease (n=12, 66.7%±13.6%) compared to those with non-mediastinal disease (n=54, 96.0%±2.8%) (p =0.001).
Retroperitoneal GCTs are very rare comprising <5% of all EGGCTs [1]. We had six cases of retro- peritoneal GCTs which included one case of mixed GCT (teratoma and YST) and five cases of teratoma. One of our cases of mature teratoma was associated with multiple somatic malignancies including squamous cell carcinoma, chondrosarcoma, and neuroendocrine tumor at presentation. Very few case reports have shown the simultaneous occurrence of carcinoma and sarcoma. Sarcomas more typically follows chemotherapy or late relapse [20]. Oosterhuis et al. [21] have reported a case of intestinal-type adenocarcinoma and a low-grade leiomyosarcoma in a late recurrence 19 years after the initial diagnosis of testicular GCT [21].
Adults (Post- pubertal)
In this age group, the mediastinum emerged as the commonly involved site (56%) with a male pre-dominance (male: female ratio of 2.7:1), a stark contrast to the other two subgroups. A study by Moran and Suster [3] found that out of 322 cases of mediastinal GCTs, 320 were noted in males. Unlike in other EGGCTs, the grading of immature teratomas and clinical staging, particularly in mediastinal lesions assumes greater prognostic significance [1]. In our study, two cases presented at stage II with pleural infiltration, and one at stage III with lung metastasis. All mediastinal teratomas in our study were mature teratomas, with three cases associated with somatic malignancies, all of which were adenocarcinomas. Fan J et al. [22] also observed that 90% of somatic malignancies arising from teratomas were adenocarcinomas. Two of our cases with somatic malignancies succumbed within seven and six months post-surgery respectively (Case 7 and 8) indicating the aggressive nature of this disease. A study on male EGGCTs by Busch et al. [23] showed that the 5-year overall survival in adult EGGCTs ranged from 40% to 90% and is more favorable for retroperitoneal seminomatous tumors than mediastinal non-seminomatous tumors.
In Conclusion, germ cell tumors must be considered in the differential diagnosis, as they can manifest across all age groups at various extragonadal locations. Age, histological subtype, and stage remain pivotal prognostic parameters in our study. Pathologists play a crucial role not only in clinching the correct diagnosis but also in evaluating the various prognostic factors that contribute to assessing the disease’s outcome.
Acknowledgements
We acknowledge Dr.Vijay CR (Biostatistics, Kidwai Memorial Institute of Oncology) for assisting in do-ing the statistical analysis of our research work.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for- profit sectors.
The authors declare no conflict of interest.
References
- Extragonadal germ cell tumors: a review with emphasis on pathologic features, clinical prognostic variables, and differential diagnostic considerations McKenney JK , Heerema-McKenney A, Rouse RV . Advances in Anatomic Pathology.2007;14(2). CrossRef
- Pediatric germ cell tumors Rescorla FJ , Breitfeld PP . Current Problems in Cancer.1999;23(6). CrossRef
- Primary germ cell tumors of the mediastinum: I. Analysis of 322 cases with special emphasis on teratomatous lesions and a proposal for histopathologic classification and clinical staging Moran CA , Suster S. Cancer.1997;80(4).
- International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group Wilkinson PM , Read G, et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.1997;15(2). CrossRef
- The Borderland of Embryology and Pathology Willis RA . Bulletin of the New York Academy of Medicine.1950;26(7).
- Extragonadal germ cell tumors. In Raghavan D, Scher HI, Leibel SA, Lange PH (ed.): Principles and Practice of Genitourinary Oncology. Philadelphia, PA: Lippincott- Raven 1997 Shivdasani RA , Kantoff PW . ;:751-764.
- Abdominal, retroperitoneal and sacrococcygeal tumours of the newborn and the very young infant. Report from the Kiel Paediatric Tumour Registry Harms D, Schmidt D, Leuschner I. European Journal of Pediatrics.1989;148(8). CrossRef
- Prognostic factors in children with extragonadal malignant germ cell tumors: a pediatric intergroup study Marina N, London WB , Frazier AL , Lauer S, Rescorla F, Cushing B, Malogolowkin MH , et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2006;24(16). CrossRef
- Congenital teratoma: a clinicopathologic study of 22 fetal and neonatal tumors Heerema-McKenney A, Harrison MR , Bratton B, Farrell J, Zaloudek C. The American Journal of Surgical Pathology.2005;29(1). CrossRef
- Treatment of childhood germ cell tumors. Review of the St. Jude experience from 1979 to 1988 Marina N, Fontanesi J, Kun L, Rao B, Jenkins JJ , Thompson EI , Etcubanas E. Cancer.1992;70(10). CrossRef
- Gonadal and extragonadal germ cell neoplasia of childhood Dehner LP . Human Pathology.1983;14(6). CrossRef
- Perinatal (fetal and neonatal) germ cell tumors Isaacs H. Journal of Pediatric Surgery.2004;39(7). CrossRef
- Epidemiologic analysis of 1,442 children and adolescents registered in the German germ cell tumor protocols Schneider DT , Calaminus G, Koch S, Teske C, Schmidt P, Haas RJ , Harms D, Göbel U. Pediatric Blood & Cancer.2004;42(2). CrossRef
- Complete surgical excision is effective treatment for children with immature teratomas with or without malignant elements: A Pediatric Oncology Group/Children's Cancer Group Intergroup Study Marina NM , Cushing B, Giller R, Cohen L, Lauer SJ , Ablin A, Weetman R, et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.1999;17(7). CrossRef
- Occult malignancy in neonatal sacrococcygeal teratomas. A report from a Combined Pediatric Oncology Group and Children's Cancer Group study Hawkins E, Issacs H, Cushing B, Rogers P. The American Journal of Pediatric Hematology/Oncology.1993;15(4).
- Sacrococcygeal teratomas: the UK Children's Cancer Study Group's experience. I. Neonatal Huddart SN , Mann JR , Robinson K, Raafat F, Imeson J, Gornall P, Sokal M, et al . Pediatric Surgery International.2003;19(1-2). CrossRef
- A pathologic analysis of lesions following modern chemotherapy for metastatic germ-cell tumors Ulbright TM , Roth LM . Pathology Annual.1990;25 Pt 1.
- Germ-cell tumors in childhood and adolescence. GPOH MAKEI and the MAHO study groups Göbel U, Schneider DT , Calaminus G, Haas RJ , Schmidt P, Harms D. Annals of Oncology: Official Journal of the European Society for Medical Oncology.2000;11(3). CrossRef
- Prognostic factors in children with extracranial germ cell tumors treated with cisplatin-based chemotherapy Kim J, Lee NH , Lee SH , Yoo KH , Sung KW , Koo HH , Seo J, Lee S. Korean Journal of Pediatrics.2015;58(10). CrossRef
- Late occurrence of malignancy following resection of a histologically mature sacrococcygeal teratoma. Report of a case and literature review Lack EE , Glaun RS , Hefter LG , Seneca RP , Steigman C, Athari F. Archives of Pathology & Laboratory Medicine.1993;117(7).
- Patient with two secondary somatic-type malignancies in a late recurrence of a testicular non-seminoma: illustration of potential and flaw of the cancer stem cell therapy concept Oosterhuis JW , Peeters SHP , Smit VTHBM , Stoop H, Looijenga LHJ , Elzevier HW , Osanto S. The International Journal of Developmental Biology.2013;57(2-4). CrossRef
- Testicular Germ Cell Tumors with Somatic-type Malignancy Fan J, Guan Y, Guo CC , Wang G. Journal of Clinical and Translational Pathology.2023;3(1). CrossRef
- Male Extragonadal Germ Cell Tumors of the Adult Busch J, Seidel C, Zengerling F. Oncology Research and Treatment.2016;39(3). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2024
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times